Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tristan Pascart | -- | 1203 | 2023-12-07 09:09:47 | | | |
| 2 | Catherine Yang | + 1 word(s) | 1204 | 2023-12-07 09:26:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pascart, T.; Budzik, J. Monosodium Crystals in Vessels of Patients with Gout. Encyclopedia. Available online: https://encyclopedia.pub/entry/52478 (accessed on 08 February 2026).
Pascart T, Budzik J. Monosodium Crystals in Vessels of Patients with Gout. Encyclopedia. Available at: https://encyclopedia.pub/entry/52478. Accessed February 08, 2026.
Pascart, Tristan, Jean-François Budzik. "Monosodium Crystals in Vessels of Patients with Gout" Encyclopedia, https://encyclopedia.pub/entry/52478 (accessed February 08, 2026).
Pascart, T., & Budzik, J. (2023, December 07). Monosodium Crystals in Vessels of Patients with Gout. In Encyclopedia. https://encyclopedia.pub/entry/52478
Pascart, Tristan and Jean-François Budzik. "Monosodium Crystals in Vessels of Patients with Gout." Encyclopedia. Web. 07 December, 2023.
Copy Citation
Cardiovascular disease in gout is a central issue, but the underlying mechanisms linking the two are unclear. The existence of monosodium (MSU) crystal deposition directly inflaming vessel walls has been recurrently suggested and challenged since the 1950s and is again a matter of active debate since recent studies using dual-energy computed tomography (DECT) suggested a higher prevalence of plaques considered to be containing MSU crystals in patients with gout.
gout
monosodium urate
vascular plaques
dual-energy computed tomography
1. Histological Evidence
Together with other organs, the CV system has been explored for potential MSU crystal deposition as early as the 1950s, and had already been at the time a source of controversy [1]. Case series from autopsies of individuals with gout had led to the identification of crystal deposits in polarized light microscopy on the intima with cellular reactions involving vessel walls as deep as the adventitia [2]. The urate nature of such crystals was quickly challenged in another case series from 11 autopsies from patients with gout, in whom visceral MSU crystal deposits could be found in several organs, but not inside vessels [1]. The authors from this study argued that prior reports may have mistaken cholesterol crystals for MSU crystals, as they can share common features of morphology but also have a negative birefringence in polarized light microscopy [1]. In synovial fluids or suspected tophi, identifying needle-shaped, negatively birefringent crystals among other co-existing crystals that are essentially made of calcium (particularly calcium pyrophosphate that has a different morphology and positive birefringence) is indeed the gold standard for the diagnosis of gout [3][4]. However, tissue itself may exhibit some birefringence, making the search for potential crystals more difficult (e.g., in the cartilage), and most importantly, other crystals may frequently be present, particularly cholesterol crystals in vessels. The lone observation of negatively birefringent crystals in such tissues using polarized light microscopy is therefore not sufficient to qualify as MSU-crystal proof, and requires additional methods of chemical characterization [5]. Furthermore, the inflammatory response to MSU crystals mediated by the NLRP3 inflammasome is not specific, as the same pathway is known to be activated by cholesterol crystals in atheroma plaques [6][7][8]. The histopathological evidence of leukocyte infiltrate in vessel walls near crystals cannot ascertain their nature.
New cadaver studies were performed very recently. Dalbeth et al. reported a study of six cadaveric donors with a known history of gout (including two subjects with crystal-proven tophi), which examined the crystal deposition of the aorta and the proximal few centimeters of the brachiocephalic, common carotids, and subclavian arteries [9]. The methods were different as the analyses were not performed on tissue sections of vessel walls, but on the aspirated fluid after the injection of 100 µL of phosphate-buffered saline into the vessel wall at ten standardized sites across each sample, and analyzed by polarizing light microscopy. Microscopic analysis demonstrated plate-like negatively birefringent cholesterol crystals from vascular tissue from five of the six subjects in three sites on average per donor. Of note, a single negatively birefringent needle-shaped crystal was observed from one of the 60 standardized sites analyzed, while numerous negatively birefringent needle-shaped crystals were present in aspirates from tophaceous joint tissue.
The only convincing evidence of MSU crystal deposition in the cardiovascular system of patients with gout is on cardiac valves. A case report from an older patient with a long-lasting history of gout reported crystal deposits in a resected aortic valve, and the presence of MSU crystals was chemically ascertained with X-ray diffraction [10]. Overall, nine studies of MSU crystal deposition on cardiac valves in tophaceous patients were reported, with various levels of evidence of the true nature of the crystals, but overall, the concept of valvular micro-tophi is convincing [11].
2. Imaging Evidence: The Debate on the Evidence Gathered with DECT
The discussion on the potential existence of arterial MSU crystal deposition was renewed by the publication of DECT scans of coronary arteries and the aorta from patients with gout and controls by Klauser et al. [12] (Figure 1). The study included 59 patients with gout who were compared to 47 controls. Using the post-processing software in ‘gout mode’, vascular plaques were coded by the machine as containing MSU crystals in the aorta more commonly among patients with gout (n = 51 [86.4%]) compared with controls (n = 7 [14.9%]) (χ2 = 17.68, p < 0.001), as well as in coronary arteries (n = 19 [32.2%] vs. n = 2 [4.3%]) (χ2 = 8.97, p = 0.003), respectively. Another important finding was that the coronary calcium scores (i.e., the extent of calcified plaques) was significantly higher in patients with gout than in controls, suggesting that there may be a relationship between calcified plaques and MSU-coded plaques. The same team performed other studies with similar methodologies, including analyses using crystal-containing phantoms providing the same results, which were also found in a small study by another team using the same methodology [13][14]. Methodological issues were raised in response to the publication of the first study, but at least the question was opened [15].
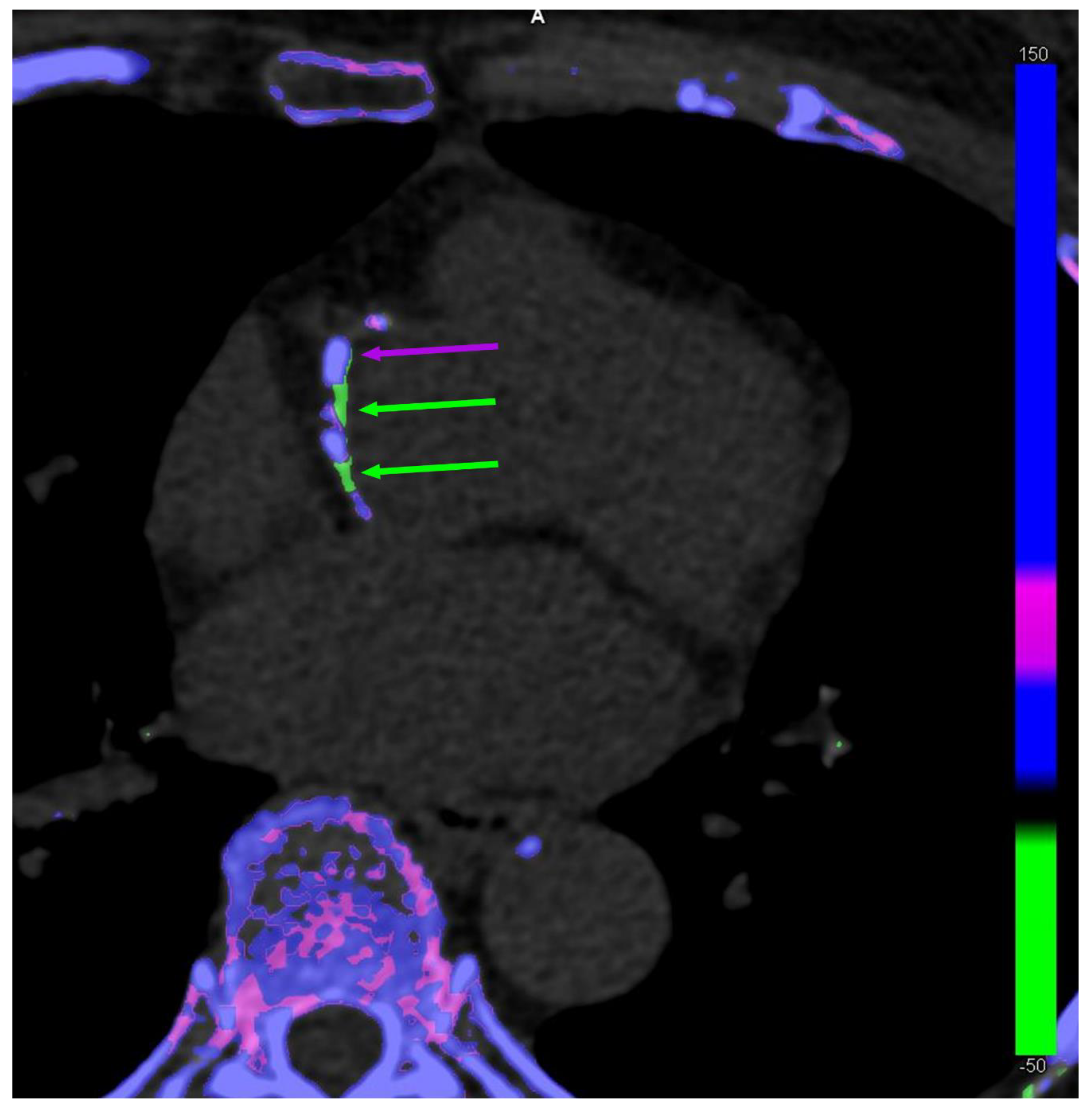
Figure 1. Dual-energy computed tomography of co-existing monosodium urate (MSU) crystal-coded (green arrows) and calcium-coded (purple arrows) coronary plaques in a 63-year-old female patient without gout.
The VASCURATE study from the researchers' team focused on popliteal artery plaques from knee DECT scans of 126 patients with gout and 26 controls [16]. The main finding of the study was that the prevalence of DECT-based vascular MSU-coded plaques was comparable in patients with gout (24.6%) and controls (23.1%; p = 0.87). In addition, the presence of such MSU-coded plaques was strongly associated with coexisting arterial calcifications (p < 0.001), but not with soft-tissue MSU deposits. Moreover, 17 patients had follow-up DECT scans during urate-lowering treatment, and vascular MSU-coded plaques remained stable despite effective ULT (p = 0.64), which decreased both serum urate levels and soft-tissue MSU volumes (p < 0.001). A single MSU-coded plaque disappeared during follow-up to be eventually coded as being calcified.
In the cadaver study (n = 6) performed by Dalbeth et al., DECT scanning of the aorta and proximal section of large arteries showed calcification in all samples, but no evidence of MSU crystal deposition [9] (Table 1).
Table 1. Summary of the evidence of vascular monosodium urate (MSU) crystal deposition in patients with gout.
| Study | Year | Subjects | Studied Arteries | MSU Crystal Present in Vessels |
Technique of Crystal Identification | Chemical Characterization of MSU Crystals |
|---|---|---|---|---|---|---|
| Traut E.F. et al. [2] | 1954 | 2 cadaveric donors with gout |
Coronary | Yes | Polarized light microscopy | No |
| Lievin M.H. et al. [1] | 1956 | 11 cadaveric donors with gout |
Coronary and Aorta | No | Polarized light microscopy | No |
| Klauser A.S. et al. [12] | 2019 | 59 Patients | Coronary and Aorta | Yes | Dual-energy CT | No |
| Barazani S. et al. [13] | 2020 | 31 patients | Coronary and Aorta | Yes | Dual-energy CT | No |
| Feuchtner G.M. et al. [14] | 2021 | 37 Patients | Coronary and Aorta | Yes | Dual-energy CT | No |
| Dalbeth N. et al. [9] | 2022 | 6 cadaveric donors | Aorta | No (doubt on 1 crystal) |
Polarized light microscopy | No |
| Pascart T. et al. [16] | 2021 | 126 patients | Popliteal arteries | No | Dual-energy CT | Non-invasive (DECT parameter analysis) |
Data from a pegloticase trial, that included sequential DECT scans and even TEP-scans in ten patients before and after treatment with pegloticase, were recently presented at the 2022 American College of Rheumatology Convergence meeting [17]. The total volume of DECT-based MSU-coded plaques did not significantly decrease over time, while the authors reported a statistically significant decrease in SUV mean (p = 0.0003) and SUV max (p = 0.0090) across all arteries studied after treatment with pegloticase. Such results would suggest that inflammation in the arterial wall is reduced after massive MSU crystal debulking, unrelated to any evolution of what is considered to be composed of MSU crystals by DECT.
References
- Levin, M.H.; Lichtenstein, L.; Scott, H.W. Pathologic changes in gout; Survey of eleven necropsied cases. Am. J. Pathol. 1956, 32, 871–895.
- Traut, E.F.; Knight, A.A.; Szanto, P.B.; Passerelli, E.W. Specific vascular changes in gout. J. Am. Med. Assoc. 1954, 156, 591–593.
- Pascart, T.; Liote, F. Gout: State of the art after a decade of developments. Rheumatology 2019, 58, 27–44.
- Neogi, T.; Jansen, T.L.; Dalbeth, N.; Fransen, J.; Schumacher, H.R.; Berendsen, D.; Brown, M.; Choi, H.; Edwards, N.L.; Janssens, H.J.E.M.; et al. 2015 Gout classification criteria: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2015, 74, 1789–1798.
- Soare, T.; Iordache, A.M.; Nicolae, G.; Iordache, S.M.; Baciu, C.; Marinescu, S.; Rizac, R.I.; Militaru, M. Identification of Uric Acid Crystals Accumulation in Human and Animal Tissues Using Combined Morphological and Raman Spectroscopy Analysis. Diagnostics 2022, 12, 2762.
- Andrès, M. Gout and Cardiovascular Disease: Mechanisms, Risk Estimations, and the Impact of Therapies. Gout Urate Cryst. Depos. Dis. 2023, 1, 152–166.
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361.
- Tall, A.R.; Westerterp, M. Inflammasomes, neutrophil extracellular traps, and cholesterol. J. Lipid Res. 2019, 60, 721–727.
- Dalbeth, N.; Alhilali, M.; Riordan, P.; Narang, R.; Chhana, A.; McGlashan, S.; Doyle, A.; Andres, M. Vascular deposition of monosodium urate crystals in gout: Analysis of cadaveric tissue by dual-energy computed tomography and compensated polarizing light microscopy. Arthritis Rheumatol. 2022, 74, 1295–1296.
- Gawoski, J.M.; Balogh, K.; Landis, W.J. Aortic valvular tophus: Identification by X-ray diffraction of urate and calcium phosphates. J. Clin. Pathol. 1985, 38, 873–876.
- Khanna, P.; Johnson, R.J.; Marder, B.; LaMoreaux, B.; Kumar, A. Systemic Urate Deposition: An Unrecognized Complication of Gout? J. Clin. Med. 2020, 9, 3204.
- Klauser, A.S.; Halpern, E.J.; Strobl, S.; Gruber, J.; Feuchtner, G.; Bellmann-Weiler, R.; Weiss, G.; Stofferin, H.; Jaschke, W. Dual-Energy Computed Tomography Detection of Cardiovascular Monosodium Urate Deposits in Patients with Gout. JAMA Cardiol. 2019, 4, 1019–1028.
- Barazani, S.H.; Chi, W.-W.; Pyzik, R.; Chang, H.; Jacobi, A.; O’Donnell, T.; Fayad, Z.A.; Ali, Y.; Mani, V. Quantification of uric acid in vasculature of patients with gout using dual-energy computed tomography. World J. Radiol. 2020, 12, 184–194.
- Feuchtner, G.M.; Plank, F.; Beyer, C.; Schwabl, C.; Held, J.; Bellmann-Weiler, R.; Weiss, G.; Gruber, J.; Widmann, G.; Klauser, A.S. Monosodium Urate Crystal Deposition in Coronary Artery Plaque by 128-Slice Dual-Energy Computed Tomography: An Ex Vivo Phantom and In Vivo Study. J. Comput. Assist. Tomogr. 2021, 45, 856–862.
- Becce, F.; Ghoshhajra, B.; Choi, H.K. Identification of Cardiovascular Monosodium Urate Crystal Deposition in Patients with Gout Using Dual-Energy Computed Tomography. JAMA Cardiol. 2020, 5, 486.
- Pascart, T.; Carpentier, P.; Choi, H.K.; Norberciak, L.; Ducoulombier, V.; Luraschi, H.; Houvenagel, E.; Legrand, J.; Verclytte, S.; Becce, F.; et al. Identification and characterization of peripheral vascular color-coded DECT lesions in gout and non-gout patients: The VASCURATE study. Semin. Arthritis Rheum. 2021, 51, 895–902.
- Khanna, I.M.V.; Pyzik, R.; Kaufman, A.; Chi, W.; Bagiella, E.; Robson, P.; Ali, Y. Assessing Urate Deposition and Inflammation in the Vasculature of Gout Patients Using Dual Energy Computed Tomography and Positron Emission Tomography Pre and Post Pegloticase—A Pilot Study . Arthritis Rheumatol. 2022, 74, 3592–3594.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
532
Revisions:
2 times
(View History)
Update Date:
07 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




