
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Patricia Morales | -- | 3470 | 2023-12-07 18:18:19 | | | |
| 2 | Peter Tang | Meta information modification | 3470 | 2023-12-08 03:13:25 | | |
Video Upload Options
The demand of healthier food products and products made with natural ingredients has increased overwhelmingly, led by the awareness of human beings of the influence of food on their health, as well as by the evidence of side effects generated by different ingredients such as some additives. This is the case for several artificial colorants, especially azo colorants, which have been related to the development of allergic reactions, attention deficit and hyperactivity disorder. All the above has focused the attention of researchers on obtaining colorants from natural sources that do not present a risk for consumption and, on the contrary, show biological activity. The most representative compounds that present colorant capacity found in nature are anthocyanins, anthraquinones, betalains, carotenoids and chlorophylls.
1. Introduction
2. Principal Colorant Compounds Found in Natural Matrices
2.1. Anthocyanins
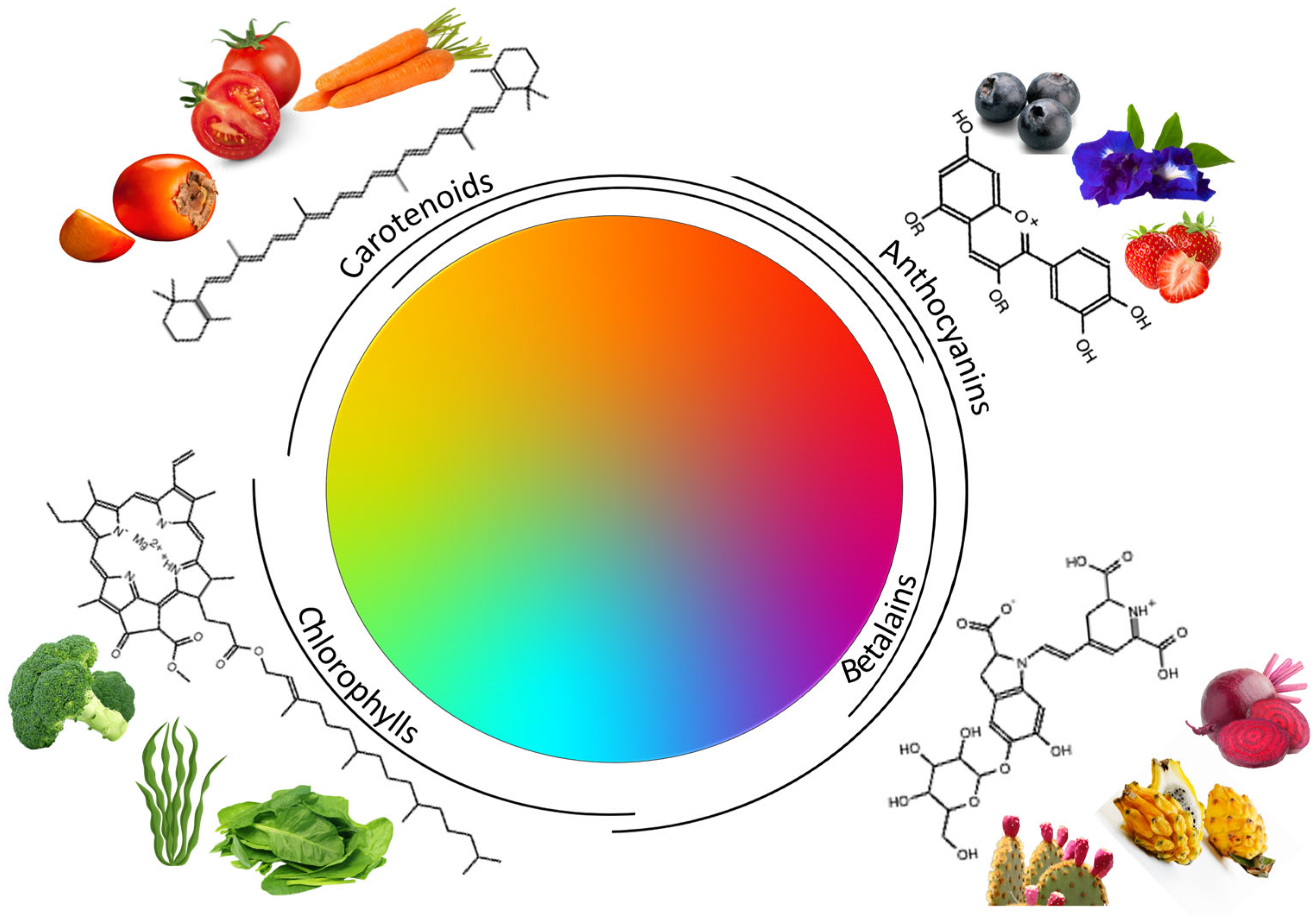
2.2. Betalains
2.3. Carotenoids
2.4. Chlorophylls
3. Source of Natural Colorants as a Potential Replacers of Artificial Food Colorants
|
Color |
Origin |
Scientific Name |
Chemical Compound |
Isolation |
E Number |
CFR Section |
|---|---|---|---|---|---|---|
|
Blue |
Butterfly pea flower extract |
Clitoria ternatea L. |
Anthocyanins |
Aqueous extraction |
NA |
73.69 |
|
Blue-green |
Spirulina extract |
Arthrospira platensis |
Phycocyanins |
NA |
73.530 |
|
|
Green |
Edible plant material, grass, lucerne and nettle extract |
Gramineae, Medicago sativa L., Urtica dioica L. |
Chlorophylls |
Solvent extraction |
E140i Cl natural green 3; magnesium chlorophyll; magnesium phaeophytin |
|
|
Green-blue |
Edible plant material, grass, lucerne and nettle extract |
Gramineae, Medicago sativa L., Urtica dioica L. |
Chlorophyllins |
Solvent extract and saponification |
E140ii Cl natural green 5; sodium chlorophyllin; potassium chlorophyllin |
|
|
Green-blue |
Edible plant material, grass, lucerne and nettle extract |
Gramineae, Medicago sativa L., Urtica dioica L. |
Copper complex of chlorophylls |
Solvent extraction and addition of a salt of copper |
E141i Cl natural green 3; copper chlorophyll; copper phaeophytin |
|
|
Green-blue |
EU: Edible plant material, grass, lucerne and nettle. US: alfalfa extract |
US: Medicago sativa L. |
Copper complex of chlorophylls |
Solvent extract, saponification, and addition of a salt of copper |
E141 (ii) CI Natural Green 5; Sodium Copper Chlorophyllin; Potassium Copper Chlorophyllin |
73.125 |
|
Yellow |
Turmeric extract |
Curcuma longa L. |
Curcumin |
Solvent extraction |
E100 Cl natural yellow 3, turmeric yellow, diferoyl methane |
73.600 |
|
Turmeric oleoresin |
Curcuma longa L. |
Curcumin |
Extraction with exact solvents |
NA |
73.615 |
|
|
Yellow-orange |
Edible plants, carrots, vegetable oils, grass, alfalfa, and nettle extract |
Daucus carota L. |
Carotenoid—Beta-carotene |
Solvent extraction |
E160a (ii) Cl food orange 5 |
73.95 |
|
Yellow-orange |
Algae extract |
Dunaliella salina Teod. |
Carotenoid—Beta-carotene |
Essential oil extraction |
E160a (iv) Cl Food orange 5 |
|
|
Yellow-brown |
Edible fruits and plants, grass, lucerne and African marigold extract |
African marigold: Tagetes erecta L. |
Carotenoids–Lutein |
Solvent extraction |
E161b Lutein; mixed carotenoids; xanthophylls |
|
|
Red-brown |
Annatto tree seeds outer coating extract |
Bixa orellana L. |
Carotenoid–bixin |
Solvent extraction |
E160b (i) Annatto; bixin; norbixin; Cl natural orange 4 |
73.30 |
|
Annatto tree seeds outer coating extract |
Bixa orellana L. |
Carotenoid–bixin |
Alkali extraction |
E160b (ii) Cl natural orange 4 |
||
|
Annatto tree seeds outer coating extract |
Bixa orellana L. |
Carotenoid–bixin |
Vegetal oil extraction |
E160b (ii) Cl natural orange 4 |
||
|
Yellow-orange |
Carrot oil |
Daucus carota L. |
Carotenoid |
Solvent extraction |
NA |
73.300 |
|
Yellow-orange |
Dried stigma powder |
Crocus sativus L. |
Carotenoid–crocin |
Dry grinding |
NA |
73.500 |
|
Red |
Ground dried paprika |
Capsicum annuum L. |
Carotenoid–capsanthin and capsorubin |
Dry grinding |
NA |
73.340 |
|
Red |
Paprika oleoresin |
Capsicum annuum L. |
Carotenoid–capsanthin and capsorubin |
Solvent extraction |
E160c |
73.345 |
|
Red |
Red tomatoes extract |
Lycopersicon esculentum L. |
Lycopene |
Solvent extraction |
E160d (ii) Natural yellow 27 |
73.585 |
|
Red beets roots extract |
Beta vulgaris L. var. Rubra |
Betalaine |
Pressing, aqueous extraction or dehydrating |
E162 Beet red |
73.40 |
|
|
Red-purple |
Grape or black carrot extract |
Vitis vinifera L., Daucus carota L. |
Anthocyanin |
Aqueous extraction |
163 |
73.169 |
|
Grape skin extract |
Vitis vinifera L. |
Anthocyanin–enocianina |
Aqueous extraction |
163 |
73.170 |
E number: European number; CFR: Code of Federal Regulations; NA: not approved.
4. Incorporation of Natural Food Colorants in Food Matrix
|
Food Color |
Natural Colorant |
Food Matrix |
Natural Sources |
Main Food Characteristics: Color and Stability |
Reference |
|---|---|---|---|---|---|
|
Red-purple |
Anthocyanins |
Yogurt |
Black rice bran extract |
Pink color with 0.2% (L*: 80.20, C*: 9.67, h: 18.34) and purplish pink with 0.6%, the highest percentage added (L*: 68.42, C*: 15.23, h: 8.73) |
[44] |
|
Yogurt |
Red onion by-products |
Time zero: 0.05 mg cya-3-gluE/g (L*: 75.27, a*: 11.20, b*: 9.55) After 4 weeks of storage 0.039 mg cya-3-gluE/g (L*: 75.19, a*: 11.35, b*: 9.75) |
[45] |
||
|
Yogurt |
Eggplant |
Color parameters of the yogurt with 1% of encapsulated extract: L*: 83.74, a*: 3.96 b*: 8.18. |
[46] |
||
|
Bakery products |
Onion skin |
Color parameters of the extracts: L*: 22.56, a*: 20.84, b*: −0.78. |
[47] |
||
|
Marmalade |
Black carrot |
Thermal processing caused a loss of anthocyanins (79.2–89.5%) |
[48] |
||
|
Red beer |
Eggplant peel extract |
Color parameters: L*: 74.67, a*: 9.86, b*: 21.76 |
[49] |
||
|
Carotenoids (lycopene) |
Butter |
Tomato skin |
Extract concentration: 20 mg/kg Color parameters, at initial time: L*: 48.78, a*: 2.36, b*: 14.17 Color parameters at 4 months of storage at 4–6 °C: L*: 48.37, a*: 2.25, b*: 14.18 |
[50] |
|
|
Mayonnaise |
Tomato skin |
Extract concentration: 50 mg/kg Color parameters, at initial time: L*: 55.82, a*: 10.32, b*: 19.95 Color parameters at 4 months of storage at 4–6 °C: L*: 53.66, a*: 9.64, b*: 19.10 |
[50] |
||
|
Ice cream |
Tomato skin |
Extract concentration: 70 mg/kg Color parameters, at initial time: L*: 84.41, a*: 11.56, b*: 28.59 Color parameters at 4 months of storage at −25 °C: 83.97, a*: 11.16, b*: 21.29 |
[50] |
||
|
Ice cream |
Tomato peel |
The ice cream prepared with 3% of extract obtained the highest score in all the parameters of the sensorial analysis |
[51] |
||
|
Pink |
Betalains |
Gummy candies |
Cactus (Opuntia ficus-indica L. Mill.) fruit pulp |
High stability of the color in the gummy candies were observed after 30 days of storage al 4 °C |
[52] |
|
Yogurt |
Pitaya |
The yogurts with 0.5% and 2% of the colorant were statistically similar to the commercial yogurts with beetroot and carmine colorants, respectively |
[53] |
||
|
Cookies |
Gomphrena globose L. |
Color parameters of samples with the lyophilized extract: L*: 56.3, a*: 22.1, b*: 8.0 Color parameters of samples with the spray dried extract: L*: 56.0, a*: 25.5, b*: 4.3 |
[54] |
||
|
Ice cream |
Gomphrena globose L. |
Color parameters: L*: 86, a*: 8, b*: 2.4 |
[55] |
||
|
Tagliatelle pasta |
Flowers of Amaranthus caudatus |
Color parameters: L*: 62, a*: 17, b*: 8 |
[55] |
||
|
Meringue cookies |
Red-fleshed pitaya peels |
Color parameters: L*: 79.6, a*: 14.5, b*: 1.24 |
[55] |
||
|
Yellow-Orange |
Carotenoids |
Dough biscuits |
Mango peel |
Color parameters of product with 20% of mango peel: L*: 52.90, a*: 7.71, b*: 22.02 |
[56] |
|
Macaroni |
Mango peel |
- |
[56] |
||
|
Green |
Chlorophylls |
Syrup |
Centella asiatica L. |
The syrups with the extracts treated with zinc and copper were stable and presented minimum change in color, mostly an increase in the h* value. |
[57] |
|
Bread |
Centella asiatica L. |
The bread with the extract treated with zinc presented a yellow–green color, while the one with the extract treated with copper presented a green color. All of them changed significantly after 7 days |
[57] |
||
|
White chocolate |
Nannochloropsis oculata D.J. Hibberd |
Samples with the encapsulated extract presented a higher quantity of chlorophylls. However, the samples with free extract presented a better stability of the color along the 28 days |
[58] |
||
|
Chewing gum |
Isochrysis galbana Pascher |
Samples showed a decrease in hardness as well as in the cohesiveness, which is advantageous for a chewing gum |
[59] |
||
|
Yogurt |
Spirulina |
The preparation with 0.25% showed the best acceptability in the sensory analysis |
[60] |
||
|
Blue |
Anthocyanins |
Cooked rice |
Butterfly pea flowers |
The incorporation of 0.6% of extract resulted in better acceptance by consumers |
[61] |
L*: lightness; a*: red/green coordinate; b*: yellow/blue coordinate; h: hue; C*: chroma.
References
- Spence, C. On the Manipulation, and Meaning(s), of Color in Food: A Historical Perspective. J. Food Sci. 2022, 88, A5–A20.
- United States Food and Drug Administration (FDA). Available online: https://www.fda.gov/food/food-ingredients-packaging/overview-food-ingredients-additives-colors (accessed on 3 May 2022).
- European Food Safety Authority (EFSA). Available online: https://www.efsa.europa.eu/en/topics/topic/food-colours (accessed on 3 May 2022).
- United States Food and Drug Administration (FDA). Available online: https://www.fda.gov/industry/color-additives/color-additives-history (accessed on 3 May 2022).
- Feingold, B.F. Hyperkinesis and Learning Disabilities Linked to the Ingestion of Artificial Food Colors and Flavors. J. Learn. Disabil. 1976, 9, 551–557.
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food Additives and Hyperactive Behaviour in 3-Year-Old and 8/9-Year-Old Children in the Community: A Randomised, Double-Blinded, Placebo-Controlled Trial. Lancet 2007, 370, 1560–1567.
- Murdoch, R.D.; Pollock, I.; Naeem, S. Tartrazine Induced Histamine Release in Vivo in Normal Subjects. J. R. Coll. Physicians 1987, 21, 257–261.
- Di Lorenzo, G.; Pacor, M.L.; Vignola, A.M.; Profita, M.; Esposito-Pellitteri, M.; Biasi, D.; Corrocher, R.; Caruso, C. Urinary Metabolites of Histamine and Leukotrienes before and after Placebo-controlled Challenge with ASA and Food Additives in Chronic Urticaria Patients. Allergy 2002, 57, 1180–1186.
- Pestana, S.; Moreira, M.; Olej, B. Safety of Ingestion of Yellow Tartrazine by Double-Blind Placebo Controlled Challenge in 26 Atopic Adults. Allergol. Immunopathol. 2010, 38, 142–146.
- Park, H.-W.; Park, C.-H.; Park, S.-H.; Park, J.Y.; Park, H.S.; Yang, H.J.; Ahn, K.-M.; Kim, K.-H.; Oh, J.-W.; Kim, K.-E.; et al. Dermatologic Adverse Reactions to 7 Common Food Additives in Patients with Allergic Diseases: A Double-Blind, Placebo-Controlled Study. J. Allergy Clin. Immunol. 2008, 121, 1059–1061.
- Amin, K.A.; Fawzia, S.A.-S. Toxicological and Safety Assessment of Tartrazine as a Synthetic Food Additive on Health Biomarkers: A Review. Afr. J. Biotechnol. 2018, 17, 139–149.
- EFSA. Scientific Opinion on the Re-Evaluation of Ponceau 4R (E 124) as a Food Additive. EFSA J. 2009, 7, 1328.
- EFSA. Scientific Opinion on the Re-Evaluation of Sunset Yellow FCF (E 110) as a Food Additive. EFSA J. 2009, 7, 1330.
- EFSA. Scientific Opinion on the Re-Evaluation of Quinoline Yellow (E 104) as a Food Additive. EFSA J. 2009, 7, 1329.
- EFSA. Statement on Allura Red AC and Other Sulphonated Mono Azo Dyes Authorised as Food and Feed Additives. EFSA J. 2013, 11, 3234.
- FDA. Color Additives and Behavioral Effects in Children. Available online: https://www.fda.gov/media/131378/download (accessed on 28 July 2023).
- Leo, L.; Loong, C.; Ho, X.L.; Raman, M.F.B.; Suan, M.Y.T.; Loke, W.M. Occurrence of Azo Food Dyes and Their Effects on Cellular Inflammatory Responses. Nutrition 2018, 46, 36–40.
- Vojdani, A.; Vojdani, C. Immune Reactivity to Food Coloring. Altern. Ther. Health Med. 2015, 21, 48–58.
- Ahmed, M.A.; Al-Khalifa, A.S.; Al-Nouri, D.M.; El-din, M.F.S. Dietary Intake of Artificial Food Color Additives Containing Food Products by School-Going Children. Saudi. J. Biol. Sci. 2021, 28, 27–34.
- Batada, A.; Jacobson, M.F. Prevalence of Artificial Food Colors in Grocery Store Products Marketed to Children. Clin. Pediatr. 2016, 55, 1113–1119.
- Stevens, L.J.; Burgess, J.R.; Stochelski, M.A.; Kuczek, T. Amounts of Artificial Food Dyes and Added Sugars in Foods and Sweets Commonly Consumed by Children. Clin. Pediatr. 2014, 54, 309–321.
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280.
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and Biological Activities of Anthocyanins. Phytochemistry 2003, 64, 923–933.
- Morata, A.; López, C.; Tesfaye, W.; González, C.; Escott, C. Anthocyanins as Natural Pigments in Beverages. In Value-Added Ingredients and Enrichments of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 14, pp. 383–428.
- Wolfe, K.L.; Liu, R.H. Structure−Activity Relationships of Flavonoids in the Cellular Antioxidant Activity Assay. J. Agric. Food Chem. 2008, 56, 8404–8411.
- Andersen, Ø.M.; Jordheim, M. Basic Anthocyanin Chemistry and Dietary Sources. In Anthocyanins in Health and Disease; Wallace, T.C., Giusti, M., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 13–90.
- Zhang, J.; Celli, G.B.; Brooks, M.S. Natural Sources of Anthocyanins. In Anthocyanins from Natural Sources: Exploiting Targeted Delivery for Improved Health; Su-Ling, M., Celli, G.B., Eds.; The Royal Society of Chemistry: London, UK, 2019; pp. 1–33.
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622.
- Zhang, Y.; Butelli, E.; Martin, C. Engineering Anthocyanin Biosynthesis in Plants. Curr. Opin. Plant Biol. 2014, 19, 81–90.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779.
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Coloring Attributes of Betalains: A Key Emphasis on Stability and Future Applications. Food Funct. 2017, 8, 1357–1372.
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520.
- Basavaraja, T.; Joshi, A.; Sethi, S.; Kaur, C.; Tomar, B.S.; Varghese, E.; Dahuja, A. Stability Enhancement of Beetroot Betalains through Copigmentation. J. Sci. Food Agric. 2022, 102, 5561–5567.
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16.
- de Freitas Santos, P.D.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of Carotenoid-Rich Materials: A Review. Food Res. Int. 2021, 147, 110571.
- Ouyang, M.; Huang, Y.; Wang, Y.; Luo, F.; Liao, L. Stability of Carotenoids and Carotenoid Esters in Pumpkin (Cucurbita maxima) Slices during Hot Air Drying. Food Chem. 2022, 367, 130710.
- Perez-Galvez, A.; Viera, I.; Roca, M. Chemistry in the Bioactivity of Chlorophylls: An Overview. Curr. Med. Chem. 2018, 24, 4515–4536.
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green Natural Colorants. Molecules 2019, 24, 154.
- EFSA. Scientific Opinion on the Re-evaluation of Chlorophylls (E 140(i)) as Food Additives. EFSA J. 2015, 13, 4089.
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Yañez, J.; Mojica, L.; Luna-Vital, D.A. Technological Applications of Natural Colorants in Food Systems: A Review. Foods 2021, 10, 634.
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural Colorants: Pigment Stability and Extraction Yield Enhancement via Utilization of Appropriate Pretreatment and Extraction Methods. Crit Rev. Food Sci. Nutr. 2017, 57, 3243–3259.
- FDA. 21 CFR Part 73 (Up to Date as of 8/08/2023) Listing of Color Additives Exempt from Certification; FDA: Silver Spring, MD, USA, 2023.
- European Commission. Commission Regulation (EU) No 231/2012 of 9 March 2012. Laying down Specifications for Food Additives Listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council; European Commission: Brussels, Belgium, 2012.
- Nontasan, S.; Moongngarm, A.; Deeseenthum, S. Application of Functional Colorant Prepared from Black Rice Bran in Yogurt. APCBEE Procedia 2012, 2, 62–67.
- Mourtzinos, I.; Prodromidis, P.; Grigorakis, S.; Makris, D.P.; Biliaderis, C.G.; Moschakis, T. Natural Food Colorants Derived from Onion Wastes: Application in a Yoghurt Product. Electrophoresis 2018, 39, 1975–1983.
- de Figueiredo Paes Barretto, F.J.; Clemente, H.A.; Santana, A.L.B.D.; da Silva Vasconcelo, M.A. Stability of Encapsulated and Non-Encapsulated Anthocyanin in Yogurt Produced with Natural Dye Obtained from Solanum melongena L. Bark. Rev. Bras. Frutic. 2020, 42.
- Stoica, F.; Condurache, N.N.; Horincar, G.; Constantin, O.E.; Turturică, M.; Stănciuc, N.; Aprodu, I.; Croitoru, C.; Râpeanu, G. Value-Added Crackers Enriched with Red Onion Skin Anthocyanins Entrapped in Different Combinations of Wall Materials. Antioxidants 2022, 11, 1048.
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Van Camp, J.; capanoglu, e. colour retention, anthocyanin stability and antioxidant capacity in black carrot (daucus carota) jams and marmalades: Effect of processing, storage conditions and in vitro gastrointestinal digestion. j. funct. foods 2015, 13, 1–10.
- Horincar, G.; Enachi, E.; Bolea, C.; Râpeanu, G.; Aprodu, I. Value-Added Lager Beer Enriched with Eggplant (Solanum melongena L.) Peel Extract. Molecules 2020, 25, 731.
- Kaur, D.; Wani, A.A.; Singh, D.P.; Sogi, D.S. Shelf Life Enhancement of Butter, Ice-Cream, and Mayonnaise by Addition of Lycopene. Int. J. Food Prop. 2011, 14, 1217–1231.
- Rizk, E.M.; El-Kady, A.T.; El-Bialy, A.R. Charactrization of Carotenoids (Lyco-Red) Extracted from Tomato Peels and Its Uses as Natural Colorants and Antioxidants of Ice Cream. Ann. Agric. Sci. 2014, 59, 53–61.
- Otálora, M.C.; de Jesús Barbosa, H.; Perilla, J.E.; Osorio, C.; Nazareno, M.A. Encapsulated Betalains (Opuntia ficus-Indica) as Natural Colorants. Case Study: Gummy Candies. LWT 2019, 103, 222–227.
- de Lima, A.C.V.; Dionisio, A.P.; de Abreu, F.A.P.; da Silva, G.S.; Junior, R.D.L.; Magalhães, H.C.R.; dos Santos Garruti, D.; da Silva Araújo, I.M.; Artur, A.G.; Taniguchi, C.A.K.; et al. Microfiltered Red–Purple Pitaya Colorant: UPLC-ESI-QTOF-MSE-Based Metabolic Profile and Its Potential Application as a Natural Food Ingredient. Food Chem. 2020, 330, 127222.
- Roriz, C.L.; Heleno, S.A.; Carocho, M.; Rodrigues, P.; Pinela, J.; Dias, M.I.; Fernandes, I.P.; Barreiro, M.F.; Morales, P.; Barros, L.; et al. Betacyanins from Gomphrena globosa L. Flowers: Incorporation in Cookies as Natural Colouring Agents. Food Chem. 2020, 329, 127178.
- Roriz, C.L.; Carocho, M.; Alves, M.J.; Rodrigues, P.; Morales, P.; Ferreira, I.C.F.R.; Heleno, S.A.; Barros, L. Betacyanins Obtained from Alternative Novel Sources as Natural Food Colorant Additives: Incorporated in Savory and Sweet Food Products. Food Funct. 2023, 14, 8775–8784.
- Ajila, C.M.; Aalami, M.; Leelavathi, K.; Rao, U.J.S.P. Mango Peel Powder: A Potential Source of Antioxidant and Dietary Fiber in Macaroni Preparations. Innov. Food Sci. Emerg. Technol. 2010, 11, 219–224.
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Molecular Structure, Stability and Cytotoxicity of Natural Green Colorants Produced from Centella Asiatica L. Leaves Treated by Steaming and Metal Complexations. Food Chem. 2017, 232, 387–394.
- Polat, D.G.; Durmaz, Y.; Konar, N.; Toker, O.S.; Palabiyik, I.; Tasan, M. Using Encapsulated Nannochloropsis oculata in White Chocolate as Coloring Agent. J. Appl. Phycol. 2020, 32, 3077–3088.
- Palabiyik, I.; Durmaz, Y.; Öner, B.; Toker, O.S.; Coksari, G.; Konar, N.; Tamtürk, F. Using Spray-Dried Microalgae as a Natural Coloring Agent in Chewing Gum: Effects on Color, Sensory, and Textural Properties. J. Appl. Phycol. 2018, 30, 1031–1039.
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina Platensis Fortification on Physicochemical, Textural, Antioxidant and Sensory Properties of Yogurt during Fermentation and Storage. LWT 2017, 84, 323–330.
- Maneechot, O.; Hahor, W.; Thongprajukaew, K.; Nuntapong, N.; Bubaka, S. A Natural Blue Colorant from Butterfly Pea (Clitoria ternatea) Petals for Traditional Rice Cooking. J. Food Sci. Technol. 2023, 60, 2255–2264.




