
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | M. Christopher Dean | -- | 3620 | 2023-12-11 13:31:03 | | | |
| 2 | Mona Zou | -1 word(s) | 3619 | 2023-12-12 08:16:37 | | | | |
| 3 | Mona Zou | + 1 word(s) | 3620 | 2023-12-12 08:30:26 | | | | |
| 4 | Mona Zou | -149 word(s) | 3471 | 2023-12-12 08:34:42 | | | | |
| 5 | Mona Zou | -1 word(s) | 3470 | 2023-12-12 08:35:09 | | |
Video Upload Options
Teeth begin to grow in the jaws before birth and continue to appear in an overlapping sequence until each is complete in length. Subsequently, the central pulp (nerve) chamber of each tooth slowly fills in with dentine and the root of the tooth continues to accumulate thin layers of cementum until the individual dies. Each of the tooth tissues, the hard enamel cap, the dentine core of the tooth and the root cementum grow incrementally and incorporate small quantities of blood-born trace elements ingested from our diet into their structure. A chronological record of zinc incorporation exists in each tooth tissue and can be visualised in thin sections, or slices, of teeth using a beam of synchrotron light. Zinc markings in teeth are especially useful and occur at birth in enamel and dentine and annually in the cementum layers. This work shows that zinc is consistently concentrated within surface enamel and in the dentine surrounding the central pulp chamber. Knowing where to sample Zn in modern and fossil teeth enables us to reconstruct a chronology of growth and to determine something about diet in the past from the remnants of different Zn isotopes contained in different foodstuffs.
1. Introduction
Zinc is an essential biological trace element. Besides this, Zn also has an affinity for bone and the three mineralised tooth tissues, enamel, dentine and cementum. Not only does the distribution of Zn vary among the three tooth tissues, but its origins also derive from different physiological, developmental and chemical processes. Ultimately, the Zn source is either from the intestinal absorption of ingested dietary Zn, or from the mother perinatally, via a direct-blood-borne maternal–placental transfer to the fetus. Zinc retrieved from tooth tissues in an archaeological and palaeontological context offers strong potential for revealing aspects of an individual’s life history, for example, as a marker of birth or of annual increments of tissue laid down during life, or as an indicator of diet. Reviewing the role and function of Zn in the body, and of how it comes to distribute differently in each of the tooth tissues, provides some insight into how and where it might best be sampled and of where its incorporation into each of the mineralised tooth tissues is likely to be tightly chronologically circumscribed or not. Fossil tooth tissues are often, if not always, altered through diagenesis, and a comparison of Zn distribution and preservation in fossils from contrasting sites may point to similarities or differences with modern teeth and so indicate where within the tooth tissues Zn of biogenic origin persists for longest.
2. Zinc as an Essential Trace Element in the Body
3. Zinc in Enamel Secretion and Maturation
4. Zinc Absorption and Maternal–Fetal Transfer
5. The Distribution of Zinc in Mineralised Tissues
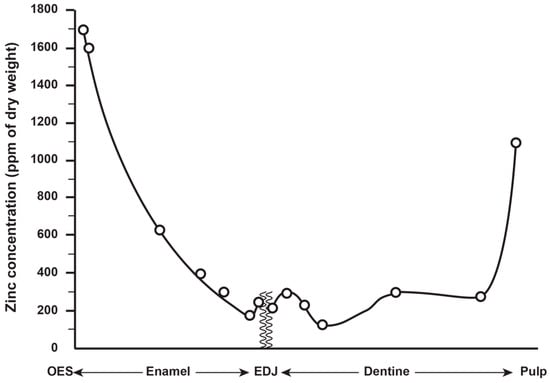
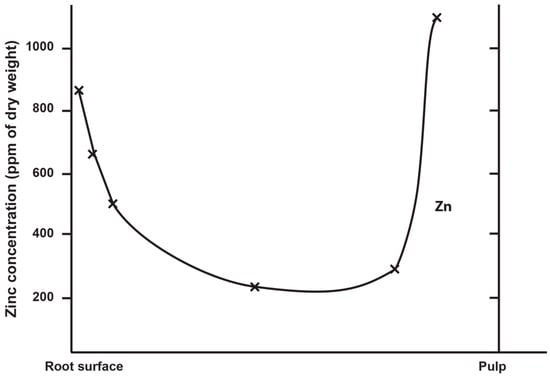
6. Zinc Incorporation into Hydroxyapatite
7. Zinc Distribution in Modern and Fossil Teeth
Zinc concentration in outer enamel is universally high across all the deciduous, permanent and fossil hominoid teeth studied so far. Where secondary dentine has formed, or is preserved, Zn concentrations are also higher than in primary dentine. Besides enamel and dentine, cementum layers also contain Zn at greater concentrations than the underlying root dentine. Figure 3 shows synchrotron X-ray fluorescence (SXRF) maps of the Zn distribution in seven permanent teeth of varying tooth types.

Figure 3. Zinc intensity maps revealed with synchrotron X-ray fluorescence (SXRF) of permanent modern great ape and human teeth, (a) Pan lower first molar tooth, (b) Pan lower third molar tooth, (c) Pongo female canine, (d) modern human upper canine (e) Gorilla lower second molar tooth, (f) Gorilla unerupted lower third molar tooth from the same individual as (e), (g) modern human lower third molar tooth. In all teeth, including the unerupted lower third molar, surface enamel is Zn-rich. In all cases, where it has formed, secondary dentine and cementum are Zn-rich. The Zn distribution in dentine in each tooth broadly follows the incremental formation pattern. Images are not to scale.
The thickness of the Zn-enriched surface enamel layer was observed not to be proportionate in thickness to the regional linear enamel thickness and varied considerably between and within teeth in both the maximum Zn concentration measured in outer enamel (197–1743 ppm), and in the rate of the exponential rise of concentration towards the surface. Zinc levels at the cusp, mid-crown and cervix of the same tooth may vary, but mid-crown measurements are as good a representation of the overall average concentrations as any, and perhaps the most consistently high. The Zn concentration range and maximum values in outer enamel overlap in the modern human, great ape and fossil hominoid deciduous and permanent tooth samples studied here. Zinc is also laid down in teeth prenatally and at the birth line (or neonatal line) in deciduous teeth and along the enamel-dentine junction (Figure 4). Figure 4 shows a thin section of a modern deciduous tooth cusp with the neonatal line as it appears in transmitted light and with synchrotron X-ray fluorescence (SXRF) to reveal the Zn distribution corresponding with the neonatal line in both enamel and dentine.

Figure 4. (a) Transmitted light micrograph and (b) matching Zn SXRF intensity image of the cuspal region of a modern human second deciduous molar tooth showing the neonatal line and enamel-dentine junction. In the SXRF Zn map, the neonatal line in both enamel (white arrows) and dentine (black arrows) is Zn-rich as is the prenatal dentine (black asterisks) and enamel-dentine junction. In prenatal enamel (white asterisks), the SXRF intensity map suggests this is Zn-depleted relative to postnatal enamel. Adapted from Figs. 2e and h in Dean et al [34].
Zinc is also tenacious compared to other trace elements contained in fossil tooth tissues and distributes within many fossil teeth exactly as it does in modern teeth. It can even be preserved at the neonatal line in fossil teeth for millions of years (Figure 5) and used as a marker of birth, with the caveat that Zn lines in enamel and dentine may also be indicative of other stress events in life.

Figure 5. (a) Transmitted light micrograph (TLM) and (b) matching Zn SXRF intensity image of the cuspal region of a fossil first molar tooth showing the neonatal line (white arrows) and enamel-dentine junction. This fossil hominoid tooth is attributed to Ekembo heseloni and dates to ~17.5 million years from Rusinga Island, Kenya.
The consistent distribution of Zn in both modern and fossil hominoid teeth allows us to take samples with greater confidence and precision. New high-resolution sampling and analytical techniques now enable access to the chronological record of Zn laid down in dentine and cementum layers through life with good prospects for tracking dietary shifts, stress events and seasonal changes far back into the archaeological and fossil records.
References
- Terrin, G.; Berni Canani, R.; Di Chiara, M.; Pietravalle, A.; Aleandri, V.; Conte, F.; De Curtis, M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients 2015, 7, 10427–10446.
- Greenberg, S.R. The Histopathology of Clinically-Important Metals. A Review. Histol. Histopathol. 1989, 4, 375–380.
- Moonga, B.S.; Dempster, D.W. Zinc Is a Potent Inhibitor of Osteoclastic Bone Resorption in Vitro. J. Bone Miner. Res. 1995, 10, 453–457.
- Lynch, R.J.M. Zinc in the Mouth, Its Interactions with Dental Enamel and Possible Effects on Caries; a Review of the Literature. Int. Dent. J. 2011, 61, 46–54.
- Ganss, B.; Jheon, A. Zinc Finger Transcription Factors in Skeletal Development. Crit. Rev. Oral Biol. Med. 2004, 15, 282–297.
- Maret, W. Escort Proteins for Cellular Zinc Ions. Nature 2022, 608, 38–39.
- Brudevold, F.; Steadman, L.T.; Spinelli, M.A.; Amdur, B.H.; Grøn, P. A Study of Zinc in Human Teeth. Arch. Oral Biol. 1963, 8, 135–144.
- Ten Cate, A.R. The Distribution of Alkaline Phosphatase in the Human Tooth Germ. Arch. Oral Biol. 1962, 7, 195–205.
- Yamaguchi, M.; Inamoto, K.; Suketa, Y. Effect of Essential Trace Metals on Bone Metabolism in Weanling Rats: Comparison with Zinc and Other Metals’ Actions. Res. Exp. Med. (Berl.) 1986, 186, 337–342.
- Balter, V.; Lamboux, A.; Zazzo, A.; Télouk, P.; Leverrier, Y.; Marvel, J.; Moloney, A.P.; Monahan, F.J.; Schmidt, O.; Albarède, F. Contrasting Cu, Fe, and Zn Isotopic Patterns in Organs and Body Fluids of Mice and Sheep, with Emphasis on Cellular Fractionation. Metallomics 2013, 5, 1470–1482.
- Klimuszko, E.; Orywal, K.; Sierpinska, T.; Sidun, J.; Golebiewska, M. The Evaluation of Zinc and Copper Content in Tooth Enamel without Any Pathological Changes—An in Vitro Study. Int. J. Nanomed. 2018, 13, 1257–1264.
- Gomez, S.; Rizzo, R.; Pozzi-Mucelli, M.; Bonucci, E.; Vittur, F. Zinc Mapping in Bone Tissues by Histochemistry and Synchrotron Radiation–Induced X-ray Emission: Correlation with the Distribution of Alkaline Phosphatase. Bone 1999, 25, 33–38.
- Kim, T.H.; Bae, C.H.; Lee, J.C.; Kim, J.E.; Yang, X.; de Crombrugghe, B.; Cho, E.S. Osterix Regulates Tooth Root Formation in a Site-Specific Manner. J. Dent. Res. 2015, 94, 430–438.
- Lu, Y.; Papagerakis, P.; Yamakoshi, Y.; Hu, J.C.-C.; Bartlett, J.D.; Simmer, J.P. Functions of KLK4 and MMP-20 in Dental Enamel Formation. Biol. Chem. 2008, 389, 695–700.
- Hubbard, M.J. Calcium Transport across the Dental Enamel Epithelium. Crit. Rev. Oral Biol. Med. 2000, 11, 437–466.
- Reynard, B.; Balter, V. Trace Elements and Their Isotopes in Bones and Teeth: Diet, Environments, Diagenesis, and Dating of Archeological and Paleontological Samples. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 416, 4–16.
- Müller, W.; Nava, A.; Evans, D.; Rossi, P.F.; Alt, K.W.; Bondioli, L. Enamel Mineralization and Compositional Time-Resolution in Human Teeth Evaluated via Histologically-Defined LA-ICPMS Profiles. Geochim. Cosmochim. Acta 2019, 255, 105–126.
- Goldberg, M.; Septier, D.; Bourd, K.; Hall, R.; Jeanny, J.-C.; Jonet, L.; Colin, S.; Tager, F.; Chaussain-Miller, C.; Garabédian, M.; et al. The Dentino-Enamel Junction Revisited. Connect. Tissue Res. 2002, 43, 482–489.
- Stock, S.R.; Finney, L.A.; Telser, A.; Maxey, E.; Vogt, S.; Okasinski, J.S. Cementum Structure in Beluga Whale Teeth. Acta Biomater. 2017, 48, 289–299.
- Jeejeebhoy, K. Zinc: An Essential Trace Element for Parenteral Nutrition. Gastroenterology 2009, 137, S7–S12.
- Jaouen, K.; Pouilloux, L.; Balter, V.; Pons, M.-L.; Hublin, J.-J.; Albarède, F. Dynamic Homeostasis Modeling of Zn Isotope Ratios in the Human Body. Metallomics 2019, 11, 1049–1059.
- Jaouen, K.; Trost, M.; Bourgon, N.; Colleter, R.; Le Cabec, A.; Tütken, T.; Elias Oliveira, R.; Pons, M.L.; Méjean, P.; Steinbrenner, S.; et al. Zinc Isotope Variations in Archeological Human Teeth (Lapa Do Santo, Brazil) Reveal Dietary Transitions in Childhood and No Contamination from Gloves. PLoS ONE 2020, 15, e0232379.
- Donangelo, C.M.; King, J.C. Maternal Zinc Intakes and Homeostatic Adjustments during Pregnancy and Lactation. Nutrients 2012, 4, 782–798.
- Takeuchi, K.; Nakagaki, H.; Toyama, Y.; Kimata, N.; Ito, F.; Robinson, C.; Weatherell, J.A.; Stösser, L.; Künzel, W. Fluoride Concentrations and Distribution in Premolars of Children from Low and Optimal Fluoride Areas. Caries Res. 1996, 30, 76–82.
- Ide-Ektessabi, A.; Shirasawa, K.; Koizumi, A.; Azechi, M. Application of Synchrotron Radiation Microbeams to Environmental Monitoring. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2004, 213, 761–765.
- Brozou, A.; Mannino, M.A.; Van Malderen, S.J.M.; Garrevoet, J.; Pubert, E.; Fuller, B.T.; Dean, M.C.; Colard, T.; Santos, F.; Lynnerup, N.; et al. Using SXRF and LA-ICP-TOFMS to Explore Evidence of Treatment and Physiological Responses to Leprosy in Medieval Denmark. Biology 2023, 12, 184.
- Obtel, N.; Le Cabec, A.; Nguyen, T.N.; Giabicani, E.; Van Malderen, S.J.M.; Garrevoet, J.; Percot, A.; Paris, C.; Dean, C.; Hadj-Rabia, S.; et al. Impact of Claudin-10 Deficiency on Amelogenesis: Lesson from a HELIX Tooth. Ann. N. Y. Acad. Sci. 2022, 1516, 197–211.
- Sánchez-Quevedo, M.; Crespo, P.; García, J.; Campos, A. X-ray Histochemistry of Zinc in Dental Tissues. Eur. Arch. Biol. 1992, 103, 47–49.
- Humphrey, L.T.; Jeffries, T.E.; Dean, M.C. Micro Spatial Distributions of Lead and Zinc in Human Deciduous Tooth Enamel. In Technique and Application in Dental Anthropology; Irish, J.D., Nelson, G.C., Eds.; Studies in Biological Anthropology; Cambridge University Press: Cambridge, UK, 2008; Volume 53, pp. 87–110.
- Humphrey, L.T.; Hassett, B.R.; Jeffries, T.E.; Dean, C.M. Trace Element Distributions in Early and Late Forming Tooth Enamel. In Proceedings of the 4th Meeting of the European Society for the Study of Human Evolution, Florence, Italy, 18–20 September 2014; p. 88.
- Humphrey, L.T.; Hassett, B.R.; Jeffries, T.E.; Dean, C.M. Micro-spatial Patterning of Lead and Zinc in Deciduous Tooth Enamel. In Proceedings of the 17th International Symposium on Dental Morphology, Bordeaux, France, 4–7 October 2017; p. 170.
- Bourgon, N.; Jaouen, K.; Bacon, A.-M.; Jochum, K.P.; Dufour, E.; Duringer, P.; Ponche, J.-L.; Joannes-Boyau, R.; Boesch, Q.; Antoine, P.-O.; et al. Zinc Isotopes in Late Pleistocene Fossil Teeth from a Southeast Asian Cave Setting Preserve Paleodietary Information. Proc. Natl. Acad. Sci. USA 2020, 117, 4675.
- Kang, D.; Amarasiriwardena, D.; Goodman, A.H. Application of Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry (LA-ICP-MS) to Investigate Trace Metal Spatial Distributions in Human Tooth Enamel and Dentine Growth Layers and Pulp. Anal. Bioanal. Chem. 2004, 378, 1608–1615.
- Dean, M.C.; Spiers, K.M.; Garrevoet, J.; Le Cabec, A. Synchrotron X-ray Fluorescence Mapping of Ca, Sr and Zn at the Neonatal Line in Human Deciduous Teeth Reflects Changing Perinatal Physiology. Arch. Oral Biol. 2019, 104, 90–102.
- Stock, S.R.; Deymier-Black, A.C.; Veis, A.; Telser, A.; Lux, E.; Cai, Z. Bovine and Equine Peritubular and Intertubular Dentin. Biomineralization 2014, 10, 3969–3977.
- Dean, M.C.; Le Cabec, A.; Spiers, K.; Zhang, Y.; Garrevoet, J. Incremental Distribution of Strontium and Zinc in Great Ape and Fossil Hominin Cementum Using Synchrotron X-ray Fluorescence Mapping. J. R. Soc. Interface 2018, 15, 20170626.
- Brudevold, F.; Steadman, L.T.; Smith, F.A. Inorganic and Organic Components of Tooth Structure. Ann. N. Y. Acad. Sci. 1960, 85, 110–132.
- Martin, R.R.; Naftel, S.J.; Nelson, A.J.; Feilen, A.B.; Narvaez, A. Synchrotron X-ray Fluorescence and Trace Metals in the Cementum Rings of Human Teeth. J. Environ. Monit. 2004, 6, 783–786.
- Martin, R.R.; Naftel, S.J.; Nelson, A.J.; Sapp III, W.D. Comparison of the Distributions of Bromine, Lead, and Zinc in Tooth and Bone from an Ancient Peruvian Burial Site by X-ray Fluorescence. Can. J. Chem. 2007, 85, 831–836.
- Stock, S.R.; Veis, A.; Telser, A.; Cai, Z. Near Tubule and Intertubular Bovine Dentin Mapped at the 250 Nm Level. J. Struct. Biol. 2011, 176, 203–211.
- Djomehri, S.I.; Candell, S.; Case, T.; Browning, A.; Marshall, G.W.; Yun, W.; Lau, S.H.; Webb, S.; Ho, S.P. Mineral Density Volume Gradients in Normal and Diseased Human Tissues. PLoS ONE 2015, 10, e0121611.
- Klevezal, G.A.; Kirillova, I.V.; Shishlina, N.I.; Sokolov, A.A.; Trunova, Y.E. Growth Layers in Tooth Dentin and Cementum: Problems and Perspectives of Their Use in the Study of Fossil and Subfossil Mammal Remains Including Humans. Doc. Archaeobiol. 2006, 4, 113–124.
- Le Cabec, A.; Tang, N.K.; Ruano Rubio, V.; Hillson, S. Nondestructive Adult Age at Death Estimation: Visualizing Cementum Annulations in a Known Age Historical Human Assemblage Using Synchrotron X-ray Microtomography. Am. J. Phys. Anthropol. 2019, 168, 25–44.
- Cerrito, P.; Bailey, S.E.; Hu, B.; Bromage, T.G. Parturitions, Menopause and Other Physiological Stressors Are Recorded in Dental Cementum Microstructure. Sci. Rep. 2020, 10, 5381.
- Seo, H.-J.; Cho, Y.-E.; Kim, T.; Shin, H.-I.; Kwun, I.-S. Zinc May Increase Bone Formation through Stimulating Cell Proliferation, Alkaline Phosphatase Activity and Collagen Synthesis in Osteoblastic MC3T3-E1 Cells. Nutr. Res. Pract. 2010, 4, 356.
- Mansell, R.E.; Hendershot, L.C. The Spectrochemical Analysis of Metals in Rat Molar Enamel, Femurs and Incisors. Arch. Oral Biol. 1960, 2, 31–37.
- Sasaki, T.; Takagi, M.; Yanagisawa, T. Structure and Function of Secretory Ameloblasts in Enamel Formation. In Proceedings of the Ciba Foundation Symposium 205; Chadwick, D.J., Cardew, G., Eds.; Novartis Foundation Symposia; John Wiley & Sons, Ltd.: Chichester, UK, 1997; pp. 32–53. ISBN 978-0-470-51530-3.
- Featherstone, J.D.B.; Nelson, D.G.A. The Effect of Fluoride, Zinc, Strontium, Magnesium and Iron on the Crystal-Structural Disorder in Synthetic Carbonated Apatites. Aust. J. Chem. 1980, 33, 2363.
- Crawford, A.W.; De Bruin, H.J. Concentration Changes in Surface Ca, P, F, Zn, Fe, and Sr During White Spot Formation. J. Dent. Res. 1983, 62, 964–968.
- Mohammed, N.R.; Mneimne, M.; Hill, R.G.; Al-Jawad, M.; Lynch, R.J.M.; Anderson, P. Physical Chemical Effects of Zinc on in Vitro Enamel Demineralization. J. Dent. 2014, 42, 1096–1104.
- Yamaguchi, M. Role of Nutritional Zinc in the Prevention of Osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254.
- Mayer, I.; Apfelbaum, F.; Featherstone, J.D.B. Zinc Ions in Synthetic Carbonated Hydroxyapatites. Arch. Oral Biol. 1994, 39, 87–90.
- Fuierer, T.A.; LoRe, M.; Puckett, S.A.; Nancollas, G.H. A Mineralization Adsorption and Mobility Study of Hydroxyapatite Surfaces in the Presence of Zinc and Magnesium Ions. Langmuir 1994, 10, 4721–4725.




