Chiral hydroxamic acid (HA) and bis-hydroxamic acid (BHA) ligands have emerged as powerful platforms for enantioselective catalysis, especially in asymmetric epoxidation and natural product synthesis. Recent advances in ligand architecture—such as phenyl-centric C₂-symmetric scaffolds—have enabled enhanced stereocontrol, broader substrate scope, and compatibility with diverse metal catalysts like Ti(IV) and V(V). These ligands not only facilitate epoxide formation with exceptional enantiomeric excess but have also proven essential in the total synthesis of complex bioactive compounds, including florfenicol, ophiodilactones, and α-bisabolol. This entry offers a detailed perspective on the evolution, application, and future prospects of chiral HA/BHA ligands in organic synthesis.
1. Introduction
The synthesis of natural products has long been a captivating pursuit in organic chemistry, spurred by the remarkable structural complexity and diverse array of biological activities exhibited by these compounds. Serving as a wellspring of inspiration, natural products are integral to the development of novel drugs, agrochemicals, and materials
[1]. Asymmetric synthesis, geared toward accessing enantiomerically pure compounds, plays a pivotal role in constructing complex natural product scaffolds with the desired stereochemistry crucial for their biological activities. Over the years, the evolution of efficient chiral ligands has revolutionized asymmetric synthesis, enabling chemists to achieve high selectivity and efficiency in their synthetic endeavors
[2]. These ligands act as molecular tools influencing the stereochemical outcome of reactions, facilitating the precise control of stereocenters. This breakthrough not only facilitates the synthesis of natural products, but also opens new avenues for the design and creation of chiral molecules tailored for diverse applications, emphasizing the profound impact of asymmetric synthesis and chiral ligand development on the continued exploration and utilization of natural products in various scientific and industrial domains.
2. Total Synthesis of Natural Compounds
2.1. Total Synthesis of α-Bisabolol
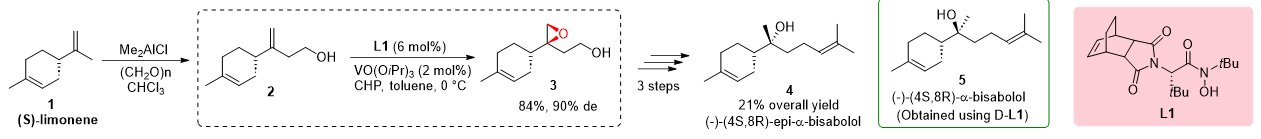
The total synthesis of (−)-α-bisabolol, a fragrance compound derived from (S)-limonene 1, was successfully achieved through the utilization of hydroxamic acid ligands L1, as demonstrated by Yamamoto in 2003. The synthetic route involved several key steps starting with the hydroxymethylation of (S)-limonene 1, resulting in the formation of (S)-alcohol 2. Diastereoselective epoxidation utilizing hydroxamic acid ligand L1 proved highly effective, yielding epoxy alcohol 3 with a good overall yield of 84% and a high diastereomeric excess of 90% (de). A subsequent reduction in epoxy alcohol 3 produced diol, which was further subjected to tosylation and coupling with isopropenyl magnesium bromide, ultimately leading to the synthesis of (−)-(4S,8S)-α-bisabolol 4. The overall yield from (S)-limonene was reported to be 21%. Additionally, the study explored the use of hydroxamic acid ligand L1, resulting in the synthesis of (−)-(4S,8R)-epi-α-Bisabolol 5 (Scheme 1).
Scheme 1. Concise and stereoselective total synthesis of (−)-(4S,8S)-α- and (−)-(4S,8R)-epi-α-bisabolol.
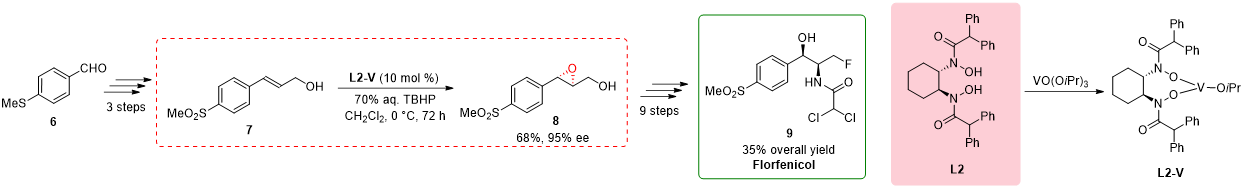
2.2. Total Synthesis of Florfenicol
The total synthesis of Florfenicol
9, as reported by Chen in 2011, involved a concise and efficient route with a significant emphasis on the utilization of a chiral bishydroxamic acid (BHA) ligand
L2 [3]. Florfenicol, a fluorinated analog of thiamphenicol, is a broad-spectrum antibiotic widely used in aquaculture species as well as various livestock species, including bovine, porcine, and chicken, for the treatment of infections.
The synthetic pathway commenced with the transformation of 4-methylthiobenzaldehyde 6, leading to the formation of allylic alcohol
7 in three sequential steps. The subsequent critical focus was directed toward the synthesis of the pivotal intermediate (
2S,
3S)-epoxide
8. While the initial consideration was the Sharpless epoxidation protocol employing anhydrous tert-butyl hydroperoxide (TBHP), its limitations for scale-up necessitated an alternative strategy. Consequently, the authors employed a modified procedure involving 1.5 equivalents of 70% aqueous TBHP and Yamamoto’s vanadium catalyst
L2-V at 0 °C for 72 h
[4]. This innovative approach successfully furnished (
2S,
3S)-epoxide
8 with a yield of 75% and an enantiomeric excess of 90%. Recrystallization enhanced further the enantiomeric excess to 95%. Subsequently, an additional nine steps were executed to achieve the desired product, Florfenicol
9, with an overall yield of 37%
[3]. The broad-spectrum antibiotic properties and widespread applications of Florfenicol highlight the importance of efficient synthetic methodologies to access such valuable compounds (
Scheme 2)
[5].
Scheme 2. Chiral BHA ligand-enabled key step in the total synthesis of Florfenicol.
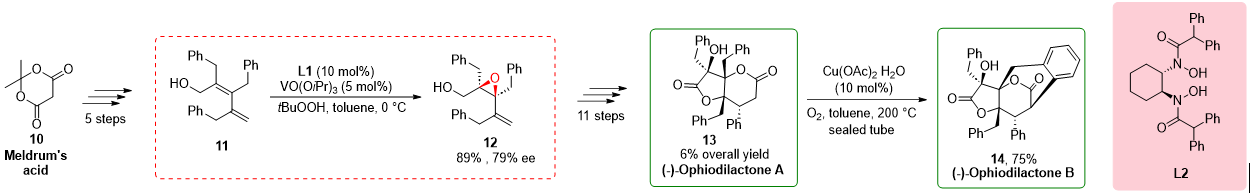
2.3. Total Synthesis of (−)-Ophiodilactones A and B
In a study conducted by Hatakeyama in 2014
[6], the total synthesis of (−)-ophiodilactones A and B was achieved using a chiral bishydroxamic acid (BHA) ligand. The synthesis involved several key steps, including Stille coupling and asymmetric epoxidation. Previous reports have shown low enantioselectivity in the epoxidation step, prompting the researchers to employ a method developed by Yamamoto and co-workers
[4]. By utilizing tert-butyl hydroperoxide and a vanadium catalyst in the presence of a BHA ligand, they successfully obtained epoxy alcohol
12 with an improved enantioselectivity of 79%. Subsequently, a sequence of 11 steps led to the synthesis of ophiodilactone A
13. The synthesis involved various transformations, including Swern oxidation, a Grignard reaction, and hydrogenation, culminating in the desired compound
13. Furthermore, ophiodilactone B
14 was obtained via the direct oxidative coupling reactions of C–H and Ar–H bonds of ophiodilactone A. The strategic use of the BHA ligand in the asymmetric epoxidation step played a pivotal role in improving the enantioselectivity and enabling the synthesis of ophiodilactones A and B. The use of the BHA ligand in the asymmetric epoxidation step played a crucial role in improving the enantioselectivity of the synthesis. By implementing the method developed by Yamamoto and co-workers, the researchers achieved higher enantioselectivity in the formation of epoxy alcohol
12. This success enabled the subsequent transformations, leading to the synthesis of γ-lactones
13 and
14 and ultimately facilitating the synthesis of ophiodilactone A
13 (
Scheme 3)
[7].
Scheme 3. Chiral BHA in total synthesis of (−)-Ophiodilactone A and (−)-Ophiodilactone B.
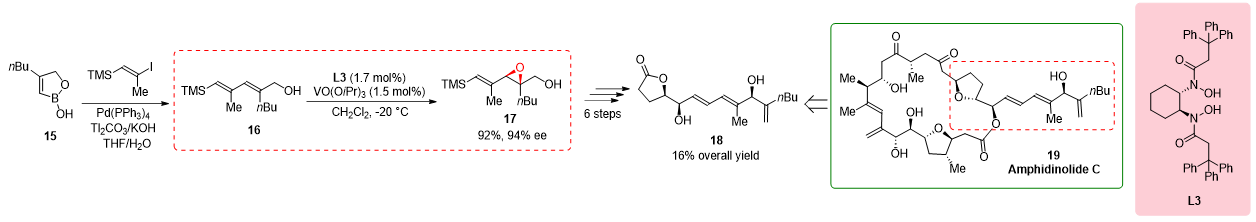
2.4. Synthesis of the Side Chain of Amphidinolide C
In the synthesis of the side chain of amphidinolide C, a complex natural product with potent antineoplastic activity, the epoxidation of allylic alcohol
16 played a crucial role in achieving the desired structure and functionality. However, the commonly used Sharpless epoxidation method did not provide the required enantioselectivity for the formation of epoxy alcohol
17. To overcome this challenge, Fenneteau et al. (2015) turned to the use of a BHA ligand, specifically the
L3-Vanadium complex, which is known for its ability to control stereoselectivity in reactions. By incorporating the BHA ligand into the epoxidation reaction, the researchers aimed to enhance the enantioselectivity and obtain the desired epoxy alcohol
17 with the desired stereochemistry. The synthesis of the side chain of amphidinolide C continued with six additional steps, ultimately yielding the desired side chain
18 (
Scheme 4)
[8].
Scheme 4. Synthesis of the side chain of amphidinolide C.
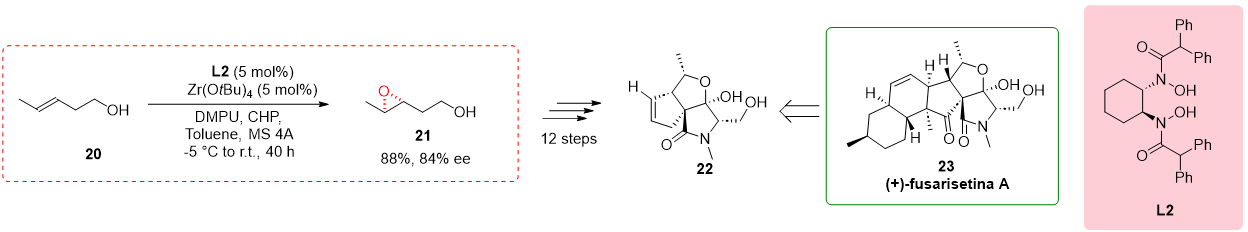
2.5. Partial Synthesis of (+)-Fusarisetin A
In their study, Kohyama et al. (2015) utilized a novel methodology in the partial synthesis of (+)-fusarisetin A, a pentacyclic fungal metabolite known for its intriguing biological properties, including its role as an acinar morphogenesis inhibitor
[9]. The researchers specifically focused on the transformation of homoallylic alcohol 20, employing the conditions described by Yamamoto and coworkers
[4]. By utilizing chiral bishydroxamic acid ligand
L2, Zn(O
tBu)
4, and DMPU, they successfully formed the corresponding epoxide
21 with an impressive enantiomeric excess of 84% and a yield of 88%. Furthermore, through an additional twelve steps, the desired compound
22 was obtained, representing a significant milestone in the synthesis of (+)-fusarisetin A. Notably, the study revealed the critical role of the metal core in controlling the enantioselectivity of the reaction, with chiral BHA ligands
L2 and hafnium catalyst leading to improved enantiomeric excesses (
Scheme 5)
[10].
Scheme 5. Partial synthesis of (+)-Fusarisetin A.
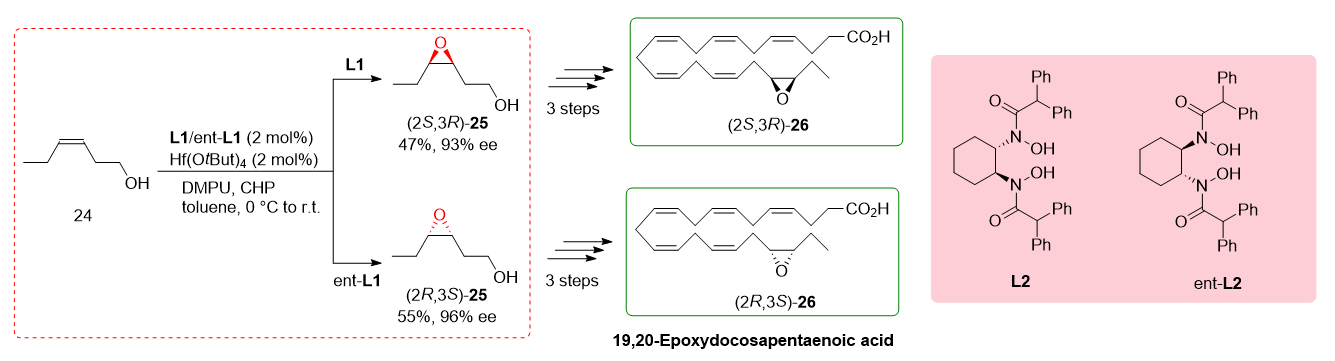
2.6. Total Synthesis of 19,20-Epoxydocosapentanoic Acid
The total synthesis of fatty acids is a challenging task, especially when large quantities of materials are required for biological assays. Within this context, the synthesis of specific unsaturated fatty acids containing an epoxide group is of particular importance as they play crucial roles as endogenous lipids
[11]. To address this challenge, Cinelli et al. reported the total synthesis of 19,20-epoxydocosapentanoic acid (19,20-EDP), which is a significant compound with biological relevance. Previous synthetic routes for fatty acids do not offer a direct method for the epoxidation of alcohols, resulting in poor outcomes. In the study, the researchers focused on the transformation of homoallylic alcohol
24 into the corresponding epoxy alcohols (
S,
R) and (
R,
S)-
25. They employed
L2 and ent-
L2 ligands in conjunction with Hf(O
tBu)
4 as the catalyst. Although the yield and enantioselectivity achieved were modest and lower than the reported values, the study represented the first report of utilizing BHA ligands in the total synthesis of these exciting compounds.
Scheme 6 illustrates the key steps involved in the total synthesis of 19,20-EDP.
Scheme 6. Hf(IV) in the total synthesis of 19,20-Epoxydocosapentanoic acid.
3. Asymmetric Epoxidation of Natural Products
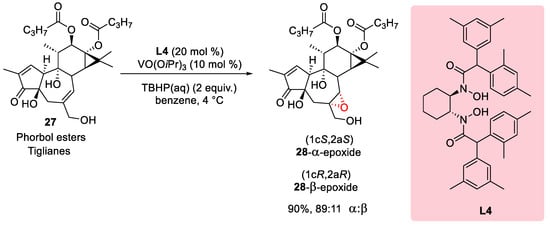
3.1. Epoxidation of Phorbol Esters
In a pivotal study conducted in 2015, Wender et al. delved into the intricate realm of diterpene epoxidation, focusing specifically on daphnanes and tiglianes at the C6–C7 position. The investigation meticulously probed the diastereoselectivity of the reaction, strategically manipulating the steric size of the ligand to unveil its impact. The researchers chose phorbol ester 27 as a test substrate, and the outcomes underscored the profound influence of ligand selection on diastereoselectivity. Scheme 7 visually encapsulates this influence, revealing that the utilization of V(V)-ligand L4 resulted in a diastereoselectivity ratio of 89:11, favoring the formation of the 28-α-epoxide.
Scheme 7. Ligand effect on Yamamoto epoxidation with Phorbol esters.
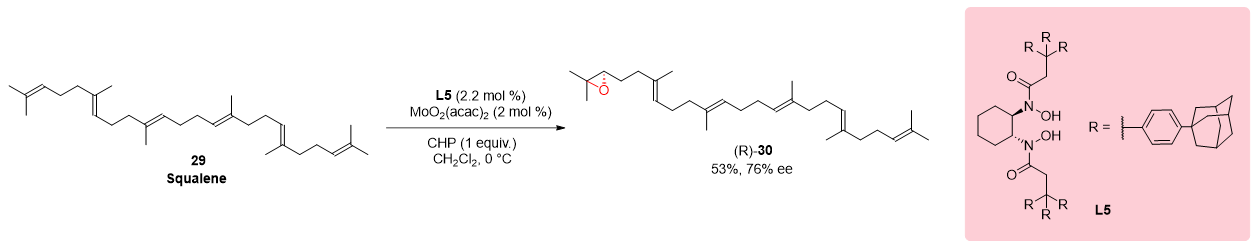
3.2. Epoxidation of Squalene
In the intricate synthetic pathway illustrated in Scheme 8, the foundational biogenetic precursor squalene 29, integral in the synthesis of steroids and polycyclic terpenoids, underwent a transformation under specified conditions. This synthetic experiment culminated in the formation of 2,3-epoxysqualene 30, showcasing a remarkable enantioselectivity of 76%. Squalene, classified as a triterpene hydrocarbon, plays a pivotal role as an intermediate in the biosynthesis of diverse biologically active molecules. To strategically introduce an epoxide group at a specific position within the squalene molecule, the researchers meticulously tailored reaction conditions conducive to this transformation. The optimization of these conditions yielded the desired 2,3-epoxysqualene 30, demonstrating a substantial enantioselectivity of 76%.
Scheme 8. Regio- and enantioselective oxidation of squalene.
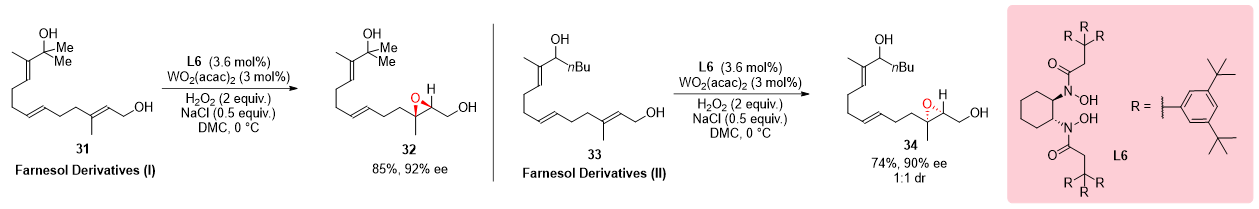
3.3. Epoxidation of Farnesol Derivatives
The regioselective synthesis of compounds featuring multiple olefin and alcohol moieties stands as a formidable challenge in organic chemistry. In a seminal study by Wang et al. in 2014, the potential of two farnesol derivatives,
31 and
33, as precursors for such intricate compounds was systematically explored using a meticulously designed catalyst system. Through the application of this catalyst system, the desired products were obtained with striking regioselectivity. The notable regioselectivity achieved in this transformation underscores the efficiency and precision of the catalyst system, unveiling novel avenues for the synthesis of complex molecules characterized by diverse functionalities (
Scheme 9)
[12].
Scheme 9. Regioselective epoxidation of farnesol derivatives.