Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alice Vilela | -- | 5008 | 2023-12-11 10:05:33 | | | |
| 2 | Mona Zou | Meta information modification | 5008 | 2023-12-12 08:07:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marques, C.; Dinis, L.; Santos, M.J.; Mota, J.; Vilela, A. Health-Promoting Compounds in Wine and Wine-Related Products. Encyclopedia. Available online: https://encyclopedia.pub/entry/52564 (accessed on 08 February 2026).
Marques C, Dinis L, Santos MJ, Mota J, Vilela A. Health-Promoting Compounds in Wine and Wine-Related Products. Encyclopedia. Available at: https://encyclopedia.pub/entry/52564. Accessed February 08, 2026.
Marques, Catarina, Lia-Tânia Dinis, Maria João Santos, João Mota, Alice Vilela. "Health-Promoting Compounds in Wine and Wine-Related Products" Encyclopedia, https://encyclopedia.pub/entry/52564 (accessed February 08, 2026).
Marques, C., Dinis, L., Santos, M.J., Mota, J., & Vilela, A. (2023, December 11). Health-Promoting Compounds in Wine and Wine-Related Products. In Encyclopedia. https://encyclopedia.pub/entry/52564
Marques, Catarina, et al. "Health-Promoting Compounds in Wine and Wine-Related Products." Encyclopedia. Web. 11 December, 2023.
Copy Citation
Health-promoting compounds in wine and wine-related products are important due to their potential benefits to human health. In contemporary food and beverage industry research, scientific investigations focus on identifying health-promoting compounds inherent in plants or derived materials. Most of these compounds belong to the polyphenol family, encompassing flavonoids and non-flavonoids. Additionally, carotenoids, antioxidants, and essential vitamins and minerals offer discernible health benefits upon consumption.
phenolic and non-phenolic compounds
climate
topography
geology
saliva pH and enzymes
sensory perception
vinegar
wine spirit
1. Health-Promoting Phenolic Compounds
1.1. Flavonoids and Non-Flavonoids
The phenolic compounds represent a heterogeneous group of chemical compounds widely studied for their human health properties. These compounds can remove free radicals and contribute to preventing cell damage due to their antioxidant properties study [1]. Among phenolic compounds are diverse subclasses of polyphenols, namely phenolic acids, flavonoids, stilbenes, lignans, and tannins [2]. Structurally, these compounds are divided into non-flavonoid and flavonoid phenolic compounds. Non-flavonoid phenolic compounds are composed of phenolic acids and stilbenes. Phenolic acids can be divided into benzoic and cinnamic acids [3]. Flavonoids can be found in the free state or polymerized with other flavonoids, sugars, or non-flavonoids. They possess a C6-C3-C6 base structure (Figure 1) and undergo modifications within the ring, forming the following structural classes: flavonol, flavones, flavanones, and anthocyanins. These compounds are responsible for several wines’ sensory characteristics, such as color [4], mouthfeel sensations (bitterness and astringency) [5], flavor, and aroma [6]. Chemically, they are critical in wine and wine-related products since they interact with wine proteins [7].
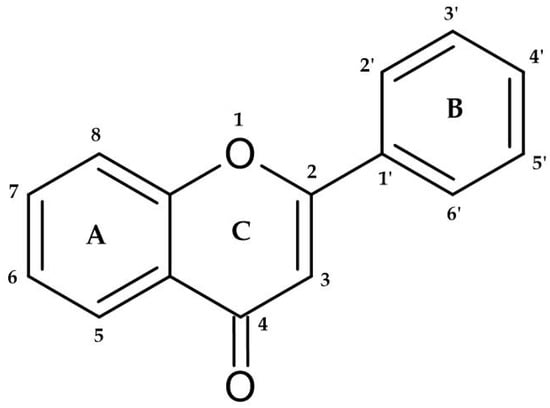
Figure 1. The base structure of flavonoids. The two C6 units denote benzyl rings (rings A and B), and the C3 unit designates the chromane ring (rings C). Depending on the hydroxylation and substitution patterns and the degree of saturation of the chromane ring, flavonoids may exhibit various substituent groups. These substituent groups play a crucial role in determining the specific categorization of each flavonoid corresponding to the labeled numbers.
Wine flavonoids can be divided into eight subcategories: anthocyanins, flavonols, flavanols, flavones, flavanones, flavanes, flavononols, and chalcones/dihydrochalcones. At the same time, nonflavonoids include phenolic acids, tannins, stilbenes, coumarins, phenyl ethanol derivatives, lignans, and neolignans [8].
In wines, people can find the phenolic compounds of grape origin comprising quantitative and qualitative data, which are influenced by variety, yield, berry area, soil, geographic origin, and climate [9]. Besides their importance to human health, they have been described as an essential tool for characterizing commercial wines and can be used in investigations on the geographical origin of wines [10].
Though most wine phenolic compounds originated from grapes, the winemaking method and yeast strain metabolic activity intervein, changing their structure, composition, quantity, and color characteristics [6][11]. For instance, yeast metabolites, such as pyruvic acid and acetaldehyde, are necessary reactants in the biological formation of anthocyanin–flavanol adducts [6][12], vitisins [12], and color-important vinyl phenol pyranoanthocyanins [6][12][13]. Saccharomyces yeasts may contribute to changes in wine color by modifying the wine pH due to organic acid metabolism (production or consumption) [11].
Enzymes produced by yeasts, such as pectinase and β-glycosidase, play a role in the winemaking process. Pectinase may affect phenolic extraction from grapes, contributing to the breakdown of pectins that bind phenolic compounds. Additionally, β-glycosidase can hydrolyze the glycosidic bond of certain phenolic compounds, releasing aglycones and influencing the sensory attributes of the wine [14][15].
Moreover, cell walls may autolyze during yeast fermentation, releasing compounds like mannoproteins. These mannoproteins can interact with wine phenolic compounds, promoting binding interactions and, in some cases, precipitation. While not all phenolic compounds have glycosidic bonds, those that do, such as anthocyanins, can be affected by β-glycosidase activity. This enzymatic action releases aglycones, contributing to the final wine product’s color intensity and flavor nuances [16].
1.2. Wine Compounds Produced by Fermentation
1.2.1. Tyrosol, Hydroxytyrosol, and Tryptophol
Bioactive products from microbial metabolisms, like tyrosol, hydroxytyrosol, and tryptophol, are natural phenolic compounds in some foods and beverages, especially wine and wine-related products [17]. The formation of tyrosol, hydroxytyrosol, and tryptophol can be influenced by external factors such as temperature, alcoholic degree, and amino acid concentration [18].
These compounds are recognized for their positive health effects, particularly as antiatherogenic, anticancer, neuroprotective, antidiabetic, lipid-regulating, and anti-obesity, and in promoting cardiovascular health-related benefits [19]. Their antimicrobial and antiviral were also investigated, particularly against COVID-19 effects, as hydroxytyrosol promotes mitochondrial function through activating mitophagy [20]; improving the mitochondria metabolism can reduce the severity of SARCS-COV-2 effects on hepatocytes’ mitochondria, ameliorating COVID-induced liver injury [21].
These compounds are considered health-promoting compounds, especially tyrosol, which can represent a natural product supplement to prevent or treat diarrhea, as it relieves diarrhea in mice by inhibiting Escherichia coli-induced activation of the NF-κB pathway in mice [22]. According to Gabbia and coworkers [23], tyrosol can also be considered a novel strategy to counteract hepatic steatosis, fibrosis, and inflammation associated with nonalcoholic steatohepatitis development by modulating the recruitment of immune cells in the liver.
After ingestion, tyrosol is partially biotransformed into hydroxytyrosol (Figure 2) [24]. The concentration of tyrosol in wine can range from 20 to 60 mg/L in red wines and up to 45 mg/L in white wines [25]. The exact concentration can vary widely among different wine varieties and styles. For example, certain red wines, such as Cabernet Sauvignon, may contain higher tyrosol due to the independent and interactive effects of Cabernet Sauvignon plant materials implanted in different geographical locations [26].
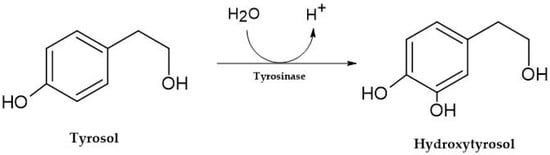
Figure 2. Biosynthesis of hydroxytyrosol.
Although olive oil is the primary source of hydroxytyrosol from the Oleuropein degradation by the action of a β-glucosidase and posterior hydrolysis in the human diet, wine is another crucial source due mainly to the yeast’s metabolism [10]. Several studies consider concentrations from 3.66 mg/L to 4.20 mg/L in red wines and 1.72 mg/L to 1.92 mg/L in white wines [27]. This bioactive compound has uncounted health-promoting properties, namely antioxidant, cardiovascular, antidiabetic, and neuroprotective effects [27]. Considering tryptophol in wine, the concentrations found in red wine range from 11.20 mg/L to 24.77 mg/L and in white wine from 4.90 mg/L to 11.26 mg/L, depending on variety [28] and fermentation parameters, namely temperature, alcoholic degree, and amino acids concentration [18]. Tryptophol shares a structural kinship with tyrosol and hydroxytyrosol as constituents of the aromatic alcohol class. Tryptophol is derived explicitly from tryptophan during the fermentation of wine. At the same time, tyrosol and hydroxytyrosol are commonly found in olive oil, and their presence in wine is typically associated with aging in wooden barrels or contact with grape skins [17][18][27].
1.2.2. Melatonin and Serotonin
Bioactive compounds like melatonin and serotonin have incredible health benefits. Melatonin (MEL) is a neurohormone (n-acetyl-5-methoxytyramine) from the pineal gland produced as a secondary metabolite in the plant kingdom. It is produced by O-serotonin methylation followed by n-methoxy tryptamine acetylation in yeast [17] and can also be synthesized from tryptophan, 5-hydroxytryptophan, serotonin, and ultimately n-acetyl serotonin. This molecule is a powerful antioxidant and is very efficient in re-establishing the circadian rhythm, helping in sleeping disorders. It protects against neurodegenerative diseases like Parkinson’s, Alzheimer’s, Huntington’s, amyotrophic lateral sclerosis, and fibrinogenesis [29].
Serotonin is formed during malolactic and alcoholic fermentation by the action of yeast and lactic acid bacteria, and it is derived from the decarboxylation of L-tryptophan [30]. According to Sagonas et al. [31], serotonin is a crucial mediator in fibrosis and vasculopathy, helping in diseases like systemic sclerosis.
Tryptophan-derived molecules, such as melatonin and serotonin (Figure 3), are present in wine at deficient concentrations, varying from picograms to ng/mL [29]. Concentrations seem low; however, these small amounts are enough in dietary intake to measure their effects by different methods [29]. These molecules have been referenced in wine, and the role of yeasts in their formation is evident [17][32]. However, there needs to be more studies regarding identifying and quantifying these molecules in some wine-related products.
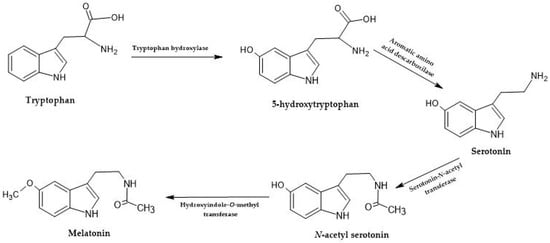
Figure 3. Tryptophan is synthesized into melatonin and serotonin through a series of enzymatic steps. Tryptophan hydroxylase is the first enzyme involved, and it adds a hydroxyl group (-OH) to tryptophan, forming 5-hydroxytryptophan (5-HTP), with the help of tetrahydrobiopterin (BH4). The following enzyme, aromatic L-amino acid decarboxylase (AADC), then removes the carboxyl group (-COOH) from 5-HTP, resulting in the formation of serotonin (5-hydroxytryptamine). To produce melatonin, serotonin is subjected to two enzymatic steps. First, serotonin N-acetyltransferase (SNAT) transfers an acetyl group (-COCH3) from acetyl coenzyme A (acetyl-CoA) to serotonin, forming N-acetyl serotonin. Then, N-acetyl serotonin methyltransferase (ASMT) catalyzes the methylation of N-acetyl serotonin using S-adenosyl methionine (SAM) as a methyl donor, resulting in the production of melatonin [17].
2. Health-Promoting Non-Phenolic Compounds
2.1. Glutathione
Due to health concerns surrounding the use of SO2, already described since the 1980s by Freedman [33] as an inducer of asthma when inhaled or ingested by sensitive subjects, even in high dilution, more attention has been given to alternative antioxidants. Ascorbic acid (AA), glutathione (GSH), and GSH-enriched commercial products are examples of alternatives. They can be added to wines and grape must, and their activity may continue throughout the vinification process [34]. However, glutathione addition is regulated by the Organisation Internationale de la Vigne et du Vin (OIV) with a limited dose of no more than 20 mg/L in the must [35]. This regulation underscores the careful consideration and adherence to international standards regarding using specific additives in winemaking processes, considering variations in regulatory frameworks across different countries. While the European Chemicals Agency (ECHA) and European Food Safety Authority (EFSA) [36][37] report no associated hazards for ascorbic acid, the OIV establishes specific maximum acceptable limits for wine treatment (250 mg/L) and residue in wine (300 mg/L) [38]. Additionally, some countries set their maximum acceptable limits, reflecting variations in regulatory standards. Despite the recommended daily dose for adults being 70 to 150 mg, these divergent limits underscore the importance of considering context-specific guidelines for ascorbic acid use [39].
Glutathione is a thiol-containing tripeptide of glutamic acid, cysteine, and glycine found in many fruits, vegetables, and beverages, including wine [40]. The natural glutathione levels in musts and wine are widely diverse, ranging from non-detectable to 100 mg/L and in wine from non-detectable to 70 mg/L [41]. Glutathione is a powerful antioxidant in wine, helping protect it from oxidative damage. It can directly scavenge free radicals and reactive oxygen species (ROS), which can cause undesirable changes in the wine’s flavor and aroma [42]. Glutathione reacts with aldehydes, like acetaldehyde, responsible for off-flavors in wine and converts them into more stable forms, limiting their accumulation in wine during storage [43]. Additionally, during bottle aging, glutathione protects varietal thiols from oxidation [44]. At the same time, its cysteinyl form can be used as a source of sulfur, increasing the concentration of polyfunctional mercaptans by reacting with trans-2-hexenal to form Glut-3MHal [45]. This process helps to enhance the wine’s aroma, assisting the preservation of compounds produced by yeasts, like esters, such as isoamyl acetate and ethyl hexanoate, terpenes (linalool, α-terpineol), and volatile thiols responsible for wine’s fruity characteristics [46] (Figure 4).
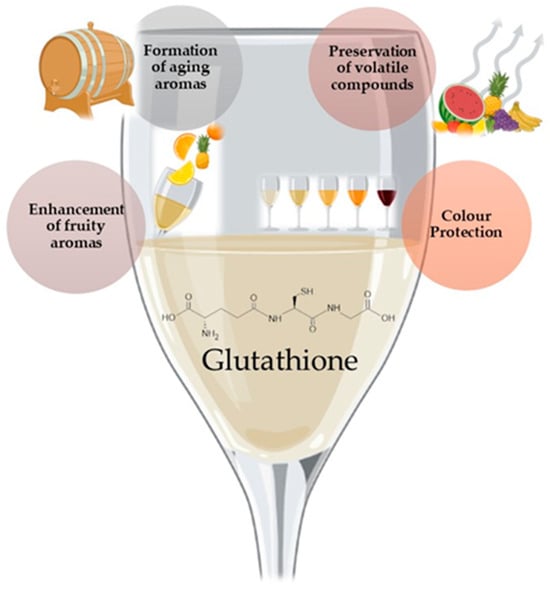
Figure 4. Schematic effect of glutathione in wine and wine-related products.
Besides enhancing wine’s fruity aroma, it also helps form aging aromas [41] (Figure 4). In the case of dry Muscat wine, Papadopoulou and coworkers [47] added 20 mg/L of glutathione, inhibiting the vanishing of linalool and α-terpineol during storage of the wine.
Glutathione also contributes to the stabilization of wine’s color intensity and stability. It can limit the formation of browning pigments by trapping o-quinones in a colorless form and restrict the production of xanthylium cation pigment precursors and o-quinone-derived phenolic compounds [44][48].
In humans, glutathione has proven to have antiaging and anti-melanogenic effects [49], and, more recently, a derived form of glutathione (ψ-GSH) can inhibit oxidative stress and neuroinflammation in an Alzheimer’s disease mouse model [50]. Chen et al. [51] studied the effects of glutathione in improving testicular spermatogenesis by inhibiting oxidative stress, mitochondrial damage, and apoptosis induced by copper deposition in mice with Wilson disease.
2.2. Vitamins
Vitamins are essential micronutrients that play a vital role in maintaining human health. Vitamin A, a fat-soluble vitamin, is present in wine and wine byproducts such as grape seeds and skins. Studies have shown that vitamin A may help prevent age-related macular degeneration and improve skin health [52].
Additionally, vitamin C is an essential vitamin with antioxidant properties that help protect cells from damage caused by free radicals. Vitamin C is critical for collagen synthesis and immune function [53], and it exhibits antioxidant activities by inhibiting lipid peroxidation and oxidative stress [54].
Vitamin E is another vitamin found in grape seeds and skins. This vitamin has been shown to have antioxidant properties that may help protect against chronic diseases such as cardiovascular disease and cancer [55].
The specific literature on vitamin concentration is scarce. Evers and coworkers [56] referenced an extensive overview of studies on water-soluble vitamins and their concentrations in grapes, grape musts, and wines. Considering this gap in the literature, Evers and coworkers [57] proposed a rapid and reliable method for analyzing vitamins in grape musts.
Although the involvement of vitamins in the development of wine aromas remains primarily unexplored, it is possible to establish some metabolic connections existing between vitamins and aromatic molecules, namely the case of pyridoxine and niacin metabolisms that have been proven to be directly linked to butanoate metabolism, a subsequent essential wine aromatic compounds derived from it, like ethyl butanoate and ethyl 2-methyl butanoate, which are odorant compounds of several wines [56].
2.3. Minerals
Several minerals are present in wine and wine-related products, and the most abundant in wine are K, Ca, Na, and Mg. The highest concentration levels can be found in K, between 500 and 1500 mg/L, followed by Ca, Mg, and Na, around 10–200 mg/L. The elements usually present in concentrations ranging from 0.1 to 10 mg/L are Al, B, Cu, Fe, Mn, Rb, Sr, and Zn. Then, ultra-trace elements below 0.1 mg/L, such as Se, Pb, and Cd [58]. These minerals play a crucial role in several physiological processes in the body and have been linked to various health benefits. Calcium is a renowned mineral for its help in bone health and muscle function and can mainly be found in grape skin at levels that can reach 0.8 mg/g fresh weight [59]. While potassium is vital for maintaining fluid and electrolyte balance and regulating blood pressure [60], magnesium has been associated with reduced risk of type 2 diabetes mellitus [61][62] and cardiovascular mortality [63].
While these concentrations may not be exceptionally high compared to other food sources, regular consumption of moderate amounts of wine and wine-related products can contribute to overall mineral intake and provide health benefits. Average wine consumption may be associated as a human health promoter, partly due to the minerals present; however, excessive intake of these minerals can harm human health [58]. It is crucial to note that some metals in these products, such as Pb and Cd, are toxic and unhealthy for human consumption. To safeguard public health, regulations require the analysis of metal content and permissible concentrations in wine. Furthermore, compliance with diverse health protection regulations in different countries necessitates the identification of potentially hazardous substances. These include Cd, Pb, Hg, Al, Tl, As, Sb, S, and several organometallic compounds of Pb and As. [64][65].
In addition, minerals were also detected in wine-related products. In wine vinegar, Paneque and coworkers [66] determined 21 minerals (Al, As, B, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, P, Pb, S, Sr, V, and Zn) in Andalusian wine vinegar by an inductively coupled plasma–optical emission spectroscopy (ICP-OES) method.
The concentrations of minerals in wine and wine-related products can vary depending on several factors, including the grape maturity, variety, soil type, climatic conditions [67], and winemaking process; therefore, the concentrations of minerals can consequently be used to discriminate the geographic origin of wines [68].
3. Sensory Perception of Health-Promoting Compounds
3.1. Health-Promoting Compound’s Terroir-Dependent Sensory Attributes
Terroir plays a significant role in shaping the wine flavor profile. The unique combination of climate, soil, topography, and biodiversity in a specific wine-growing region contributes to the distinctiveness and complexity of wines produced from that terroir [69]. The climate of a particular wine-growing region dramatically influences the flavor characteristics of the grapes. Cool temperatures make wines with higher acidity and lighter fruit flavors, while temperatures yield riper, fuller-bodied wines with bolder fruit flavors [70]. The composition and structure of the soil impact the mineral content and nutrient availability of the grapevines. Different soils contribute distinct flavors and textures to the grapes, which can be reflected in the wine [69][71]. For example, volcanic soils may impart a smoky or mineral note, while limestone soils can add a chalky or flinty character [72]. The topography of a vineyard, such as its altitude, slope, and exposure to sunlight, influences factors like drainage, sun exposure, and airflow. These factors can affect grape ripening, acidity, and flavor development. For instance, grapes grown on a steep slope might experience better sun exposure, resulting in riper fruit flavors [73], whereas insolation of grape bunches followed by reillumination enhances the flavor of aromatic grape cultivars. This hypothesis was tested by combining gene expression and metabolic analysis of the monoterpene and flavonol synthesis pathways in Vitis vinifera L. cv. Riesling [74]. The presence of diverse flora and fauna in and around vineyards can contribute to the overall health and balance of the ecosystem. This biodiversity can indirectly impact wine flavor by promoting natural pest control, improving soil health, and influencing the vineyard environment [75].
Descriptive analysis (DA) is commonly employed to provide a detailed profile of wine’s sensory characteristics [76]. However, it can also serve the purpose of characterizing the typical qualities of a particular wine [77]. A study by King et al. [78] exemplified this by utilizing DA to represent Malbec wines from various vineyard sites in California and Mendoza. This inter-country study revealed that variations in altitude can impact the sensory attributes of a wine, highlighting its role as a factor in terroir and regional typicity. Another survey by Geffroy et al. [79] employed a similar approach and identified the characteristic peppery notes and higher rotundone concentrations in French Gamay wines originating from vineyards in cooler climate regions. In contrast, Kustos et al. [80] explored the regional typicity of Australian Chardonnay and Shiraz wines. They concluded that regional differences alone were insufficient to fully describe the sensory distinctions of the samples, emphasizing the need to consider winemaking elements as well.
3.2. Influence of pH, Saliva (Enzymes) Biochemistry, and Oral Microflora on the Sensory Perception of Health-Promoting Compounds
Investigating the factors influencing flavor perception can provide insight into promoting healthy food choices and maintaining good nutrition. In addition to food and beverage characteristics, oral physiology, specifically the saliva [81] and microbiome [82], significantly shapes flavor perception.
Considered the “mirror of the body,” saliva is a complex biological fluid of enormous importance for humans [83][84]. The constitution of saliva is based on approximately 99% water and the remainder in inorganic and organic compounds. The former includes sodium, potassium, calcium, magnesium, chlorides, and carbonates. Regarding the organic compounds, these encompass enzymes—for example, α-amylase, lipase, lysozyme, and esterases—and various proteins, such as immunoglobulins, mucins (glycoproteins), proline-rich proteins, and hormones, among others [85][86][87]. According to recent data [88], the pH of saliva is between 6.2 and 7.4. Slightly acidic or basic, saliva is a highly viscoelastic fluid. It has unique properties that allow the performance of numerous functions, such as lubrication, wetting, emulsification, and changes in surface tension to prevent friction between particles and oral surfaces [89][90]. The above properties also facilitate chewing, digestion, homeostasis, and taste perception and have antimicrobial properties and buffering capacity [83][89][90]. Like other bodily fluids, saliva has a buffering ability to absorb or release hydrogen ions (H+) to regulate their concentration changes and stabilize the pH value [91][92]. A study conducted in 2022 [93], which studied the influence of solutions (aqueous and hydroalcoholic) and drinks, found that saliva influences the pH change. In this study, there were differences in the pH values according to the beverage analyzed; in the case of red and white wines, the pH decreased after contact with the taster’s saliva. This result may be related to the fact that the wines are composed of different acids and in different concentrations. Tartaric, malic, lactic, and citric acids, among others present in wine, may regulate the wine’s pH and confer a buffering capacity [91]. Depending on the acid components present, they may also inhibit the effect of saliva on pH.
Salivary proteins are crucial in the oral cavity, where the various components of saliva interact to create new compounds. These proteins perform multiple functions such as oral digestion (amylases and lipases), detoxification (proline-rich proteins and statins), defense against microorganisms (immunoglobulins and peroxidases), lubrication of the oral cavity (mucins), and the transportation of taste molecules (lipocalins) [93].
An essential protein found in human saliva is α-amylase, which is involved in the breakdown of carbohydrates and starches during the digestive process [94][95][96]. This enzyme is an endoglycohydrolase encoded by the Amy1 gene. It hydrolyses internal α-1,4-glucoside bonds of starch to the disaccharide maltose and moderate-length oligosaccharides called boundary dextrins. Amylase is inactivated at the acidic pH of the gastric lumen. Still, it is more stable in the presence of the pH of oral saliva or in solutions where the pH is closer to the natural pH of saliva—between 6.2 and 7.4 [88]. A study carried out in 2023 [93] reinforces the previous statement, since in aqueous and hydroalcoholic sucrose solutions tested—where the pH was between 5.67 and 6.19, respectively—there was a high activity of α-amylase, showing that this is protected when in contact with solutions and capable of assisting in the digestion of carbohydrates and starch.
Wine has numerous compounds in its constitution that can influence amylase activity. Within the study by Santos and coworkers [93], red wine, generally considered fruity and sweeter, showed higher activity of this enzyme than white wine. The compounds responsible for the enzyme activity are directly related to the sweetness of the wine and are mainly sugars (glucose and fructose), glycols, and glycerol. Moreover, some wines may contain residual sugars that contribute even more to the sweetness of fortified wines. On the other hand, there are compounds capable of inhibiting the activity of amylase, as is the case of tannins. These can bind to proteins, including enzymes, and form stable complexes. Thus, tannins are believed to inhibit α-amylase activity [93]. Regarding aromatic compounds, they tend to be adsorbed by the mucosal film. However, the presence of tannins and their aggregation with the mucosal film could lead to disturbances in the interaction with the aromatic compounds [97].
Variation in the activity and concentration of the α-amylase enzyme is linked to stress, making it a valuable marker for predicting stress levels, whether physical or psychological [75][98][99]. The secretion of the enzyme by the salivary glands is directly related to the activation of the autonomic nervous system [74][75][77]. Therefore, it is safe to say that the activity and concentration of this enzyme depend on the degree of stress to which the individual is subjected during the saliva collection process [77][87][96]. Furthermore, the methods used to determine and interpret the behavior of the α-amylase enzyme must also be considered and adapted to this study’s objective.
The enzyme lipase catalyzes the hydrolysis of ester bonds in the structure of triglycerides (lipids) and releases monoglycerides and free fatty acids [94][100][101]. Lipids are the main constituents of fat; it is possible to state that lipase is one of the main factors influencing the taste perception of fat, impacting its aroma and taste [86][100]. The concentration of the lipase enzyme in human saliva is low [94], and its activity is also related to the composition of the beverages being tasted [93]. Working with complex drinks that contain triglycerides and fatty acids in their composition, coming from both the raw material (grapes, wine) and the fermentative activity of yeast [102][103], will lead to more significant action of this enzyme. Regarding aroma, the concentration of short-chain fatty acids, especially the volatile fatty acids isovaleric and butyric, exceeding the threshold of sensory detection, can form an unpleasant smell (such as cheese or sweaty feet aroma) in beer and wine, thus being considered a defect often of microbiological origin [104].
Another crucial factor in taste perception, apart from the influence of saliva and its constituents, is the oral microbiota composition [82][88]. The human mouth hosts hundreds of bacterial species and, according to the Human Oral Microbiome Database (HOMD), contains over 750 species of prokaryotes. The bacterial diversity varies between different regions of the oral cavity, with the saliva and tongue microbiome showing the most outstanding richness, dominated by the genera Rothia, Prevotella, Streptococcus, Veillonella, Fusobacterium, Neisseria, and Haemophilus [105].
Two dominating mechanisms have been suggested that support the role of the oral microbiota in taste sensitivity: (i) Biofilm formation that can limit the access of tastants to their receptors; (ii) microorganisms' metabolic activity that can mediate sensitivity by metabolizing in the mouth, tastants derived from food or saliva, or by the production of bioactive metabolites [106].
Two studies [107][108] have shown a strong correlation between taste perception, microbiota composition, and wine consumption. This conclusion is because polyphenols and oenological extracts (mainly red wine and grape seed byproducts) are effective antimicrobials against certain bacterial species. A study conducted by [82] compared the tongue microbiome of professional wine tasters and non-professional wine tasters and found that the microbiome of the first one, from the area further back of the tongue, had a higher abundance in specific bacterial genera such as Streptococcus, Veillonella, and Prevotella. On the other hand, this study also concluded that the microbiome of the dorsum of the tongue is influenced by the age and gender of the taster, and the frequency of wine-tasting sessions is negatively associated with microbiome diversity.
Besides all these factors, human genetics and physiology must also be considered. In some leading wine and wine-related products, phenolic compounds taste bitter. So, despite their healthy properties, many people do not like to eat plant-derived food, like vegetables, because of the bitterness associated with polyphenols [109]. Therefore, studying the sensory properties of polyphenol compounds is a prominent subject relevant to people’s food choices and the chemopreventive potential of food. It has been shown that hydrolyzable tannin and anthocyanins could be the primary compounds responsible for the bitterness of fruits and derived products, such as red wine [110]. Sweet and bitter transduction pathways have been discovered in parallel and share many mechanisms. In humans, bitter compounds bind to a variety of ~25 different receptors of the T2R family that can form both homomeric and heteromeric complexes [111]. Bitter perception is initiated by TAS2Rs, a family of G-protein-coupled receptors, codded by 25 bitter taste receptor family genes expressed on the surface of taste buds. The protein has an active site susceptible to the taste compound’s molecular structure [112]. However, different polyphenol compounds activate different combinations of bitter taste receptors, highlighting the complex relationships between bitter taste receptors, perception, evolution, and health. A reduction in selective pressure during human development has also been observed across the TAS2R family, possibly due to evolved changes in human biology and behavior [111][112].
The chemical structure of the taste compound can also influence its sensory perception. In general, amino acids (a.a) possess primary taste properties, in which aspartic acid and glutamic acid directly contribute to sour taste. These amino acids in the derived form of salt are umami, savory, and meaty. Meanwhile, L-amino acids with hydrophobic side chains, particularly leucine, isoleucine, tyrosine, and valine, are attributed to bitter taste [113][114]. D-amino acids, such as proline, alanine, lysine, glycine, serine, and threonine, are less commonly found than their more prevalent L-amino acid counterparts in nature, reflecting the prevailing predominance of L-amino acids in biochemical processes. Despite their relative scarcity, these D-form amino acids are recognized as having a sweet taste [115]. Thus, not only do natural sugars activate the sweet taste receptor T1R2/T1R3, but ligands with distinct chemical structures, such as amino acids and proteins, may bind to different domains of the sweet taste receptors [116]. The conformational change in the receptors upon ligand binding activates intracellular downstream signaling [117].
Regarding minerals, ions such as iron and copper may facilitate a retronasal perception of “metallic flavor perception,” defined as a combination of taste and retronasal odor [118]. This phenomenon occurs due to mineral salivary protein oxidation and the production of oxidation-related aldehydes related to odorant lipids.
4. Extraction Techniques of Nutraceutical Compounds in Wine and Wine-Related Products
Extraction is a process widely used in the food industry to increase the quality of food products. Since primordial times, long extraction periods and low efficiency led to the emergence of analytical methodologies [119][120].
The most basic and common technique for analyte extraction is solid–liquid extraction (SLE) or leaching. This unitary process allows the separation of substances in a solid matrix using organic solvents, forming a solution with the target analytes [121]. Soxhlet extraction (SOX) is an example of SLE, in which the solid sample is placed in a SOX thimble with an organic solvent. A condensation environment is created by heating under the reflux of the solvent. This exhaustive extraction takes about 12–24 hours and uses large quantities of purified solvent in addition to the extensive time [1].
For liquid samples, ubiquitously used is liquid–liquid extraction (LLE), a process of transferring a dissolved substance from one liquid phase to another liquid phase. Usually, one phase is water, and the other is a non-polar organic solvent (immiscible or partially miscible solvent) [119].
As the complexity of products and food matrices to be analyzed has increased, there has been a growing demand for alternative extraction methods that are more efficient, faster, and more environmentally friendly with fewer solvents. These techniques can extract compounds from solid or liquid samples and are considered green extraction methods. There are several alternatives to conventional extraction methods.
Although some of these methods use organic solvents, compared to traditional methods, the amounts are reduced, and energy expenditure is lower than in conventional methods [1]. Other solutions exist beyond the alternative ways to extract health-promoting compounds in wine and wine-related products, such as green/sustainable solvents.
Ionic liquids (ILs) are salts liquid at or near room temperature due to mixing organic cations with organic or inorganic anions, i.e., a combination of molecules or at-oms electrified. Deep eutectic solvents (DESs) are another type of ionic liquid. Two or more substances make a eutectic mixture with lower melting points than any components. Natural eutectic solvents (NADESs) are a mixture naturally occurring in plants and animals. DESs and NADESs have high thermal stability and solubility and low toxicity [1][122].
After extracting nutraceutical compounds, purification, and chemical characterization are common steps that can be taken to study further and develop the extracted compounds. The purification step removes unwanted impurities, and the chemical characterization involves identifying and quantifying the compounds extracted.
References
- Vilela, A.; Pinto, T. Grape Infusions: Between Nutraceutical and Green Chemistry. Sustain. Chem. 2021, 2, 441–466.
- Cassidy, A.; Minihane, A.-M. The Role of Metabolism (and the Microbiome) in Defining the Clinical Efficacy of Dietary Flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22.
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine—Stabili-zation and Treatments; John Wiley and Sons: Hoboken, NJ, USA, 2006; Available online: https://books.google.pt/books?id=a03C-aFy2jsC (accessed on 4 September 2023).
- Morata, A.; Escott, C.; Loira, I.; Del Fresno, J.M.; Gonzalez, C.; Suarez-Lepe, J.Á. Influence of Saccharomyces and non-Saccharomyces yeasts in the formation of pyranoanthocyanins and polymeric pigments during red winemaking. Molecules 2019, 24, 4490.
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19.
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of non-Saccharomyces yeasts to wine freshness. A review. Biomolecules 2019, 10, 34.
- Caldeira, M.; Perestrelo, R.; Barros, A.; Bilelo, M.; Morête, A.; Câmara, J.; Rocha, S. Allergic Asthma Exhaled Breath Metabolome: A Challenge for Comprehensive Two-Dimensional Gas Chromatography. J. Chromatogr. A 2012, 1254, 87–97.
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and their variation in red wines II. Antho-cyanin-derived pigments and their color evolution. Molecules 2012, 17, 1483–1519.
- Jovanović-Cvetković, T.; Sredojević, M.; Natić, M.; Grbić, R.; Akšić, M.F.; Ercisli, S.; Cvetković, M. Exploration and Comparison of the Behavior of Some Indigenous and International Varieties (Vitis vinifera L.) Grown in Climatic Conditions of Herzegovina: The Influence of Variety and Vintage on Physico-Chemical Characteristics of Grapes. Plants 2023, 12, 695.
- Radovanović, B.C.; Radovanović, A.N.; Souquet, J.-M. Phenolic Profile and Free Radical-Scavenging Activity of Cabernet Sauvi-gnon Wines of Different Geographical Origins from the Balkan Region. J. Sci. Food Agric. 2010, 90, 2455–2461.
- Vilela, A. Modulating Wine Pleasantness Throughout Wine-Yeast Co-Inoculation or Sequential Inoculation. Fermentation 2020, 6, 22.
- Ruta, L.L.; Farcasanu, I.C. Anthocyanins and Anthocyanin-Derived Products in Yeast-Fermented Beverages. Antioxidants 2019, 8, 182.
- Morata, A.; Vejarano, R.; Ridolfi, G.; Benito, S.; Palomero, F.; Uthurry, C.; Tesfaye, W.; González, C.; Suárez-Lepe, J. Reduction of 4-ethylphenol production in red wines using HCDC+ yeasts and cinnamyl esterases. Enzym. Microb. Technol. 2013, 52, 99–104.
- Espejo, F. Role of commercial enzymes in wine production: A critical review of recent research. J. Food Sci. Technol. 2020, 58, 9–21.
- Cosme, F.; Inês, A.; Vilela, A. Microbial and Commercial Enzymes Applied in the Beverage Production Process. Fermentation 2023, 9, 385.
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 1435.
- Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46.
- Bordiga, M.; Lorenzo, C.; Pardo, F.; Salinas, M.R.; Travaglia, F.; Arlorio, M.; Coïsson, J.D.; Garde-Cerdán, T. Factors Influencing the Formation of Histaminol, Hydroxytyrosol, Tyrosol, and Tryptophol in Wine: Temperature, Alcoholic Degree, and Amino Ac-ids Concentration. Food Chem. 2016, 197, 1038–1045.
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001.
- Dong, Y.; Yu, M.; Wu, Y.; Xia, T.; Wang, L.; Song, K.; Zhang, C.; Lu, K.; Rahimnejad, S. Hydroxytyrosol Promotes the Mitochondrial Function through Activating Mitophagy. Antioxidants 2022, 11, 893.
- Akbari, H.; Taghizadeh-Hesary, F. COVID-19 induced liver injury from a new perspective: Mitochondria. Mitochondrion 2023, 70, 103–110.
- Yu, F.; Guo, J.; Ren, H.L.; Lu, S.; He, Z.; Chang, J.; Hu, X.; Shi, R.; Jin, Y.; Li, Y.; et al. Tyrosol Inhibits NF-ΚB Pathway in the Treatment of Enterotoxigenic Escherichia coli-Induced Diarrhea in Mice. Microb. Pathog. 2023, 176, 105944.
- Gabbia, D.; Sayaf, K.; Colognesi, M.; Zanotto, I.; Russo, F.; De Martin, S. Tyrosol Attenuates Hepatic Steatosis and Fibrosis in a Mouse Model of NASH. Dig. Liver Dis. 2023, 55, S66.
- Rodríguez-Morató, J.; Boronat, A.; Fitó, M.; De la Torre, R. Effects of White Wine and Tyrosol on Circulating Ceramides in Individuals at Cardiovascular Risk. Curr. Dev. Nutr. 2021, 5, 365.
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718.
- Muñoz, F.; Urvieta, R.; Buscema, F.; Rasse, M.; Fontana, A.; Berli, F. Phenolic Characterization of Cabernet Sauvignon Wines from Different Geographical Indications of Mendoza, Argentina: Effects of Plant Material and Environment. Front. Sustain. Food Syst. 2021, 5, 700642.
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive Compounds in Wine: Resveratrol, Hydroxytyrosol and Melatonin: A Review. Food Chem. 2012, 130, 797–813.
- Gil, C.; Gómez-Cordovés, C. Tryptophol Content of Young Wines Made from Tempranillo, Garnacha, Viura and Airén Grapes. Food Chem. 1986, 22, 59–65.
- Marhuenda, J.; Villaño, D.; Arcusa, R.; Zafrilla, P. Melatonin in Wine and Beer: Beneficial Effects. Molecules 2021, 26, 343.
- Albu, C.; Letitia, E.R.; Gabriel-Lucian, R. Assessment of Melatonin and Its Precursors Content by an HPLC-MS/MS Method from Different Romanian Wines. ACS Omega 2020, 5, 27254–27260.
- Sagonas, I.; Daoussis, D. Serotonin and Systemic Sclerosis. An Emerging Player in Pathogenesis. Jt. Bone Spine 2022, 89, 105309.
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos, E.; Garcia-Parrilla, M.C. Melatonin: A New Bioactive Com-pound in Wine. J. Food Compos. Anal. 2011, 24, 603–608.
- Freedman, B.J. Sulphur Dioxide in Foods and Beverages: Its Use as a Preservative and Its Effect on Asthma. Respir. Med. 1980, 74, 128–134.
- Lyu, X.; Del Prado, D.R.; Araujo, L.D.; Quek, S.-Y.; Kilmartin, P.A. Effect of glutathione addition at harvest on Sauvignon Blanc wines. Aust. J. Grape Wine Res. 2021, 27, 431–441.
- OIV. Resolutions OIV-OENO 445-2015. In Proceedings of the 13th OIV General Assembly. Available online: https://www.oiv.int/public/medias/1686/oiv-oeno-445-2015-en.pdf (accessed on 25 May 2023).
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of vitamin C (ascorbic acid and sodium calcium ascorbyl phosphate) as a feed additive for all animal species based on a dossier submitted by VITAC EEIG. EFSA J. 2013, 11, 3103.
- European Chemicals Agency. Ascorbic Acid. 2023. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.000.061 (accessed on 21 November 2023).
- OIV. Maximum Acceptable Limits. 2023. Available online: https://www.oiv.int/standards/international-code-of-oenological-practices/annexes/maximum-acceptable-limits (accessed on 21 November 2023).
- Abdullah, M.; Jamil, R.T.; Attia, F.N. Vitamin C (Ascorbic Acid). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Dienes-Nagy, Á.; Vuichard, F.; Belcher, S.; Blackford, M.; Rösti, J.; Lorenzini, F. Simultaneous Quantification of Glutathione, Glutathione Disulfide and Glutathione-S-Sulfonate in Grape and Wine Using LC-MS/MS. Food Chem. 2022, 386, 132756.
- Kritzinger, E.C.; Bauer, F.F.; du Toit, W.J. Role of Glutathione in Winemaking: A Review. J. Agric. Food Chem. 2013, 61, 269–277.
- Lambert-Royo, M.I.; Ubeda, C.; Del Barrio-Galán, R.; Sieczkowski, N.; Canals, J.M.; Peña-Neira, A.; Cortiella, M.G.I. The Diversity of Effects of Yeast Derivatives during Sparkling Wine Aging. Food Chem. 2022, 390, 133174.
- Webber, V.; Dutra, S.V.; Spinelli, F.R.; Carnieli, G.J.; Cardozo, A.; Vanderlinde, R. Effect of Glutathione during Bottle Storage of Sparkling Wine. Food Chem. 2017, 216, 254–259.
- Nikolantonaki, M.; Julien, P.; Coelho, C.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Gougeon, R.D. Impact of Glutathione on Wines Oxidative Stability: A Combined Sensory and Metabolomic Study. Front. Chem. 2018, 6, 182.
- Clark, A.C.; Deed, R.C. The Chemical Reaction of Glutathione and Trans-2-Hexenal in Grape Juice Media to Form Wine Aroma Precursors: The Impact of pH, Temperature, and Sulfur Dioxide. J. Agric. Food Chem. 2018, 66, 1214–1221.
- Binati, R.L.; Larini, I.; Salvetti, E.; Torriani, S. Glutathione Production by Non-Saccharomyces Yeasts and Its Impact on Winemaking: A Review. Food Res. Int. 2022, 156, 111333.
- Papadopoulou, D.; Roussis, I.G. Inhibition of the decline of linalool and α-terpineol in muscat wines by glutathione and N-acetylcysteine. Ital. J. Food Sci. 2001, 13, 413–419.
- Tsai, P.-C.; Araujo, L.D.; Tian, B. Varietal Aromas of Sauvignon Blanc: Impact of Oxidation and Antioxidants Used in Winemaking. Fermentation 2022, 8, 686.
- Weschawalit, S.; Thongthip, S.; Phutrakool, P.; Asawanonda, P. Glutathione and Its Antiaging and Antimelanogenic Effects. Clin. Cosmet. Investig. Dermatol. 2017, 10, 147–153.
- Raza, A.; Xie, W.; Kim, K.-H.; Rao Dronamraju, V.; Williams, J.; Vince, R.; More, S.S. Dipeptide of ψ-GSH Inhibits Oxidative Stress and Neuroinflammation in an Alzheimer’s Disease Mouse Model. Antioxidants 2022, 11, 1075.
- Chen, K.; Wu, L.; Liu, Q.; Tan, F.; Wang, L.; Zhao, D.; Fang, X.; Liu, X.; Liu, J.; Han, H. Glutathione Improves Testicular Spermatogenesis through Inhibiting Oxidative Stress, Mitochondrial Damage, and Apoptosis Induced by Copper Deposition in Mice with Wilson Disease. Biomed. Pharm. 2023, 158, 114107.
- Zasada, M.; Budzisz, E. Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments. Adv. Dermatol. Allergol. 2019, 36, 392–397.
- Fernández-Pachón, M.S.; Villaño, D.; García-Parrilla, M.C.; Troncoso, A.M. Antioxidant Activity of Wines and Relation with Their Polyphenolic Composition. Anal. Chim. Acta 2004, 513, 113–118.
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Bächler, J.P. Decrease in Oxidative Stress through Supplementation of Vitamins C and E Is Associated with a Reduction in Blood Pressure in Patients with Essential Hypertension. Clin. Sci. 2008, 114, 625–634.
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total Antioxidant Capacity as a Tool to Assess Redox Status: Critical View and Experimental Data. Free. Radic. Biol. Med. 2000, 29, 1106–1114.
- Evers, M.S.; Roullier-Gall, C.; Morge, C.; Sparrow, C.; Gobert, A.; Alexandre, H. Vitamins in Wine: Which, What for, and How Much? Compr. Rev. Food Sci. Food Saf. 2021, 20, 2991–3035.
- Evers, M.S.; Hervé, A.; Morge, C.; Sparrow, C.; Gobert, A.; Roullier-Gall, C. Exploring the Unexplored: A Characterization of Vitamins and Vitamers in White Grape Musts by High-Performance Liquid Chromatography. Food Chem. 2023, 398, 133860.
- Grindlay, G.; Mora, J.; Gras, L.; de Loos-Vollebregt, M.T.C. Atomic Spectrometry Methods for Wine Analysis: A Critical Evaluation and Discussion of Recent Applications. Anal. Chim. Acta 2011, 691, 18–32.
- Huang, X.-M.; Huang, H.-B.; Wang, H.-C. Cell Walls of Loosening Skin in Post-Veraison Grape Berries Lose Structural Polysaccharides and Calcium While Accumulate Structural Proteins. Sci. Hortic. 2005, 104, 249–263.
- McCormick, J.A.; Topf, J.; Tomacruz, I.D.; Grimm, P.R. A New Understanding of Potassium’s Influence Upon Human Health and Renal Physiology. Adv. Kidney Dis. Health 2023, 30, 137–147.
- Kocyigit, E.; Akturk, M.; Koksal, E. Relationships between Serum and Dietary Magnesium, Calcium, and Metabolic Parameters in Women with Type 2 Diabetes Mellitus. Clin. Nutr. ESPEN 2023, 54, 304–310.
- Soriano-Pérez, L.; Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Pedraza-Chaverri, J. Magnesium and Type 2 Diabetes Mellitus: Clini-cal and Molecular Mechanisms. Health Sci. Rev. 2022, 4, 100043.
- Siddiqui, M.; Pasha, A.; Kochar, K.; Junarta, J.; Fischman, D.L. Effect of high potassium diet on cardiovascular mortality: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2022, 79, 1606.
- Brescia, M.A.; Caldarola, V.; De Giglio, A.; Benedetti, D.; Fanizzi, F.P.; Sacco, A. Characterization of the geographical origin of Italian red wines based on traditional and nuclear magnetic resonance spectrometric determinations. Anal. Chim. Acta 2002, 458, 177–186.
- Jos, A.; Moreno, I.; González, A.G.; López-Artíguez, M.; Cameán, A.M. Study of the mineral profile of Catalonian “brut” cava using atomic spectrometric methods. Eur. Food Res. Technol. 2004, 218, 448–451.
- Paneque, P.; Morales, M.L.; Burgos, P.; Ponce, L.; Callejón, R.M. Elemental Characterisation of Andalusian Wine Vinegars with Protected Designation of Origin by ICP-OES and Chemometric Approach. Food Control 2017, 75, 203–210.
- Versari, A.; Laurie, V.F.; Ricci, A.; Laghi, L.; Parpinello, G.P. Progress in Authentication, Typification and Traceability of Grapes and Wines by Chemometric Approaches. Food Res. Int. 2014, 60, 2–18.
- Shimizu, H.; Akamatsu, F.; Kamada, A.; Koyama, K.; Iwashita, K.; Goto-Yamamoto, N. Variation in the Mineral Composition of Wine Produced Using Different Winemaking Techniques. J. Biosci. Bioeng. 2020, 130, 166–172.
- van Leeuwen, C. Terroir: The Effect of the Physical Environment on Vine Growth, Grape Ripening and Wine Sensory Attributes. In Managing Wine Quality; Elsevier: Amsterdam, The Netherlands, 2010; pp. 273–315.
- Goode, J. Wine Science: The Application of Science in Winemaking; Mitchell Beazley: London, UK, 2014; Available online: https://books.google.pt/books?id=RYoKngEACAAJ (accessed on 4 September 2023).
- van Leeuwen, C.; Roby, J.-P.; De Rességuier, L. Soil-Related Terroir Factors: A Review. OENO One 2018, 52, 173–188.
- Robinson, J.; Harding, J.; Vouillamoz, J. Wine Grapes: A Complete Guide to 1368 Vine Varieties, Including Their Origins and Flavours; Penguin Books Ltd.: London, UK, 2013; Available online: https://books.google.pt/books?id=YGTnD2wGn94C (accessed on 5 June 2023).
- Robinson, J.; Harding, J. The Oxford Companion to Wine; Oxford University Press: Oxford, UK, 2015.
- Friedel, M.; Frotscher, J.; Nitsch, M.; Hofmann, M.; Bogs, J.; Stoll, M.; Dietrich, H. Light Promotes Expression of Monoterpene and Flavonol Metabolic Genes and Enhances Flavour of Winegrape Berries (Vitis vinifera L. Cv. Riesling). Aust. J. Grape Wine Res. 2016, 22, 409–421.
- Johnson, H.; Robinson, J. The World Atlas of Wine, 7th ed.; Octopus Books: Ottawa, ON, Canada, 2013; Available online: https://books.google.pt/books?id=0AXrwQEACAAJ (accessed on 5 June 2023).
- Marques, C.; Correia, E.; Dinis, L.-T.; Vilela, A. An overview of sensory characterization techniques: From classical descriptive analysis to the emergence of novel profiling methods. Foods 2022, 11, 255.
- Gonzaga, L.; Capone, D.L.; Bastian, S.E.P.; Jeffery, D.W. Defining Wine Typicity: Sensory Characterisation and Consumer Perspectives. Aust. J. Grape Wine Res. 2021, 27, 246–256.
- King, E.S.; Stoumen, M.; Buscema, F.; Hjelmeland, A.K.; Ebeler, S.E.; Heymann, H.; Boulton, R.B. Regional Sensory and Chemical Characteristics of Malbec Wines from Mendoza and California. Food Chem. 2014, 143, 256–267.
- Geffroy, O.; Buissière, C.; Lempereur, V.; Chatelet, V. A Sensory, Chemical and Consumer Study of the Peppery Typicality of French Gamay Wines from Cool-Climate Vineyards. OENO One 2016, 50, 35.
- Kustos, M.; Gambetta, J.M.; Jeffery, D.W.; Heymann, H.; Goodman, S.; Bastian, S.E.P. A Matter of Place: Sensory and Chemical Characterization of Fine Australian Chardonnay and Shiraz Wines of Provenance. Food Res. Int. 2020, 130, 108903.
- Muñoz-González, C.; Feron, G.; Canon, F. Main Effects of Human Saliva on Flavour Perception and the Potential Contribution to Food Consumption. Proc. Nutr. Soc. 2018, 77, 423–431.
- Duarte-Coimbra, S.; Forcina, G.; Pérez-Pardal, L.; Beja-Pereira, A. Characterization of Tongue Dorsum Microbiome in Wine Tas-ters. Food Res. Int. 2023, 163, 112259.
- Martina, E.; Campanati, A.; Diotallevi, F.; Offidani, A. Saliva and Oral Diseases. J. Clin. Med. 2020, 9, 466.
- Schwartz, M.; Neiers, F.; Feron, G.; Canon, F. The Relationship between Salivary Redox, Diet, and Food Flavor Perception. Fron-Tiers Nutr. 2021, 7, 612735.
- Genovese, A.; Piombino, P.; Gambuti, A.; Moio, L. Simulation of Retronasal Aroma of White and Red Wine in a Model Mouth System. Investigating the Influence of Saliva on Volatile Compound Concentrations. Food Chem. 2009, 114, 100–107.
- Mosca, A.C.; Chen, J. Food-Saliva Interactions: Mechanisms and Implications. Trends Food Sci. Technol. 2017, 66, 125–134.
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A Review of Selected Studies That Determine the Physical and Chemical Properties of Saliva in the Field of Dental Treatment. BioMed Res. Int. 2018, 2018, 6572381.
- Schwartz, M.; Canon, F.; Feron, G.; Neiers, F.; Gamero, A. Impact of Oral Microbiota on Flavor Perception: From Food Processing to in-mouth Metabolization. Foods 2021, 10, 2006.
- Gittings, S.; Turnbull, N.; Henry, B.; Roberts, C.J.; Gershkovich, P. Characterisation of Human Saliva as a Platform for Oral Dissolution Medium Development. Eur. J. Pharm. Biopharm. 2015, 91, 16–24.
- Boehm, M.W.; Yakubov, G.E.; Stokes, J.R.; Baier, S.K. The Role of Saliva in Oral Processing: Reconsidering the Breakdown Path Paradigm. J. Text. Stud. 2020, 51, 67–77.
- Obreque-Slier, E.; Espínola-Espínola, V.; López-Solís, R. Wine PH Prevails over Buffering Capacity of Human Saliva. J. Agric. Food Chem. 2016, 64, 8154–8159.
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314.
- Santos, M.J.; Correia, E.; Vilela, A. Exploring the Impact of α-Amylase Enzyme Activity and pH on Flavor Perception of Alcohol-ic Drinks. Foods 2023, 12, 1018.
- Morquecho-Campos, P.; Bikker, F.J.; Nazmi, K.; de Graaf, K.; Laine, M.L.; Boesveldt, S. Impact of Food Odors Signaling Specific Taste Qualities and Macronutrient Content on Saliva Secretion and Composition. Appetite 2019, 143, 104399.
- Ali, N.; Nater, U.M. Salivary Alpha-Amylase as a Biomarker of Stress in Behavioral Medicine. Int. J. Behav. Med. 2020, 27, 337–342.
- Contreras-Aguilar, M.D.; Mateo, S.V.; Tecles, F.; Hirtz, C.; Escribano, D.; Cerón, J.J. Changes Occurring on the Activity of Salivary Alpha-Amylase Proteoforms in Two Naturalistic Situations Using a Spectrophotometric Assay. Biology 2021, 10, 227.
- Ployon, S.; Morzel, M.; Belloir, C.; Bonnotte, A.; Bourillot, E.; Briand, L.; Lesniewska, E.; Lherminier, J.; Aybeke, E.; Canon, F. Mechanisms of Astringency: Structural Alteration of the Oral Mucosal Pellicle by Dietary Tannins and Protective Effect of BPRPs. Food Chem. 2018, 253, 79–87.
- Moran, W. Terroir—The human factor. Aust. NZ Wine Ind. J. 2001, 16, 32–51.
- Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Subiela, S.; Martínez-Miró, S.; Rubio, M.; Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Influence of the Way of Reporting Alpha-Amylase Values in Saliva in Different Naturalistic Situations: A Pilot Study. PLoS ONE 2017, 12, e0180100.
- Canon, F.; Neiers, F.; Guichard, E. Saliva and Flavor Perception: Perspectives. J. Agric. Food Chem. 2018, 66, 7873–7879.
- Jacob, J.J.; Suthindhiran, K. Immobilisation of Lipase Enzyme onto Bacterial Magnetosomes for Stain Removal. Biotechnol. Rep. 2020, 25, e00422.
- Gordon, R.; Power, A.; Chapman, J.; Chandra, S.; Cozzolino, D. A Review on the Source of Lipids and Their Interactions during Beer Fermentation That Affect Beer Quality. Fermentation 2018, 4, 89.
- Restrepo, S.; Espinoza, L.; Ceballos, A.; Urtubia, A. Production of Fatty Acids during Alcoholic Wine Fermentation under selected temperature and Aeration Conditions. Am. J. Enol. Vitic. 2019, 70, 169–176.
- Schumaker, M.R.; Diako, C.; Castura, J.C.; Edwards, C.G.; Ross, C.F. Influence of Wine Composition on Consumer Perception and Acceptance of Brettanomyces Metabolites Using Temporal Check-All-That-Apply Methodology. Food Res. Int. 2019, 116, 963–972.
- Rabe, A.; Salazar, M.G.; Michalik, S.; Fuchs, S.; Welk, A.; Kocher, T.; Völker, U. Metaproteomics Analysis of Microbial Diversity of Human Saliva and Tongue Dorsum in Young Healthy Individuals. J. Oral Microbiol. 2019, 11, 1654786.
- Rud, I.; Almli, V.L.; Berget, I.; Tzimorotas, D.; Varela, P. Taste Perception and Oral Microbiota: Recent Advances and Future Perspectives. Curr. Opin. Food Sci. 2023, 51, 101030.
- Esteban-Fernández, A.; Zorraquín-Peña, I.; González de Llano, D.; Bartolomé, B.; Moreno-Arribas, M.V. The Role of Wine and Food Polyphenols in Oral Health. Trends Food Sci. Technol. 2017, 69, 118–130.
- Le Roy, C.I.; Wells, P.M.; Si, J.; Raes, J.; Bell, J.T.; Spector, T.D. Red Wine Consumption Associated with Increased Gut Microbiota α-Diversity in 3 Independent Cohorts. Gastroenterology 2020, 158, 270–272.e2.
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435.
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different Phenolic Compounds Activate Distinct Hu-man Bitter Taste Receptors. J. Agric. Food Chem. 2013, 61, 1525–1533.
- Wooding, S.P.; Ramirez, V.A. Global Population Genetics and Diversity in the TAS2R Bitter Taste Receptor Family. Front. Genet. 2022, 13, 952299.
- Wooding, S.P.; Ramirez, V.A.; Behrens, M. Bitter taste receptors: Genes, evolution and health. Evol. Med. Public Health 2021, 9, 431–447.
- Temussi, P.A. The good taste of peptides. Pept. Sci. 2012, 18, 73–82.
- Hakimi, S.; Kari, N.M.; Ismail, N.; Ismail, M.N.; Ahmad, F. Evaluation of taste active peptides and amino acids from anchovy proteins in fish sauce by in silico approach. Food Sci. Biotechnol. 2022, 31, 767–785.
- Bassoli, A.; Borgonovo, G.; Caremoli, F.; Mancuso, G. The Taste of D- and L-Amino Acids: In Vitro Binding Assays with Cloned Human Bitter (TAS2Rs) and Sweet (TAS1R2/TAS1R3) Receptors. Food Chem. 2014, 150, 27–33.
- DuBoi, G.E. Molecular mechanism of sweetness sensation. Physiol. Behav. 2016, 164, 453–463.
- Von Molitor, E.; Riedel, K.; Krohn, M.; Hafner, M.; Rudolf, R.; Cesetti, T. Sweet Taste Is Complex: Signaling Cascades and Circuits Involved in Sweet Sensation. Front. Hum. Neurosci. 2021, 15, 667709.
- Ömür-Özbek, P.; Dietrich, A.M.; Duncan, S.E.; Lee, Y.W. Role of Lipid Oxidation, Chelating Agents, and Antioxidants in Metallic Flavor Development in the Oral Cavity. J. Agric. Food Chem. 2012, 60, 2274–2280.
- Jiang, B.; Tsao, R.; Li, Y.; Miao, M. Food Safety: Food Analysis Technologies/Techniques. In Encyclopedia of Agriculture and Food Systems; Academic Press: Cambridge, MA, USA, 2014; pp. 273–288.
- Zhang, X.L.; Zheng, Y.; Xia, M.L.; Wu, Y.N.; Liu, X.J.; Xie, S.K.; Wu, Y.F.; Wang, M. Knowledge Domain and Emerging Trends in Vinegar Research: A Bibliometric Review of the Literature from WOSCC. Foods 2020, 9, 166.
- Chanioti, S.; Liadakis, G.; Tzia, C. Solid–Liquid Extraction. In Food Engineering Handbook; Varzakas, T., Tzia, C., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 253–286.
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
525
Revisions:
2 times
(View History)
Update Date:
12 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




