Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Angela Racioppo | -- | 4127 | 2023-12-07 11:18:44 | | | |
| 2 | Wendy Huang | Meta information modification | 4127 | 2023-12-07 13:14:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Racioppo, A.; D’amelio, A.; De Santis, A.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Role of Plant Growth-Promoting Bacteria in Abiotic-Stress Reduction. Encyclopedia. Available online: https://encyclopedia.pub/entry/52486 (accessed on 12 March 2026).
Racioppo A, D’amelio A, De Santis A, Bevilacqua A, Corbo MR, Sinigaglia M. Role of Plant Growth-Promoting Bacteria in Abiotic-Stress Reduction. Encyclopedia. Available at: https://encyclopedia.pub/entry/52486. Accessed March 12, 2026.
Racioppo, Angela, Annalisa D’amelio, Alessandro De Santis, Antonio Bevilacqua, Maria Rosaria Corbo, Milena Sinigaglia. "Role of Plant Growth-Promoting Bacteria in Abiotic-Stress Reduction" Encyclopedia, https://encyclopedia.pub/entry/52486 (accessed March 12, 2026).
Racioppo, A., D’amelio, A., De Santis, A., Bevilacqua, A., Corbo, M.R., & Sinigaglia, M. (2023, December 07). Role of Plant Growth-Promoting Bacteria in Abiotic-Stress Reduction. In Encyclopedia. https://encyclopedia.pub/entry/52486
Racioppo, Angela, et al. "Role of Plant Growth-Promoting Bacteria in Abiotic-Stress Reduction." Encyclopedia. Web. 07 December, 2023.
Copy Citation
Soil degradation is a global problem and refers to the reduction or loss of the biological and economic productive capacity of the soil resource. Plant growth-promoting bacteria (PGPB) could be a low-cost and long-term solution to restore soil fertility, as they provide a wide range of benefits in agriculture, including increasing crop productivity, improving soil nutrient levels and inhibiting the growth of pathogens.
plant growth-promoting bacteria
abiotic stress
salinity
soil
drought
1. Introduction
Agricultural productivity in marginal areas is affected by several environmental stresses, which can be divided into abiotic and biotic stresses. Salinity, drought, flooding, HM contamination, temperature extremes and pH are the major abiotic stresses. PGPB are known to alleviate the negative effects of stress on plants by influencing their stress response processes. Based on scientific evidence, it can be stated that the use of PGPB formulations is beneficial for plant development and is a way to transform damaged and fallow soils into healthy ones [1]. After focusing on the general mechanisms of action of PGPB, the following sections provide an overview of the specific effects of some of these mechanisms on biotic and abiotic stresses (drought, salinity and soil contamination) that affect agricultural productivity in marginal areas (Figure 1).
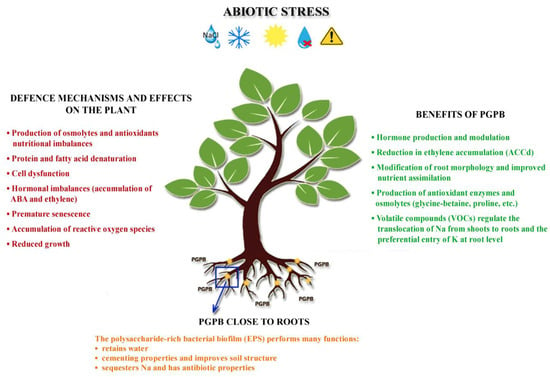
Figure 1. Plant defence mechanisms and beneficial effects of PGPB under stressful conditions.
2. PGPB—Plant Growth Promoters in High-Salinity Soils
Salinisation is one of the problems faced by marginal soils for a variety of reasons. Soils can be affected by natural or secondary salinity. In the former case, salt enrichment is often inherited from the material from which the soil is derived (igneous rocks of the lithosphere) and subsequently promoted by climatic conditions and hydrological events that favoured the deposition of large amounts of salts in sedimentary rocks, surface and subsurface waters, seas and oceans [2]. Natural saline soils are also found along marine coastlines, where infiltration of surface water tables and/or marine aerosols enrich soils with salt (NaCl) over a range of several hundred metres to a few kilometres. Secondary salinisation, on the other hand, results from the careless anthropogenic management of soils that are already vulnerable to this threat, with the use of inappropriate water and irrigation methods and inappropriate fertilisation, the advancement of the salt wedge due to over-exploitation (and misuse) of groundwater, and inadequate soil drainage conditions [3]. In Italy, salinisation affects many lowland areas, particularly coastal areas. Among the southern regions, Apulia, Sicily and Sardinia (to a lesser extent, Basilicata, Calabria and Campania) are the areas most affected by this phenomenon. In Apulia, the most exposed areas are the coastal ones, in particular the Gargano, the Murge Baresi, the Salento, the Ionian–Taranto arc and the Adriatic coast. These areas are characterised by the presence of high concentrations of salts caused by the overexploitation of coastal aquifers for agricultural, industrial and civil purposes. The use of water for irrigation has a negative impact on soil fertility and production, in terms of both yield and product quality.
Tolerance to moderate salinity (4–8 dS m−1) is a characteristic of Mediterranean plants, with some species showing sensitivity to salt stress but good adaptation to drought [4]. For example, Ficus carica L. has moderate salt tolerance, and growth under saline conditions does not result in a major reduction in biomass but is characterised by a reduction in relative water and chlorophyll content [5]. The growth of Beta vulgaris L. and Sacharum officinarum L. was increased under conditions of moderate salinity, but their photosynthetic activity and nutrient uptake were limited [6]. Furthermore, reduced flower and seed production under salinity conditions was observed in some Mediterranean crops such as chickpea and grapevine.
Salinity reduces the ability of plants to absorb water from the soil (osmotic stress) and leads to increased concentrations of ions such as Na+ and Cl− in cells, which can exceed the toxic thresholds (ionic stress) [7]. Osmotic and ionic stress reduces cell expansion and causes nutrient imbalances and oxidative stress that affects plant growth, development and survival [8]. Due to the presence of excess soluble salts, plants struggle to absorb water from the soil because the circulating solution is so concentrated that it creates a high osmotic potential, as a result of which plant roots, instead of absorbing water, release it, causing dehydration. This phenomenon inhibits key plant metabolic processes, such as photosynthesis, protein synthesis and lipid metabolism, and adversely affects productivity. Several strategies have been implemented to combat salt stress, including the use of salt resistance genes in conventional crops, but these are only effective under laboratory conditions. Another approach is the pre-treatment of biological materials with specific and selective chemicals such as ascorbic acid, nitric oxide, phosphoric acid and glycine betaine [9] or with physical effectors such as UV-B irradiation [10]. However, although effective, these treatments are not recommended for sustainable agriculture. A possible sustainable solution could be the use of soil bacterial and fungal communities that colonise plant roots and stimulate their growth (PGPB). Although studies in the Mediterranean, and particularly in the Apulian context, are very scarce, a recent study demonstrated the beneficial effect of PGPB on durum wheat under drought and water stress conditions, improving photosynthetic efficiency, grain yield and plant height [11]. Under saline conditions, bacterial inoculation consistently improved nutrient uptake and increased plant biomass compared to non-inoculated plants. The ability of PGPB to improve the growth and yield of many crops, some of them typical of the Apulian context, grown under saline conditions outside the Apulian region, was reported in several studies. Bacterial inoculation was also shown to improve photosynthetic parameters in sugar beet [12][13], and similar results were obtained in tomato, rice and wheat [14][15]. PGPB can alleviate salinity stress by modifying stress-induced physiological changes in plants through various mechanisms, such as the regulation of the synthesis of various phytohormones, including IAA, ACC-deaminase, EPSs and volatile organic compounds, atmospheric nitrogen fixation, nitrogen solubilisation and the solubilisation of mineral phosphate [16][17]. Therefore, harnessing the potential of PGPB could improve crop performance in saline soils [18].
The main strategy used by plants to tolerate salt stress is the translocation of sodium into vacuoles, thereby reducing the amount of sodium in the cytoplasm. A recent study showed that PGPB increase the expression of genes encoding the plasma membrane protein salt overly sensitive exchanger 1 (SOS1) and other proteins related to the SOS pathway [19]. Similarly, in wheat plants subjected to salinity stress and treatment with Dietzia natronolimnaea, a significant increase in the expression of SOS1 (localised on the plasma membrane) and SOS4 was observed compared to non-inoculated plants [20]. The process of adaptation to stress involves most metabolic processes in plants, but it is generally accepted that plant hormones such as ABA, SA acid and ETH play an important role in activating the signalling cascade associated with several genes involved in enhancing salt tolerance [21]. Research conducted on tomato under salt stress condition showed that the use of two Bacillus species (B. aryabhattai H19-1 and B. mesonae H20-5) increased carotenoid content, proline and ABA levels and antioxidant enzyme activities [22]. ACC-deaminase production by PGPB is the most studied mechanism by which plants alleviate salinity stress in soils by reducing the levels of ETH precursors, which tend to increase in plants under stress [23]. ACC-deaminase-producing Pseudomonas spp. promoted the growth of Citrus macrophylla under salinity stress conditions, contributed to the accumulation of IAA in leaves and inhibited the accumulation of chloride and proline in roots [24]. The results from a study of Bacillus ACC-deaminase species (B. methylotrophicus, B. siamensis) in wheat showed the ability of PGPB to enhance wheat seed germination and seedling growth at different NaCl concentrations. This suggests the potential use of these species to improve crop growth in agricultural systems under salinity stress [25]. Furthermore, several studies confirmed the beneficial effects of halotolerant IAA-producing and halophilic bacterial strains on Triticum aestivum [26] and Brassica napus [27] under salinity stress conditions. These microorganisms were able to increase plant biomass, provide additional IAA uptake and induce salt tolerance by reducing the ETH levels.
Another recent study on Brassica napus reported the effect of seed inoculation with five different species of PGPB, i.e., Azospirillum brasilense, Arthrobacter globiformis, Burkholderia fariambia, Herbaspirillum seropedicae and Pseudomonas spp. (separately). Plants inoculated with PGPB showed increased development, reduced water loss due to low membrane damage, increased antioxidant activity and increased synthesis of osmolytic proline; moreover, no deleterious effects on their photosynthetic apparatus were reported [28]. Positive results were also obtained in a study on spinach (Spinacia oleracea L.). Inoculation of plants with halotolerant (Pseudomonas spp., Thalassobacillus spp., Terribacillus spp.) and chitinolytic (Pseudomonas spp., Sanguibacter spp., Bacillus spp.) strains improved plant growth and reduced salinity [29].
PGPB are also able to help plants to reduce salt stress through the formation of biofilms. Biofilms are one of the most important protective strategies against adverse and unpredictable environmental conditions. Sessile PGPB are therefore better able to survive and interact with plants than planktonic cells. In fact, these microorganisms were shown to be more resistant to antimicrobial compounds, drought and UV radiation [30]. Microorganisms can develop biofilms on a wide range of materials, such as roots and soil, improving crop and soil performance. In addition, increased EPSs production supports biofilm formation and improves tolerance to abiotic stresses such as salinity. EPSs-producing PGPB can bind Na+ ions by reducing their availability, thereby reducing salt stress, increasing K+ uptake and improving water uptake [31]. The production of EPSs also results in changes in the cell envelope, with an increase in water retention and the regulation of carbon sources [32].
This was confirmed by several studies conducted on different crops (barley, sunflower) [33]. In particular, sunflower plants inoculated with Pseudomonas plecoglossicida PB5 and Bacillus licheniformis AP6, two strains capable of forming biofilms, were more resistant to salt stress than non-inoculated plants [34].
The application of a silicon fertiliser (potassium silicate, K2SiO3) was shown to ameliorate the negative effects of various biotic and abiotic stresses on plants, including salinity [35]. Silicon can enhance salinity tolerance in plants by improving Na+ and K+ homeostasis, nutrient status, ROS-scavenging enzyme activity and photosynthetic efficiency [36][37][38]. Mahmood et al. [39] reported that treatment with PGPB combined with the foliar application of a silicon fertiliser led to better tolerance to salinity stress in mung bean plants compared to plants treated with PGPB or the silicon fertiliser alone. Subsequently, Al-Garni et al. [40] showed that the combined application of two strains of Pseudomonas (Pseudomonas pseudoalcaligenes and P. putida) with a silicon fertiliser alleviated the salinity stress in coriander by increasing relative water content, photosynthetic pigment concentrations, peroxidase activity, total biomass, salt tolerance index and total phenolic content. The combined application of PGPB and a silicon fertiliser seems to be a feasible and promising strategy to improve plant performance in salt-affected farmlands [41]. The above strategies involving the use of PGPB and halotolerant bacteria are useful for remediating saline soils and improving plant growth under salinity stress.
3. PGPB—Effects of Drought Stress
Climate change is affecting crop production around the world. High temperatures combined with a lack of rainfall lead to drought, and this effect is more pronounced on marginal soils. Drought alters not only plant responses to pathogens, but also plant microbial communities. Drought stress, therefore, has a major impact on the quantity and quality of harvests, reducing the world’s food supply, and is one of the most serious problems in agriculture [42]. It has been estimated that half of the world’s arable land will be affected by drought in the first half of 2050, and this is closely linked to global temperature increases [43]. In several regions, and especially in semi-arid areas, the increase in frequency, duration and intensity of droughts, mainly driven by climate change dynamics, is expected to drastically reduce the current freshwater supplies, limiting crop development and yields, especially where agriculture is highly dependent on irrigation. The impact of climate change on water yields is already evident in drought-prone regions of the Mediterranean basin (Spain, Malta, Italy, Greece and Turkey). The hot and dry climate and the variability of rainfall intensity pose serious problems for the use of water resources in many regions of the Mediterranean basin, including Apulia. As far as the region of Apulia is concerned, the most vulnerable areas are located in the central and southern parts, which are characterised by a high percentage of vegetables and fruit trees (the most vulnerable crops). On the other hand, north-central Apulia (the Capitanata and Terre d’Apulia consortia) is less vulnerable, mainly due to the greater presence of vineyards and olive groves (more tolerant to water stress) [44].
Plants exposed to drought stress show different response mechanisms in the form of morphological, physiological and biochemical changes. In general, drought stress affects seed germination, plant growth and yield, transpiration rate, net photosynthesis rate, stomatal conductance, relative leaf water content and water potential [45][46][47].
Plants can use various strategies to avoid or tolerate water shortages, such as reducing transpiration and photosynthesis, enhancing the action of phytohormones and diverting energy into developing a stronger root system instead of producing new leaves [48].
Nitrogen, like many other micronutrients, is involved in several essential biochemical pathways that occur in plants, such as chlorophyll production and photosynthesis [49]. Plants have natural mechanisms to carry out N fixation, but this is a process that is highly susceptible to drought stress, leading to reduced growth rates [50]; furthermore, in the absence of water, nitrate reductase activity is reduced, resulting in poor uptake of the available nitrogen [51]. It is a common practice to use synthetic fertilisers to improve crop nitrogen uptake on drought-prone soils, but a sustainable alternative could be the use of PGPB. These bacteria regulate plant growth under drought stress conditions both directly (increased phytohormone production and nutrient availability) and indirectly (induction of systemic resistance (ISR), suppression of pathogens, synthesis of lytic enzymes) and secrete various compounds such as osmolytes, antioxidants, phytohormones, etc., that improve the osmotic potential of roots under drought stress conditions [52][53]. In drought-stressed and low-moisture soils, diazotrophic PGPB such as cyanobacteria, Azospirilium and Azoarcus [54] can produce and make nitrogen available to plants by enhancing nitrogen production, uptake and accumulation in plant tissues and soil. A recent study demonstrated the efficacy of a co-inoculum of Bradyrhizobium japonicum USDA110 and the PGPR P. putida NUU8 in soybean (G. max L.), showing increased nitrogen accumulation in plant tissues (+35%) and soil (+23%) compared to the control under drought stress conditions [55].
The critical response to biotic and abiotic stresses is based on the action of phytohormones, which slow down vital plant functions by reducing energy loss. Phytohormones play a key role in regulating the plant response to biotic and abiotic stresses and include auxins, ETH, GA and ABA [56]. PGPB can modulate the levels of these hormones to improve drought stress resistance. One of the most important supporting activities of PGPB is the development of the root system to increase the uptake of water and macro- and micronutrients from the soil through the production of IAA [57]. Many studies demonstrated the production of these compounds by various microorganisms such as Azospyrillium, Pseudomonas, Bacillus and Staphylococcus spp. [58][59].
Wheat under drought conditions inoculated with B. megaterium and B. licheniformis showed different growth patterns due to the different production rates of IAA and ACC- deaminase, which were higher in B. megaterium. The study showed higher root system development in wheat inoculated with B. megaterium than in wheat inoculated with B. licheniformis [60].
Another study confirmed that B. megaterium applied to Arabidospis taliana showed a high rate of IAA production, and under drought stress conditions, plants inoculated with the microorganism showed a 1.2-–3.0-fold increase in many plant growth parameters, such as fresh weight, dry weight, etc. PGPB activity of B. megaterium in maize, as demonstrated by Romero-Munar et al. [61], was enhanced when PGPB were co-inoculated with Rhizophagus irregularis, an arbuscular fungus, under drought and high-temperature stress conditions. The study showed that the double inoculation increased plant growth by 19%, while plants inoculated with B. megaterium alone showed an insignificant growth increase compared to the uninoculated control plants.
Another interesting compound produced by PGPB that may effectively assist IAA in modulating the response to phytohormones and, consequently, plant growth is ACC-deaminase. The rate of ACC-deaminase production by PGPB strains increases when stress conditions are more intense, as shown by Aguilera-Torres et al. [62]. PGPB isolated from two different sites at an altitude of 2050 m showed an interesting difference in ACC-deaminase activity, which was five-fold higher in the most stressed soil. The main activity of ACC-deaminase is the reduction of ethylene and related stress, and several studies demonstrated a positive relationship between ACC-deaminase activity and plant growth.
A study by Ojuederie et al. [63] on the growth-promoting activity of PGPB strains inoculated into maize under drought stress conditions emphasised the enhancing activity of ACC-deaminase. Among the three inoculated microorganisms, Pseudomonas sp. MRBP13 showed higher ACC-deaminase activity and stress reduction than the other microorganisms. In cluster bean (Cyamopsis tetragonoloba L.), increased ACC-deaminase activity was confirmed by ETH reduction. Pseudomonas stutzeri (AK17) and Paenibacillus polymyxa (KM6), which are producers of ACC-deaminase, led to lower ETH accumulation in inoculated plants than in non-inoculated controls. Furthermore, the activity of these PGPB increased the relative water content (RWC). RWC is a useful parameter for determining and monitoring the health of a plant under drought stress conditions and takes into account water uptake and water lost through transpiration [64].
An interesting characteristic of PGPB is the ability to produce EPSs. This trait, typical of some PGPB, can increase stress resistance under drought conditions by improving soil water retention [65]. In maize inoculated with Bacillus velenenzis, the combined effect of ACC-deaminase activity and EPSs production resulted in increased root length, fresh and dry weight and plant growth parameters [66].
The role of EPSs in alleviating drought stress was also investigated in two wheat cultivars, Johar-16 and Gold-16, using EPSs-producing PGPB and IAA [67]. The strains tested were a Chryseobacterium sp. (LEW3), an Acinetobacter sp. (LEW9) and a Klebsiella sp. (LEW16). The plants inoculated with LEW16 had a larger root diameter than the uninoculated control for both varieties, and root growth was about 27% higher for both wheat varieties.
4. PGPB—Improvement of Plant Growth on Polluted Marginal Land
The ‘health’ of soils, particularly agricultural soils, is reflected in the health of consumers and is challenged every day by human activities that cause soil pollution. The deliberate or accidental introduction of hazardous substances into soil can alter the specific soil characteristics to such an extent that not only its protective functions but also its productive and ecological functions are impaired. Soil contamination may be localised in limited areas corresponding to known sources of contamination (contaminated sites) or may be due to inputs of contaminants whose origin cannot be identified or to the presence of multiple sources of pollution, e.g., agricultural practices, mainly related to the use of plant protection products, atmospheric emissions from industrial, civil and roadside installations and accumulation of nutrients in soils. The presence of contaminated sites is a problem common to all industrialised countries and results from the presence of anthropogenic activities that can lead to local soil contamination through spills, leaks from facilities/reservoirs and improper waste management. The latest State of the Environment study by the Regional Agency for Environmental Prevention and Protection (ARPA) showed that Apulia is still the region with the highest industrial emissions in Italy, despite the fact that air quality regulations (e.g., on dioxins) are stricter than in the rest of the country. Critical results were highlighted in the Taranto, Bari, Brindisi, Barletta–Andria–Trani and Foggia provinces. In Apulia, there are different sites of national importance where the environmental situation is serious, which include Manfredonia, Brindisi and Bari [68]. Significant levels of HMs, particularly chromium (Cr), were found in more than 400 hectares of soil in the Altamura and Gravina areas [69]. This contamination is the result of the inappropriate disposal of a wide range of wastes, the sources of which are likely to be of various origins: urban, industrial, hospital, agricultural, etc. The pollutants recognised by the Food and Agriculture Organization of the United Nations (FAO) are HMs, metalloids, radionuclides, synthetic organic compounds such as pesticides and polycyclic aromatic hydrocarbons, pathogenic bacteria and emerging pollutants such as pharmaceuticals and personal care products [70][71].
PGPB are involved in the soil remediation mechanism both indirectly, by promoting plant growth through phytoremediation [72][73][74], and directly, by activating internal cell mechanisms. Not all PGPB are able to biodegrade HMs, as shown in several studies that reported the negative effects of HMs on the physiology, metabolic activity and survival of microorganisms [75][76]. Resistance to metals is, of course, a necessary condition for the survival of microorganisms in contaminated soils and their restorative activity. The main mechanisms used by PGPB to restore heavy-metal-contaminated soils include detoxifying activities such as accumulation, precipitation, transformation. Bioaccumulation can be divided into two phases: bacterial binding to a heavy metal and subsequent HM inclusion into the bacterial cell through the cell membrane, resulting in a reduction in the contaminant levels in the soil [77]. Once inside the cell, through facilitated transport or passive diffusion, HMs are transformed or degraded to less toxic forms through oxidation, chelation or methylation [41].
Potential ameliorative abilities in the presence of heavy-metal stress by Pseudomonas spp., Bacillus spp., Acinetobacter spp., Luteibacter spp., Azotobacter spp., Trichoderma spp., etc., have been studied [78]. Arsenic stress-ameliorating activity of Pseudomonas oleovorans was observed in rice, as reported by Anand et al. [79]. The activity of the microorganism not only was effective for plant health but also was able to reduce the amount of As in soil and in rice shoots, roots and grains. A recent study demonstrated high arsenite (As(III)) and arsenate (As(V)) tolerance and efficient As(V) reduction and As(III) efflux activity in P. putida ARS1, an endophyte isolated from rice grown in arsenic-contaminated soil [80]. Genome sequencing revealed that P. putida ARS1 possesses two sets of chromosomal arsenic resistance genes (arsRCBH), which contribute to efficient As(V) reduction and As(III) efflux, resulting in high arsenic resistance. Furthermore, the co-culture of P. putida ARS1 and Wolffia globosa (a strong arsenic accumulator with high potential for arsenic phytoremediation) increased arsenic accumulation in W. globosa by 69% and resulted in the removal of 91% of arsenic (from an initial concentration of 0.6 mg/L As(V)) from water within 3 days. As observed by Marwa et al. [81], arsenic contamination can also be bioremediated by other PGPB such as Bacillus spp. and Acinetobacter spp. Bacillus flexus and Acinetobacter junii are generally classified as PGPB due to their ability to solubilise phosphate and produce siderophores, IAA and ACC-deaminase; moreover, the strains showed growth in the presence of 150 mmol L−1 As(V) and 70 mmol L−1 As(III).
Enhancement activities were also evaluated in more critical situations, such as multimetal contamination [82]. Klebsiella spp. M2 and Kluyvera spp. M8 showed detoxification activities in the presence of cadmium (Cd) and lead (Pb) by means of phosphate solubilisation mechanisms. The increase in available phosphate promoted the precipitation of Cd and Pb as phosphate compounds, reducing the heavy metal uptake by plant roots.
Mercury (Hg) is considered by the Agency for Toxic Substances and Disease Registry (ATSDR) to be the third most hazardous heavy metal found in contaminated soils [83]. More recently, a study by Chang et al. [84] focused on the mechanisms of Hg(II) resistance and sequestration by Pseudomonas sp. AN-B15. According to the authors, the main mechanisms involved are volatilisation and conversion of Hg(II) to mercury sulphide (HgS) and sulfhydryl mercury, but the biological pathways underlying these mechanisms are unknown.
With regard to direct bioremediation action against other HMs, the bibliography is limited, and the conducted studies focused on phytoextraction mechanisms and the support of PGPB in this bioremediation activity [85][86][87][88]. The bioremediation studies are not limited to HMs, but also concern the search for sustainable PGPB-mediated solutions against contamination by organic pollutants and pesticides. Particular attention has been paid to glyphosate, a widely used herbicide with known carcinogenic activities [89][90][91].
Very few studies have been conducted to assess the simultaneous growth-promoting and glyphosate-detoxifying effects of PGPB. A recent study compared eleven PGPB strains and selected five microorganisms capable of simultaneously enhancing maize (Z. mays) growth and degrading glyphosate at various concentrations [92]. Enterobacter ludwigii, Pseudomonas aeruginosa, Klebsiella variicola, Enterobacter cloacae and Serratia liquefaciens led to a reduction in glyphosate levels after 28 days under axenic conditions, using two concentrations of glyphosate, i.e., 100 and 200 mg/kg.
PGPB have also been tested for their ability to reduce the concentration of organic contaminants such as polycyclic aromatic hydrocarbons (PHAs) and gasoline by-products such as diesel in contaminated soils. In vitro and in vivo, Bacillus marsiflavi bac144 showed the ability to degrade hydrocarbons. In vitro, the microorganism was able to reduce the concentration of hydrocarbons by 65%, while in vivo, in maize, the degradation rate was increased by 46% [93]. Besides Bacillus spp., also Pseudomonas spp. degrade petroleum hydrocarbons. The detoxification effect investigated by Ambust et al. [94] is based on the ability of Pseudomonas spp. SA3 to produce a biosurfactant. As described by the authors, these microorganisms have the ability to reduce surface tension. This facilitates the reduction of hydrophobic compounds such as hydrocarbons.
References
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of Plant Growth Promoting Rhizobacteria for Sustainable Development in Agriculture. Microbiol. Res. 2018, 206, 131–140.
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-96189-7.
- Otlewska, A.; Migliore, M.; Dybka-Stępień, K.; Manfredini, A.; Struszczyk-Świta, K.; Napoli, R.; Białkowska, A.; Canfora, L.; Pinzari, F. When Salt Meddles between Plant, Soil, and Microorganisms. Front. Plant Sci. 2020, 11, 553087.
- De Ollas, C.; Morillón, R.; Fotopoulos, V.; Puértolas, J.; Ollitrault, P.; Gómez-Cadenas, A.; Arbona, V. Facing Climate Change: Biotechnology of Iconic Mediterranean Woody Crops. Front. Plant Sci. 2019, 10, 427.
- Sadder, M.T.; Alshomali, I.; Ateyyeh, A.; Musallam, A. Physiological and Molecular Responses for Long Term Salinity Stress in Common Fig (Ficus carica L.). Physiol. Mol. Biol. Plants 2021, 27, 107–117.
- Hossain, M.S.; ElSayed, A.I.; Moore, M.; Dietz, K.-J. Redox and Reactive Oxygen Species Network in Acclimation for Salinity Tolerance in Sugar Beet. J. Exp. Bot. 2017, 68, 1283–1298.
- Shah, A.N.; Tanveer, M.; Abbas, A.; Fahad, S.; Baloch, M.S.; Ahmad, M.I.; Saud, S.; Song, Y. Targeting Salt Stress Coping Mechanisms for Stress Tolerance in Brassica: A Research Perspective. Plant Physiol. Biochem. 2021, 158, 53–64.
- Pankaj, U.; Singh, G.; Verma, R.K. Microbial Approaches in Management and Restoration of Marginal Lands. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 295–305. ISBN 978-0-12-818258-1.
- El-Esawi, M.; Alaraidh, I.; Alsahli, A.; Ali, H.; Alayafi, A.; Witczak, J.; Ahmad, M. Genetic Variation and Alleviation of Salinity Stress in Barley (Hordeum vulgare L.). Molecules 2018, 23(2488).
- Dhanya Thomas, T.T.; Dinakar, C.; Puthur, J.T. Effect of UV-B Priming on the Abiotic Stress Tolerance of Stress-Sensitive Rice Seedlings: Priming Imprints and Cross-Tolerance. Plant Physiol. Biochem. 2020, 147, 21–30.
- Yaghoubi Khanghahi, M.; Leoni, B.; Crecchio, C. Photosynthetic Responses of Durum Wheat to Chemical/Microbiological Fertilization Management under Salt and Drought Stresses. Acta Physiol. Plant. 2021, 43, 123.
- Redondo-Gómez, S.; Romano-Rodríguez, E.; Mesa-Marín, J.; Sola-Elías, C.; Mateos-Naranjo, E. Consortia of Plant-Growth-Promoting Rhizobacteria Isolated from Halophytes Improve the Response of Swiss Chard to Soil Salinization. Agronomy 2022, 12, 468.
- Zhou, N.; Zhao, S.; Tian, C.-Y. Effect of Halotolerant Rhizobacteria Isolated from Halophytes on the Growth of Sugar Beet (Beta vulgaris L.) under Salt Stress. FEMS Microbiol. Lett. 2017, 364, fnx091.
- Babar, M.; Saif-ur-Rehman; Rasul, S.; Aslam, K.; Abbas, R.; Athar, H.-R.; Manzoor, I.; Kashif Hanif, M.; Naqqash, T. Mining of Halo-Tolerant Plant Growth Promoting Rhizobacteria and Their Impact on Wheat (Triticum aestivum L.) under Saline Conditions. J. King Saud Univ. Sci. 2021, 33, 101372.
- Taj, Z.; Challabathula, D. Protection of Photosynthesis by Halotolerant Staphylococcus Sciuri ET101 in Tomato (Lycoperiscon esculentum) and Rice (Oryza sativa) Plants During Salinity Stress: Possible Interplay Between Carboxylation and Oxygenation in Stress Mitigation. Front. Microbiol. 2021, 11, 547750.
- Kang, S.-M.; Khan, A.L.; Waqas, M.; Asaf, S.; Lee, K.-E.; Park, Y.-G.; Kim, A.-Y.; Khan, M.A.; You, Y.-H.; Lee, I.-J. Integrated Phytohormone Production by the Plant Growth-Promoting Rhizobacterium Bacillus tequilensis SSB07 Induced Thermotolerance in Soybean. J. Plant Interact. 2019, 14, 416–423.
- Ilangumaran, G.; Smith, D.L. Plant Growth Promoting Rhizobacteria in Amelioration of Salinity Stress: A Systems Biology Perspective. Front. Plant Sci. 2017, 8, 1768.
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; AL-Harrasi, A. Plant Growth Promoting Bacteria as an Alternative Strategy for Salt Tolerance in Plants: A Review. Microbiol. Res. 2018, 209, 21–32.
- Poria, V.; Dębiec-Andrzejewska, K.; Fiodor, A.; Lyzohub, M.; Ajijah, N.; Singh, S.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) Integrated Phytotechnology: A Sustainable Approach for Remediation of Marginal Lands. Front. Plant Sci. 2022, 13, 999866.
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant Growth Promoting Rhizobacteria Dietzia Natronolimnaea Modulates the Expression of Stress Responsive Genes Providing Protection of Wheat from Salinity Stress. Sci. Rep. 2016, 6, 34768.
- Ramakrishna, W.; Rathore, P.; Kumari, R.; Yadav, R. Brown Gold of Marginal Soil: Plant Growth Promoting Bacteria to Overcome Plant Abiotic Stress for Agriculture, Biofuels and Carbon Sequestration. Sci. Total Environ. 2020, 711, 135062.
- Yoo, S.-J.; Weon, H.-Y.; Song, J.; Sang, M.K. Induced Tolerance to Salinity Stress by Halotolerant Bacteria Bacillus Aryabhattai H19-1 and B. Mesonae H20-5 in Tomato Plants. J. Microbiol. Biotechnol. 2019, 29, 1124–1136.
- Sofy, M.R.; Aboseidah, A.A.; Heneidak, S.A.; Ahmed, H.R. ACC Deaminase Containing Endophytic Bacteria Ameliorate Salt Stress in Pisum Sativum through Reduced Oxidative Damage and Induction of Antioxidative Defense Systems. Environ. Sci. Pollut. Res. 2021, 28, 40971–40991.
- Vives-Peris, V.; De Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root Exudates: From Plant to Rhizosphere and Beyond. Plant Cell Rep. 2020, 39, 3–17.
- Amna; Ud Din, B.; Sarfraz, S.; Xia, Y.; Kamran, M.A.; Javed, M.T.; Sultan, T.; Hussain Munis, M.F.; Chaudhary, H.J. Mechanistic Elucidation of Germination Potential and Growth of Wheat Inoculated with Exopolysaccharide and ACC- Deaminase Producing Bacillus Strains under Induced Salinity Stress. Ecotoxicol. Environ. Saf. 2019, 183, 109466.
- Orhan, F. Alleviation of Salt Stress by Halotolerant and Halophilic Plant Growth-Promoting Bacteria in Wheat (Triticum aestivum). Braz. J. Microbiol. 2016, 47, 621–627.
- Saghafi, D.; Ghorbanpour, M.; Lajayer, B.A. Efficiency of Rhizobium Strains as Plant Growth Promoting Rhizobacteria on Morpho-Physiological Properties of Brassica napus L. under Salinity Stress. J. Soil Sci. Plant Nutr. 2018, 18, 253–268.
- Rossi, M.; Borromeo, I.; Capo, C.; Glick, B.R.; Del Gallo, M.; Pietrini, F.; Forni, C. PGPB Improve Photosynthetic Activity and Tolerance to Oxidative Stress in Brassica napus Grown on Salinized Soils. Appl. Sci. 2021, 11, 11442.
- Anees, M.; Qayyum, A.; Jamil, M.; Rehman, F.U.; Abid, M.; Malik, M.S.; Yunas, M.; Ullah, K. Role of Halotolerant and Chitinolytic Bacteria in Phytoremediation of Saline Soil Using Spinach Plant. Int. J. Phytoremediation 2020, 22, 653–661.
- Amaya-Gómez, C.V.; Porcel, M.; Mesa-Garriga, L.; Gómez-Álvarez, M.I. A Framework for the Selection of Plant Growth-Promoting Rhizobacteria Based on Bacterial Competence Mechanisms. Appl. Environ. Microbiol. 2020, 86, e00760-20.
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement Using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus Licheniformis QA1 and Enterobacter Asburiae QF11 Isolated from Chenopodium Quinoa Willd. Microorganisms 2020, 8, 948.
- Kaushal, M.; Wani, S.P. Rhizobacterial-Plant Interactions: Strategies Ensuring Plant Growth Promotion under Drought and Salinity Stress. Agric. Ecosyst. Environ. 2016, 231, 68–78.
- Kasim, W.A.; Gaafar, R.M.; Abou-Ali, R.M.; Omar, M.N.; Hewait, H.M. Effect of Biofilm Forming Plant Growth Promoting Rhizobacteria on Salinity Tolerance in Barley. Ann. Agric. Sci. 2016, 61, 217–227.
- Yasmeen, T.; Ahmad, A.; Arif, M.S.; Mubin, M.; Rehman, K.; Shahzad, S.M.; Iqbal, S.; Rizwan, M.; Ali, S.; Alyemeni, M.N.; et al. Biofilm Forming Rhizobacteria Enhance Growth and Salt Tolerance in Sunflower Plants by Stimulating Antioxidant Enzymes Activity. Plant Physiol. Biochem. 2020, 156, 242–256.
- Alzahrani, Y.; Kuşvuran, A.; Alharby, H.F.; Kuşvuran, S.; Rady, M.M. The Defensive Role of Silicon in Wheat against Stress Conditions Induced by Drought, Salinity or Cadmium. Ecotoxicol. Environ. Saf. 2018, 154, 187–196.
- Rios, J.J.; Martínez-Ballesta, M.C.; Ruiz, J.M.; Blasco, B.; Carvajal, M. Silicon-Mediated Improvement in Plant Salinity Tolerance: The Role of Aquaporins. Front. Plant Sci. 2017, 8, 948.
- Li, Y.-T.; Zhang, W.-J.; Cui, J.-J.; Lang, D.-Y.; Li, M.; Zhao, Q.-P.; Zhang, X.-H. Silicon Nutrition Alleviates the Lipid Peroxidation and Ion Imbalance of Glycyrrhiza Uralensis Seedlings under Salt Stress. Acta Physiol. Plant. 2016, 38, 96.
- Garg, N.; Bhandari, P. Interactive Effects of Silicon and Arbuscular Mycorrhiza in Modulating Ascorbate-Glutathione Cycle and Antioxidant Scavenging Capacity in Differentially Salt-Tolerant Cicer Arietinum L. Genotypes Subjected to Long-Term Salinity. Protoplasma 2016, 253, 1325–1345.
- Mahmood, S.; Daur, I.; Al-Solaimani, S.G.; Ahmad, S.; Madkour, M.H.; Yasir, M.; Hirt, H.; Ali, S.; Ali, Z. Plant Growth Promoting Rhizobacteria and Silicon Synergistically Enhance Salinity Tolerance of Mung Bean. Front. Plant Sci. 2016, 7, 876.
- Al-Garni, S.M.S.; Khan, M.M.A.; Bahieldin, A. Plant Growth-Promoting Bacteria and Silicon Fertilizer Enhance Plant Growth and Salinity Tolerance in Coriandrum sativum. J. Plant Interact. 2019, 14, 386–396.
- Etesami, H. Can Interaction between Silicon and Plant Growth Promoting Rhizobacteria Benefit in Alleviating Abiotic and Biotic Stresses in Crop Plants? Agric. Ecosyst. Environ. 2018, 253, 98–112.
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic Stress in Crop Production. Int. J. Mol. Sci. 2023, 24, 6603.
- Fadiji, A.E.; Orozco-Mosqueda, M.D.C.; Santos-Villalobos, S.D.L.; Santoyo, G.; Babalola, O.O. Recent Developments in the Application of Plant Growth-Promoting Drought Adaptive Rhizobacteria for Drought Mitigation. Plants 2022, 11, 3090.
- Ronco, P.; Zennaro, F.; Torresan, S.; Critto, A.; Santini, M.; Trabucco, A.; Zollo, A.L.; Galluccio, G.; Marcomini, A. A Risk Assessment Framework for Irrigated Agriculture under Climate Change. Adv. Water Resour. 2017, 110, 562–578.
- Luo, Q.; Xie, H.; Chen, Z.; Ma, Y.; Yang, H.; Yang, B.; Ma, Y. Morphology, Photosynthetic Physiology and Biochemistry of Nine Herbaceous Plants under Water Stress. Front. Plant Sci. 2023, 14, 1147208.
- Tang, H.; Zhu, H. Specific Changes in Morphology and Dynamics of Plant Mitochondria under Abiotic Stress. Horticulturae 2022, 9, 11.
- Ali, H.; Sallahuddin, N.; Ahmed Shamsudin, N.H.; Mohd Zain, N.A.; Ibrahim, M.H.; Yaacob, J.S. Abiotic Stress Induces Morphological, Physiological, and Genetic Changes in Orthosiphon Stamineus Benth. In Vitro Cultures. Horticulturae 2022, 8, 153.
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of Waterlogging Tolerance in Plants: Research Progress and Prospects. Front. Plant Sci. 2021, 11, 627331.
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-Plant-Microbe Interactions in Stressed Agriculture Management: A Review. Pedosphere 2017, 27, 177–192.
- Freitas, V.F.D.; Cerezini, P.; Hungria, M.; Nogueira, M.A. Strategies to Deal with Drought-Stress in Biological Nitrogen Fixation in Soybean. Appl. Soil Ecol. 2022, 172, 104352.
- Gloser, V.; Dvorackova, M.; Mota, D.H.; Petrovic, B.; Gonzalez, P.; Geilfus, C.M. Early Changes in Nitrate Uptake and Assimilation Under Drought in Relation to Transpiration. Front. Plant Sci. 2020, 11, 602065.
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Ihsan, M.; Laiq, M.; Ullah, S.; Fahad, S.; et al. Drought Tolerance Improvement in Plants: An Endophytic Bacterial Approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397.
- Ullah, A.; Akbar, A.; Luo, Q.; Khan, A.H.; Manghwar, H.; Shaban, M.; Yang, X. Microbiome Diversity in Cotton Rhizosphere Under Normal and Drought Conditions. Microb. Ecol. 2019, 77, 429–439.
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits That Go Far beyond Biological Nitrogen Fixation. AMB Express 2018, 8, 73.
- Jabborova, D.; Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; et al. Co-Inoculation of Rhizobacteria Promotes Growth, Yield, and Nutrient Contents in Soybean and Improves Soil Enzymes and Nutrients under Drought Conditions. Sci. Rep. 2021, 11, 22081.
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone Signaling and Crosstalk in Regulating Drought Stress Response in Plants. Plant Cell Rep. 2021, 40, 1305–1329.
- Yasmin, H.; Naeem, S.; Bakhtawar, M.; Jabeen, Z.; Nosheen, A.; Naz, R.; Keyani, R.; Mumtaz, S.; Hassan, M.N. Halotolerant Rhizobacteria Pseudomonas Pseudoalcaligenes and Bacillus Subtilis Mediate Systemic Tolerance in Hydroponically Grown Soybean (Glycine max L.) against Salinity Stress. PLoS ONE 2020, 15, e0231348.
- Yaadesh, S.; Tomar, G.S.; Kaushik, R.; Prasanna, R.; Grover, M. Azospirillum–Bacillus Associations: Synergistic Effects on in Vitro PGP Traits and Growth of Pearl Millet at Early Seedling Stage under Limited Moisture Conditions. Biotech 2023, 13, 90.
- Sati, D.; Pande, V.; Samant, M. Plant-Beneficial Bacillus, Pseudomonas, and Staphylococcus Spp. from Kumaon Himalayas and Their Drought Tolerance Response. Front. Sustain. Food Syst. 2023, 7, 1085223.
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S.; et al. Drought-Tolerant Bacillus megaterium Isolated from Semi-Arid Conditions Induces Systemic Tolerance of Wheat under Drought Conditions. Plant Cell Rep. 2022, 41, 549–569.
- Romero-Munar, A.; Aroca, R.; Zamarreño, A.M.; García-Mina, J.M.; Perez-Hernández, N.; Ruiz-Lozano, J.M. Dual Inoculation with Rhizophagus Irregularis and Bacillus megaterium Improves Maize Tolerance to Combined Drought and High Temperature Stress by Enhancing Root Hydraulics, Photosynthesis and Hormonal Responses. Int. J. Mol. Sci. 2023, 24, 5193.
- Aguilera-Torres, C.; Riveros, G.; Morales, L.V.; Sierra-Almeida, A.; Schoebitz, M.; Hasbún, R. Relieving Your Stress: PGPB Associated with Andean Xerophytic Plants Are Most Abundant and Active on the Most Extreme Slopes. Front. Microbiol. 2023, 13, 1062414.
- Ojuederie, O.B.; Babalola, O.O. Growth Enhancement and Extenuation of Drought Stress in Maize Inoculated with Multifaceted ACC Deaminase Producing Rhizobacteria. Front. Sustain. Food Syst. 2023, 6, 1076844.
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The Effect of Drought Stress on the Leaf Relative Water Content and Tuber Yield of a Half-Sib Family of ‘Katahdin’-Derived Potato Cultivars. Breed. Sci. 2016, 66, 328–331.
- Le Gall, S.; Bérard, A.; Page, D.; Lanoe, L.; Bertin, N.; Doussan, C. Increased Exopolysaccharide Production and Microbial Activity Affect Soil Water Retention and Field Performance of Tomato under Water Deficit. Rhizosphere 2021, 19, 100408.
- Nadeem, S.M.; Ahmad, M.; Tufail, M.A.; Asghar, H.N.; Nazli, F.; Zahir, Z.A. Appraising the Potential of EPS -producing Rhizobacteria with ACC -deaminase Activity to Improve Growth and Physiology of Maize under Drought Stress. Physiol. Plant. 2021, 172, 463–476.
- Latif, M.; Bukhari, S.A.H.; Alrajhi, A.A.; Alotaibi, F.S.; Ahmad, M.; Shahzad, A.N.; Dewidar, A.Z.; Mattar, M.A. Inducing Drought Tolerance in Wheat through Exopolysaccharide-Producing Rhizobacteria. Agronomy 2022, 12, 1140.
- Tateo, A.; Campanaro, V.; Amoroso, N.; Bellantuono, L.; Monaco, A.; Pantaleo, E.; Rinaldi, R.; Maggipinto, T. Predicting Air Quality from Measured and Forecast Meteorological Data: A Case Study in Southern Italy. Atmosphere 2023, 14, 475.
- Brunetti, G.; Soler-Rovira, P.; Farrag, K.; Senesi, N. Tolerance and Accumulation of Heavy Metals by Wild Plant Species Grown in Contaminated Soils in Apulia Region, Southern Italy. Plant Soil 2009, 318, 285–298.
- FAO; UNEP. Global Assessment of Soil Pollution: Report; FAO: Rome, Italy; UNEP: Nairobi, Kenya, 2021; ISBN 978-92-5-134469-9.
- Rodríguez, N.; McLaughlin, M.; Pennock, D. Soil Pollution: A Hidden Reality; FAO: Rome, Italy, 2018; ISBN 978-92-5-130505-8.
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the Molecular Mechanisms for the Enhanced Phytoremediation of Heavy Metals through Plant Growth Promoting Rhizobacteria: A Review. J. Environ. Manag. 2020, 254, 109779.
- Sharma, P. Efficiency of Bacteria and Bacterial Assisted Phytoremediation of Heavy Metals: An Update. Bioresour. Technol. 2021, 328, 124835.
- Nedjimi, B. Phytoremediation: A Sustainable Environmental Technology for Heavy Metals Decontamination. SN Appl. Sci. 2021, 3, 286.
- Jarosławiecka, A.K.; Piotrowska-Seget, Z. The Effect of Heavy Metals on Microbial Communities in Industrial Soil in the Area of Piekary Śląskie and Bukowno (Poland). Microbiol. Res. 2022, 13, 626–642.
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J. Toxicol. 2018, 2018, 2568038.
- Gupta, R.; Khan, F.; Alqahtani, F.M.; Hashem, M.; Ahmad, F. Plant Growth–Promoting Rhizobacteria (PGPR) Assisted Bioremediation of Heavy Metal Toxicity. Appl. Biochem. Biotechnol. 2023.
- Wang, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Natarajan, D.; Ma, Y. Plant Growth-Promoting Bacteria in Metal-Contaminated Soil: Current Perspectives on Remediation Mechanisms. Front. Microbiol. 2022, 13, 966226.
- Anand, V.; Kaur, J.; Srivastava, S.; Bist, V.; Dharmesh, V.; Kriti, K.; Bisht, S.; Srivastava, P.K.; Srivastava, S. Potential of Methyltransferase Containing Pseudomonas Oleovorans for Abatement of Arsenic Toxicity in Rice. Sci. Total Environ. 2023, 856, 158944.
- Wang, Z.-W.; Yang, G.; Chen, J.; Zhou, Y.; Núñez Delgado, A.; Cui, H.-L.; Duan, G.-L.; Rosen, B.P.; Zhu, Y.-G. Fundamentals and Application in Phytoremediation of an Efficient Arsenate Reducing Bacterium Pseudomonas Putida ARS1. J. Environ. Sci. 2024, 137, 237–244.
- Marwa, N.; Singh, N.; Srivastava, S.; Saxena, G.; Pandey, V.; Singh, N. Characterizing the Hypertolerance Potential of Two Indigenous Bacterial Strains (Bacillus Flexus and Acinetobacter Junii) and Their Efficacy in Arsenic Bioremediation. J. Appl. Microbiol. 2019, 126, 1117–1127.
- Qin, S.; Zhang, H.; He, Y.; Chen, Z.; Yao, L.; Han, H. Improving Radish Phosphorus Utilization Efficiency and Inhibiting Cd and Pb Uptake by Using Heavy Metal-Immobilizing and Phosphate-Solubilizing Bacteria. Sci. Total Environ. 2023, 868, 161685.
- Rahman, Z.; Singh, V.P. The Relative Impact of Toxic Heavy Metals (THMs) (Arsenic (As), Cadmium (Cd), Chromium (Cr)(VI), Mercury (Hg), and Lead (Pb)) on the Total Environment: An Overview. Environ. Monit. Assess. 2019, 191, 419.
- Chang, J.; Yan, Z.; Dong, J.; Wu, X.; Meng, Z.; Shi, Y.; Chen, J. Mechanisms Controlling the Transformation of and Resistance to Mercury(II) for a Plant-Associated Pseudomonas Sp. Strain, AN-B15. J. Hazard. Mater. 2022, 425, 127948.
- Makarova, A.S.; Nikulina, E.; Fedotov, P. Induced Phytoextraction of Mercury. Sep. Purif. Rev. 2022, 51, 174–194.
- González, D.; Blanco, C.; Probanza, A.; Jiménez, P.A.; Robas, M. Evaluation of the PGPR Capacity of Four Bacterial Strains and Their Mixtures, Tested on Lupinus Albus Var. Dorado Seedlings, for the Bioremediation of Mercury-Polluted Soils. Processes 2021, 9, 1293.
- Robas, M.; Jiménez, P.A.; González, D.; Probanza, A. Bio-Mercury Remediation Suitability Index: A Novel Proposal That Compiles the PGPR Features of Bacterial Strains and Its Potential Use in Phytoremediation. Int. J. Environ. Res. Public. Health 2021, 18, 4213.
- Durand, A.; Maillard, F.; Alvarez-Lopez, V.; Guinchard, S.; Bertheau, C.; Valot, B.; Blaudez, D.; Chalot, M. Bacterial Diversity Associated with Poplar Trees Grown on a Hg-Contaminated Site: Community Characterization and Isolation of Hg-Resistant Plant Growth-Promoting Bacteria. Sci. Total Environ. 2018, 622–623, 1165–1177.
- Crump, K.; Crouch, E.; Zelterman, D.; Crump, C.; Haseman, J. Accounting for Multiple Comparisons in Statistical Analysis of the Extensive Bioassay Data on Glyphosate. Toxicol. Sci. 2020, 175, 156–167.
- Ogunbiyi, O.D.; Akamo, D.O.; Oluwasanmi, E.E.; Adebanjo, J.; Isafiade, B.A.; Ogunbiyi, T.J.; Alli, Y.A.; Ayodele, D.T.; Oladoye, P.O. Glyphosate-Based Herbicide: Impacts, Detection, and Removal Strategies in Environmental Samples. Groundw. Sustain. Dev. 2023, 22, 100961.
- Pérez-Fernández, M.A.; Calvo-Magro, E.; Valentine, A. Benefits of the Symbiotic Association of Shrubby Legumes for the Rehabilitation of Degraded Soils under Mediterranean Climatic Conditions. Land Degrad. Dev. 2016, 27, 395–405.
- Mohy-Ud-Din, W.; Akhtar, M.J.; Bashir, S.; Asghar, H.N.; Nawaz, M.F.; Chen, F. Isolation of Glyphosate-Resistant Bacterial Strains to Improve the Growth of Maize and Degrade Glyphosate under Axenic Condition. Agriculture 2023, 13, 886.
- Saeed, M.; Ilyas, N.; Bibi, F.; Jayachandran, K.; Dattamudi, S.; Elgorban, A.M. Biodegradation of PAHs by Bacillus Marsiflavi, Genome Analysis and Its Plant Growth Promoting Potential. Environ. Pollut. 2022, 292, 118343.
- Ambust, S.; Das, A.J.; Kumar, R. Bioremediation of Petroleum Contaminated Soil through Biosurfactant and Pseudomonas Sp. SA3 Amended Design Treatments. Curr. Res. Microb. Sci. 2021, 2, 100031.
More
Information
Subjects:
Soil Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
07 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





