Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elham Afghani | -- | 2675 | 2023-12-07 02:07:50 | | | |
| 2 | Catherine Yang | Meta information modification | 2675 | 2023-12-07 02:16:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ning, K.; Salamone, A.; Manos, L.; Lafaro, K.J.; Afghani, E. Diagnosis of Serous Cystadenoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/52466 (accessed on 07 February 2026).
Ning K, Salamone A, Manos L, Lafaro KJ, Afghani E. Diagnosis of Serous Cystadenoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/52466. Accessed February 07, 2026.
Ning, Kylie, Ashley Salamone, Lindsey Manos, Kelly J. Lafaro, Elham Afghani. "Diagnosis of Serous Cystadenoma" Encyclopedia, https://encyclopedia.pub/entry/52466 (accessed February 07, 2026).
Ning, K., Salamone, A., Manos, L., Lafaro, K.J., & Afghani, E. (2023, December 07). Diagnosis of Serous Cystadenoma. In Encyclopedia. https://encyclopedia.pub/entry/52466
Ning, Kylie, et al. "Diagnosis of Serous Cystadenoma." Encyclopedia. Web. 07 December, 2023.
Copy Citation
Incidental pancreatic cysts are highly prevalent, with management dependent on the risk of malignant progression. Serous cystadenomas (SCAs) are the most common benign pancreatic cysts seen on imaging. They have typical morphological patterns but may also show atypical features that mimic precancerous and cancerous cysts.
serous cystadenoma
pancreatic cysts
CT
MRI
1. Morphologic Features of SCA
Imaging plays a vital role in differentiating pancreatic cysts. Certain features allow for SCA to stand out from other pancreatic cysts. For example, SCAs do not communicate with the pancreatic duct as opposed to IPMNs. SCAs also have four distinct morphological patterns: microcystic, macrocystic, a combination of microcystic and macrocystic, and solid. Microcystic SCAs demonstrate multiple small cysts less than 2 cm in size separated by thin septations. This particular morphology is seen in approximately 50% of SCAs. These lesions may also have a fibrous central scar and calcification seen in up to 30% of microcystic SCAs, giving them a “sunburst” appearance. CT may show a circumvascular sign, which is a result of its hypervascularity. A recent study evaluated the CT features of 71 patients with pancreatic cysts, of which 30 were SCAs, 21 with MCNs, and 20 with branch duct IPMNs who subsequently underwent resection with confirmed surgical pathology, finding that the presence of a central scar or calcification and/or circumvascular sign is 97–100% specific for SCA but has a sensitivity ranging from 23.3 to 76.7%. The presence of the circumvascular sign alone has the highest sensitivity of the three [1]. Microcystic SCAs may also have a honeycomb appearance, indicating multiple tiny cysts.
Macrocystic SCAs, also known as oligocystic patterns, consist of multiloculated cysts >2 cm separated by thin septations. This pattern accounts for approximately 30% of all SCAs. They lack a central scar but demonstrate external lobulations similar to microcystic SCA [2][3][4]. This pattern is more likely found in the head and may cause symptoms such as jaundice or obstruction of the common bile duct [5]. Furthermore, macrocystic pattern SCAs are most easily confused with other pancreatic cysts and are challenging to differentiate. A multicenter study showed that 31% of macrocystic SCAs were misidentified as IPMN, PanNet, MCN, pseudocysts, or ductal adenocarcinomas [6]. Approximately 20% of SCAs consist of microcystic and macrocystic morphology.
The solid variant of SCA is depicted by a solid hypervascular lesion with or without cystic lesions. This pattern accounts for 5% of all SCAs. On histopathology, the cells are arranged in nests, sheets, and trabeculae, separated by thick fibrous bands. The solid lesion within an SCA may also indicate intratumoral hemorrhage, similar to a pancreatic neuroendocrine tumor; solid pseudopapillary neoplasm; or metastatic renal cell carcinoma [2][3][4][7]. The former is significantly smaller when comparing solid SCA to a neuroendocrine tumor and shows wash-in and wash-out enhancement patterns [8].
Despite having distinct features that may distinguish them from other cysts, there may be atypical features such as the presence of parenchyma atrophy, distal location to the lesion, dilation of the upstream pancreatic duct from mass effect, vascular invasion, and invasion of adjacent structures [4][9]. This wide range of morphologies in imaging poses a diagnostic dilemma as it mimics precancerous and cancerous lesions.
2. Cross-Sectional Imaging
Traditionally, CT scans have been used in diagnosing cysts, but increasingly, MRIs are becoming more popular. CT and MRI scans can identify the typical characteristics distinguishing SCAs from other cysts. However, each modality has its advantages and disadvantages. MRI is superior to CT when identifying septations, the presence of a solid component, pancreatic ductal communication, and magnification of the macrocystic nature on T2-weighed images [10][11]. In contrast, CTs can depict calcifications and hypervascularity but provide insufficient soft tissue contrast and spatial resolution, which does not allow for the identification of the microcystic appearance and SCAs less than 2 cm [4][7][9]. Figure 1 shows features of SCA on CT and MRI. Moreover, CT is quick and available in many places, whereas MRIs may not be available, are prone to motion artifacts, take much longer in the scanner, and are more expensive. Recently, there has also been concern about gadolinium deposits in the brain [12]. In blinded studies, radiologists could accurately diagnose SCAs between 23 and 82% [13][14][15]. Given the limitations, combining the two imaging modalities may provide better characterization. Regardless, SCAs often cannot solely be diagnosed based on cross-sectional imaging, and further evaluation is needed.
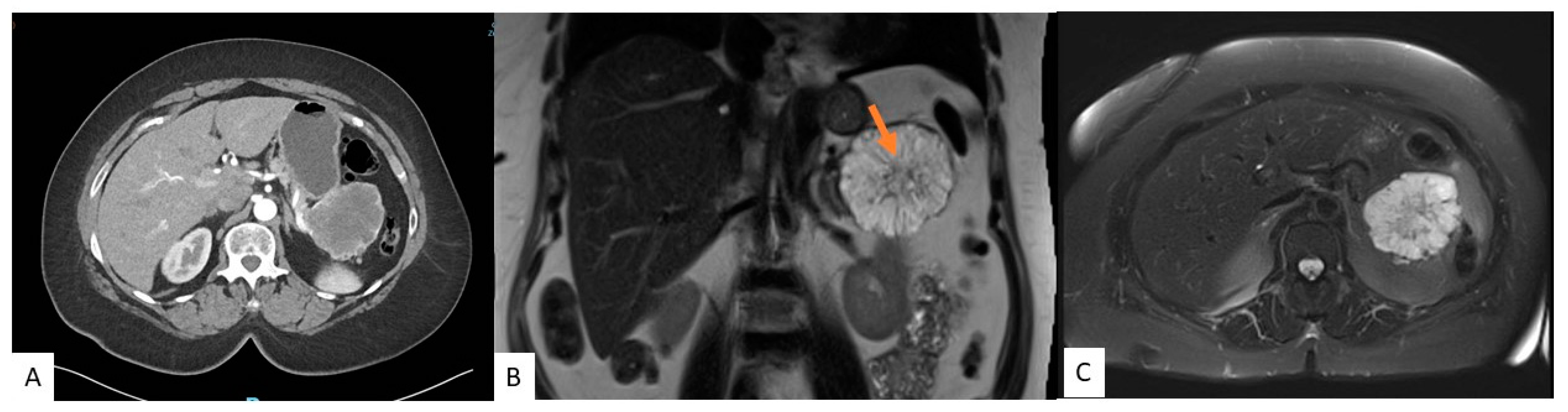
Figure 1. A 54-year-old female with an incidental pancreatic cyst. CT image showing a heterogeneously enhancing lesion with numerous small internal cysts in the tail of the pancreas measuring 6.4 cm × 7.3 cm × 5.4 cm (AP × TV × CC) (A). MRI coronal (B) and axial (C) views of a microcystic lesion in the tail of the pancreas with enhancing septations and central scar (arrow).
Radiomics-based approaches and deep learning have been developing in the era of artificial intelligence. They use various images and extract clinical data using an algorithm to predict the type of cyst. In a 2019 study, artificial intelligence provided sensitivity and specificity of 91.9% and 92.9% respectively, in differentiating benign from precancerous cysts [16], although this was limited by a small sample size. The use of specific radiomic features have shown to be superior to standard radiologic features in diagnosing SCA [17]. A recent study showed radiomic-based approaches have an equivalent performance as an academic radiologist with more than 25 years of experience [18].
18F-FDG PET/CT has shown to be promising in the preoperative diagnosis of pancreatic cysts while it is currently not recommended in guidelines [19][20][21]. It relies on the uptake of glucose metabolism by cancer cells. Sperti and colleagues were the first to assess its reliability in differentiating benign from malignant cysts [19]. In later studies, they assessed the reliability of 18F-FDG PET/CT in determining benign cysts from IPMNs. A recent comparison showed that 18F-FDG PET/CT has a diagnostic accuracy of 94% in diagnosing malignant from benign cysts compared to 77% for multidetector CT and 87% for MRI. This study was a prospective single-center study of 31 pancreatic cysts, of which 22 underwent surgical resection with proven histopathology, 4 of which were confirmed SCAs [22] This was in contrast to an earlier retrospective study of 68 patients who underwent 18F-FDG PET/CT, of which 21 underwent surgical resection. The latter study found the sensitivity and specificity to be 57% and 85%, respectively [23]. However, 18F-FDG PET/CT is prone to false positive results, especially with pancreatitis. Further large cohort studies are needed to show the efficacy of 18F-FDG PET/CT in differentiating SCAs from precancerous or cancerous cysts.
3. Endoscopic Ultrasound
Endoscopic ultrasound (EUS) has become more widely utilized as a diagnostic modality in differentiating pancreatic cysts as benign, precancerous, or cancerous, with a diagnostic accuracy of 50–75% [24]. Its high-resolution imaging can delineate classic SCA features, such as the honeycomb feature of multiple small microcysts and multiple compartments [25] (Figure 2). However, it is affected by variable interobserver agreement and is operator dependent. A large study looking at interobserver agreement between endosonographers found that out of all the cysts, only SCAs were met with some certainty in diagnosis. The agreement between endosonographers for SCAs was considered moderately good while the agreement was only considered to be fair for the other types of cysts. Despite this, only 47% of the SCAs were correctly identified [26]. Another major limitation is that EUS is not particularly useful in distinguishing between precancerous cysts, such as IPMN or MCN, and macrocystic SCAs [27]. This is an issue, as misdiagnosis could lead to a drastically different treatment plan. EUS and fine needle aspiration (FNA) is a more valuable tool when compared to EUS alone in diagnosing a pancreatic cyst, having a higher sensitivity and specificity (97% and 100%, respectively) in determining if a cyst should be resected [28].
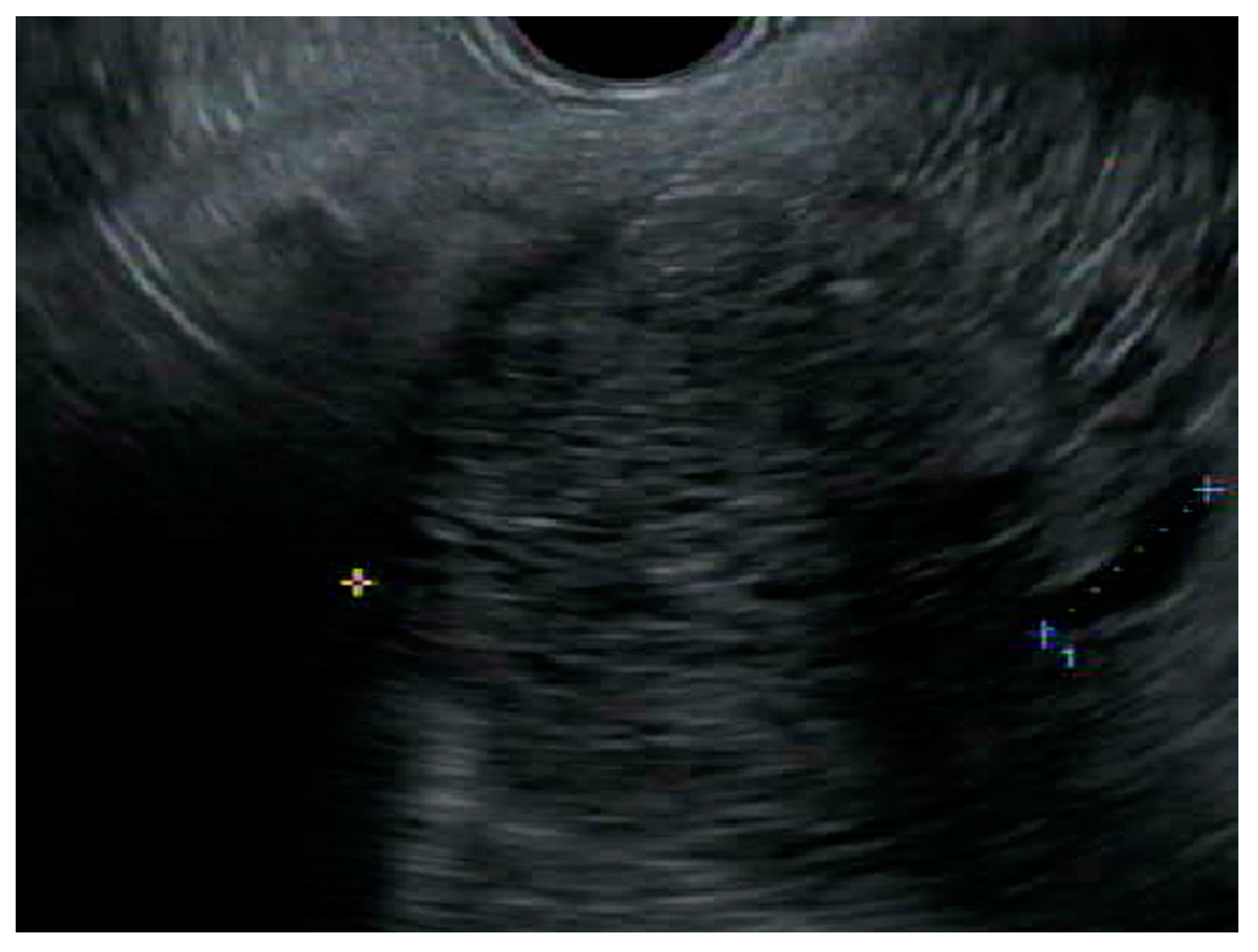
Figure 2. Endoscopic ultrasound image of a microcystic anechoic structure in the body of the pancreas measuring 7 cm × 7.1 cm that was well defined, multi chambered, with thin walls and septations.
4. Cyst Fluid Analysis
Cyst fluid drawn during EUS-FNA is useful in narrowing down a diagnosis for pancreatic cysts based on multiple components, such as cytological features or the presence of mucin, CEA, glucose, amylase, and molecular markers. Cyst fluid analysis has proven useful over the past years. It is increasingly used in various ways as technology improves and costs and turnaround times for analysis decrease, yet there is one major limitation. Most components of cyst fluid analysis have varied cutoffs and no set guidelines for what values may correlate to what specific type of cyst; however, studies have shown that value ranges (higher vs. lower) may be useful in distinguishing broadly between benign and precancerous cysts. Therefore, many components of cyst fluid analysis discussed may not be able to differentiate for SCA specifically but can still be useful in determining management. Another larger limitation is that microcystic SCAs are not amendable to cyst fluid analysis given the multiple tiny cysts.
On gross appearance, cyst fluid is usually clear-yellow and has low viscosity due to the absence of mucin when compared to mucinous cysts such as IPMNs and MNCs [29]. The presence of neoplastic cuboidal epithelium with abundant cytoplasm on cytology is specific for SCA but is only seen in 10% of cases [30]. Typically, they are unicellular to acellular with a clear or hemorrhagic background due to the highly vascular epithelium and highly vascularized fibrous septa. Cell nuclei are usually observed to be centrally located with smooth nuclear contours, a round to oval shape, have indistinct nucleoli, and have evenly distributed chromatin. Other typical characteristics include a lack of atypia, mitotic activity, and necrosis [30][31]. Some literature suggests that the presence of hemosiderin-laden macrophages may be a good surrogate marker for SCA as well. However, it is rare for cytology results to show histiocytes with hemosiderin [27][30]. Unfortunately, there are many challenges posed with obtaining a clear diagnosis from cytology, one being that many procedures such as transduodenal and transgastric approaches during EUS FNA sometimes cause GI contamination and background mucin, leading to misdiagnosis of the SCA as a mucinous cyst such as IPMN or MCN. Additionally, SCAs are occasionally mistaken for mucinous cysts with atypia due to degenerative gastric and intestinal epithelium exhibiting some cellular atypia [30]. These challenges in addition to SCA’s low cellularity and lack of defining characteristics make relying on cytology alone difficult. Thus, results should be considered in combination with CEA, immunostaining, or other measures.
Cyst fluid carcinoembryonic antigen (CEA) analysis is widely available. It can discriminate between benign and precancerous cysts. Still, due to the lack of correlation between CEA levels and dysplasia or cyst growth, CEA values cannot indicate much besides whether a cyst is mucin producing [32]. CEA < 0.5 may be indicative of a benign cyst such as SCA or pseudocyst, whereas >192 ng/mL may indicate a precancerous cyst such as IPMN or MCN with a specificity of 84% and sensitivity of 73% [33]. However, pancreatic neuroendocrine tumors may also have a low CEA.
More recently, cyst fluid analysis of glucose is becoming favorable as it is rapid, widely available, and inexpensive. Since glucose is a common metabolite found in pancreatic cyst fluid, glucose tests can also provide information about cyst types. Precancerous cysts have lower glucose levels due to the hypothesis that glycolysis is the primary cellular ATP source in cancer cells in conjunction with the known finding that pancreatic tumor cells require high glucose levels for metabolism [34]. However, studies have used variable cutoff values with varying results. A recent systematic review and meta-analysis showed intracystic glucose ≤50 mg/dL has a pooled sensitivity and specificity of 90.5% and 88%, respectively [35]. Furthermore, there is variability in glucose testing, whether by colorimetry in a lab or bedside glucometer. Further studies are needed to validate the correlation between techniques.
In a meta-analysis comparing glucose to CEA, glucose testing had significantly higher sensitivity and diagnostic accuracy than CEA alone (91% vs. 56% and 94% vs. 85%, respectively) in differentiating between precancerous cysts, such as IPMNs and MCN, and non-mucin-producing cysts, such as SCA and pseudocyst [36]. Other studies have also shown similar findings [34]. Park and colleagues found SCAs had higher median glucose concentrations when compared to pseudocysts (98 mg/dL and 23 mg/dL, respectively) [37]. While this proves promising at the moment, very few studies have found glucose levels for the detection of specific types of cysts, as Park and colleagues have shown. Given the widespread availability, cheaper cost, and quick turnaround time of glucose testing, it has the potential to be useful in excluding the diagnosis of IPMNs or MCNs. However, while glucose may be useful for differentiating between benign and precancerous cysts, values need to be further examined to see if glucose testing can diagnose a cyst as an SCA vs. other benign cysts specifically.
Cyst fluid amylase, a digestive enzyme found in the pancreas, is a valuable marker suggestive of ductal communication [38]. As expected, amylase is typically very low because SCAs do not communicate with the pancreatic duct [38]. While amylase can be useful in distinguishing between benign vs. precancerous cysts and even between MCNs and IPMNs, currently, there are no specific values that may determine SCAs specifically [38]. Amylase levels of <250 U/L may be indicative of an SCA, IPMN, MCN, or pancreatic neuroendocrine tumor with a sensitivity of 44% and specificity of 98% and are thus only helpful in excluding pseudocysts [30]. Many studies exclude pseudocysts when looking at amylase since it is expected that the amylase levels are high due to the nature of pseudocysts accumulating pancreatic digestive juices [39], but it is assumed that amylase levels >250 U/L are likely to be pseudocysts. Interestingly, one study found that by analyzing cyst fluid from their cohort with a CEA <30, they discovered that amylase <350 could detect 85% of SCA [30].
The use of molecular markers is becoming increasingly popular. While the other components of cyst fluid analysis mentioned do not have concrete values and ways to distinguish between specific types of cysts, molecular markers offer the advantage of indicating specific types of cysts, commonly ones such as IPMN, MCN, or SCA. The presence of a VHL mutation and the absence of a KRAS, GNAS, or RNF43 mutation has a 71–100% sensitivity and 91–100% specificity of correctly identifying SCA preoperatively [40][41]. In a recent study, a VHL mutation had 100% PPV and 98% NPV of SCA. Furthermore, the presence of VHL with TP53 or TERT promoter mutations correlated with SCAs with interval growth in size [41]. While there is great promise in studies showing that SCAs do not have mutations in any other genes but VHL when conducting a molecular markers panel and that VHL can be highly specific and sensitive for diagnosing SCA, there is a caveat [41][42]. Pancreatic neuroendocrine tumors (PanNets) arise in 8–17% of patients with VHL disease and also may account for about 25% of PanNets that are analyzed for molecular markers [42][43]. Patients with SCAs and VHL mutations may actually have a mixed serous-neuroendocrine tumor [42]. Therefore, to avoid confusion of a diagnosis between PanNet and SCA in those who are found to have a VHL mutation, other diagnostic tools should be used in combination.
Unfortunately, not all SCAs arise from VHL mutations and therefore they will not have any known molecular markers. However, the diagnosis of SCA can be made based on the absence of other markers, for example, the absence of the CTNNB1 mutation, which is specific for solid pseudopapillary, lack of KRAS or GNAS mutations, being specific for a mucinous cyst, as well as the use of other advanced endoscopic techniques to characterize the vascular network of the cyst walls.
5. Confocal Laser Endomicroscopy
Confocal laser endomicroscopy (nCLE) is a technique using real-time visualization of a pancreatic cyst. A miniprobe is inserted through a 19-gauge FNA needle and captures microscopic images of the cyst epithelial lining. It can detect superficial vascular networks or fern patterns, which is specific for SCA. Napoleon and colleagues were the first to describe nCLE in diagnosing SCAs. They highlighted the tightly connected tortuous blood vessels. They may also appear as white vessels on a dark or grey background, or as dark vessels on a clear background, depending on the density of blood cells within the vessels and fluorescein contrast [44] (Figure 3). nCLE has a sensitivity, specificity, and diagnostic accuracy of 99% based on a recent systematic review and meta-analysis, which pooled seven studies. However, nCLE is only available in some places and is operator dependent. It has been reported to have a pancreatitis rate of 1% [45]. Nevertheless, it is possible to distinguish SCA from precancerous cysts with the use of nCLE when all other modalities fail.
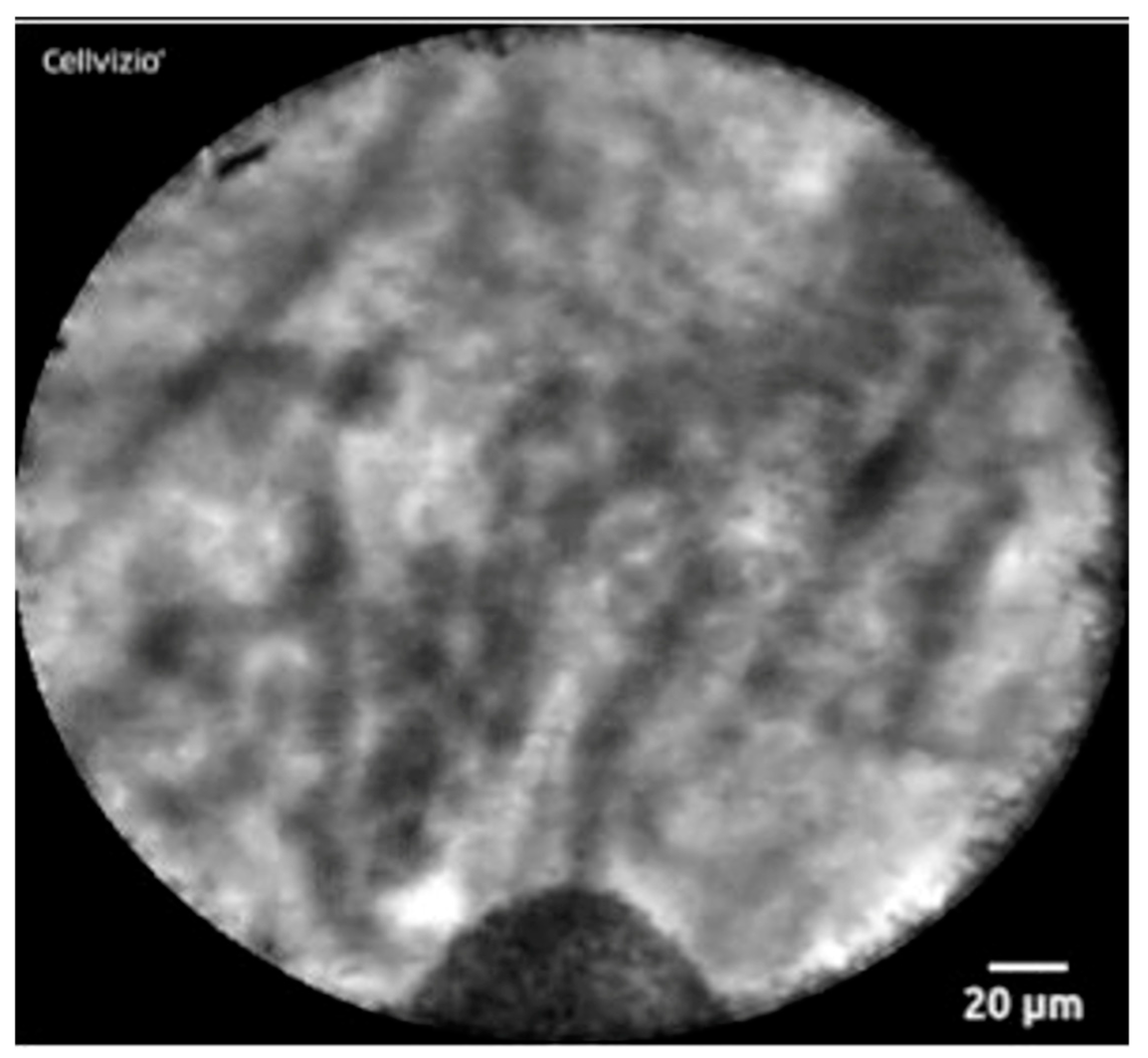
Figure 3. nCLE image of superficial vascular network or “fern pattern”.
References
- Wang, G.; Wang, Z.; Chen, H.; Zhang, D.; Wen, L. Discrimination of serous cystadenoma from mucinous cystic neoplasm and branch duct intraductal papillary mucinous neoplasm in the pancreas with CT. Abdom. Radiol. 2020, 45, 2772–2778.
- Jais, B.; Rebours, V.; Malleo, G.; Salvia, R.; Fontana, M.; Maggino, L.; Bassi, C.; Manfredi, R.; Moran, R.; Lennon, A.M.; et al. Serous cystic neoplasm of the pancreas: A multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2016, 65, 305–312.
- Kim, H.J.; Lee, D.H.; Ko, Y.T.; Lim, J.W.; Kim, H.C.; Kim, K.W. CT of serous cystadenoma of the pancreas and mimicking masses. AJR Am. J. Roentgenol. 2008, 190, 406–412.
- Chu, L.C.; Singhi, A.D.; Haroun, R.R.; Hruban, R.H.; Fishman, E.K. The many faces of pancreatic serous cystadenoma: Radiologic and pathologic correlation. Diagn. Interv. Imaging 2017, 98, 191–202.
- Egawa, N.; Maillet, B.; Schroder, S.; Mukai, K.; Kloppel, G. Serous oligocystic and ill-demarcated adenoma of the pancreas: A variant of serous cystic adenoma. Virchows Arch. 1994, 424, 13–17.
- Feller, E.; Schiffman, F.J. Extrahepatic biliary obstruction by lymphoma. Arch. Surg. 1990, 125, 1507–1509.
- Sun, H.Y.; Kim, S.H.; Kim, M.A.; Lee, J.Y.; Han, J.K.; Choi, B.I. CT imaging spectrum of pancreatic serous tumors: Based on new pathologic classification. Eur. J. Radiol. 2010, 75, 45.
- Fang, X.; Jiang, H.; Cao, K.; Li, J.; Liu, F.; Wang, L.; Lu, J.; Shao, C.; Bian, Y. Distinguishing pancreatic solid serous cystadenomas from nonfunctional pancreatic neuroendocrine tumors by computed tomography: A propensity score analysis. Medicine 2022, 101, e30523.
- Shah, A.A.; Sainani, N.I.; Kambadakone, A.R.; Shah, Z.K.; Deshpande, V.; Hahn, P.F.; Sahani, D.V. Predictive value of multi-detector computed tomography for accurate diagnosis of serous cystadenoma: Radiologic-pathologic correlation. World J. Gastroenterol. 2009, 15, 2739–2747.
- Jones, M.J.; Buchanan, A.S.; Neal, C.P.; Dennison, A.R.; Metcalfe, M.S.; Garcea, G. Imaging of indeterminate pancreatic cystic lesions: A systematic review. Pancreatology 2013, 13, 436–442.
- Bassi, C.; Salvia, R.; Molinari, E.; Biasutti, C.; Falconi, M.; Pederzoli, P. Management of 100 consecutive cases of pancreatic serous cystadenoma: Wait for symptoms and see at imaging or vice versa? World J. Surg. 2003, 27, 319–323.
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. International Society for Magnetic Resonance in Medicine Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570.
- Curry, C.A.; Eng, J.; Horton, K.M.; Urban, B.; Siegelman, S.; Kuszyk, B.S.; Fishman, E.K. CT of primary cystic pancreatic neoplasms: Can CT be used for patient triage and treatment? AJR Am. J. Roentgenol. 2000, 175, 99–103.
- Procacci, C.; Biasiutti, C.; Carbognin, G.; Accordini, S.; Bicego, E.; Guarise, A.; Spoto, E.; Andreis, I.A.; De Marco, R.; Megibow, A.J. Characterization of cystic tumors of the pancreas: CT accuracy. J. Comput. Assist. Tomogr. 1999, 23, 906–912.
- Kehagias, D.; Smyrniotis, V.; Kalovidouris, A.; Gouliamos, A.; Kostopanagiotou, E.; Vassiliou, J.; Vlahos, L. Cystic tumors of the pancreas: Preoperative imaging, diagnosis, and treatment. Int. Surg. 2002, 87, 171–174.
- Kurita, Y.; Kuwahara, T.; Hara, K.; Mizuno, N.; Okuno, N.; Matsumoto, S.; Obata, M.; Koda, H.; Tajika, M.; Shimizu, Y.; et al. Diagnostic ability of artificial intelligence using deep learning analysis of cyst fluid in differentiating malignant from benign pancreatic cystic lesions. Sci. Rep. 2019, 9, 6893.
- Yang, J.; Guo, X.; Ou, X.; Zhang, W.; Ma, X. Discrimination of Pancreatic Serous Cystadenomas From Mucinous Cystadenomas With CT Textural Features: Based on Machine Learning. Front. Oncol. 2019, 9, 494.
- Chu, L.C.; Park, S.; Soleimani, S.; Fouladi, D.F.; Shayesteh, S.; He, J.; Javed, A.A.; Wolfgang, C.L.; Vogelstein, B.; Kinzler, K.W.; et al. Classification of pancreatic cystic neoplasms using radiomic feature analysis is equivalent to an experienced academic radiologist: A step toward computer-augmented diagnostics for radiologists. Abdom Radiol. 2022, 47, 4139–4150.
- Sperti, C.; Pasquali, C.; Chierichetti, F.; Liessi, G.; Ferlin, G.; Pedrazzoli, S. Value of 18-fluorodeoxyglucose positron emission tomography in the management of patients with cystic tumors of the pancreas. Ann. Surg. 2001, 234, 675–680.
- Sperti, C.; Pasquali, C.; Decet, G.; Chierichetti, F.; Liessi, G.; Pedrazzoli, S. F-18-fluorodeoxyglucose positron emission tomography in differentiating malignant from benign pancreatic cysts: A prospective study. J. Gastrointest. Surg. 2005, 9, 22–29.
- Sperti, C.; Bissoli, S.; Pasquali, C.; Frison, L.; Liessi, G.; Chierichetti, F.; Pedrazzoli, S. 18-Fluorodeoxyglucose Positron Emission Tomography Enhances Computed Tomography Diagnosis of Malignant Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2007, 246, 932–939.
- Kauhanen, S.; Rinta-Kiikka, I.; Kemppainen, J.; Gronroos, J.; Kajander, S.; Seppanen, M.; Alanen, K.; Gullichsen, R.; Nuutila, P.; Ovaska, J. Accuracy of 18F-FDG PET/CT, Multidetector CT, and MR Imaging in the Diagnosis of Pancreatic Cysts: A Prospective Single-Center Study. J. Nucl. Med. 2015, 56, 1163–1168.
- Mansour, J.C.; Schwartz, L.; Pandit-Taskar, N.; D’Angelica, M.; Fong, Y.; Larson, S.M.; Brennan, M.F.; Allen, P.J. The utility of F-18 fluorodeoxyglucose whole body PET imaging for determining malignancy in cystic lesions of the pancreas. J. Gastrointest. Surg. 2006, 10, 1354–1360.
- Sedlack, R.; Affi, A.; Vazquez-Sequeiros, E.; Norton, I.D.; Clain, J.E.; Wiersema, M.J. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest. Endosc. 2002, 56, 543–547.
- Kaneto, H.; Endo, T.; Ozeki, I.; Itoh, H.; Sasaki, S.; Mukaiya, M.; Ikeda, K.; Koito, K.; Imai, K. Macrocystic serous cystadenoma of the pancreas: Importance of co-existent tiny cysts depicted by EUS. J. Gastroenterol. 2000, 35, 472–475.
- Ahmad, N.A.; Kochman, M.L.; Brensinger, C.; Brugge, W.R.; Faigel, D.O.; Gress, F.G.; Kimmey, M.B.; Nickl, N.J.; Savides, T.J.; Wallace, M.B.; et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest. Endosc. 2003, 58, 59–64.
- Belsley, N.A.; Pitman, M.B.; Lauwers, G.Y.; Brugge, W.R.; Deshpande, V. Serous cystadenoma of the pancreas: Limitations and pitfalls of endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer 2008, 114, 102–110.
- Frossard, J.L.; Amouyal, P.; Amouyal, G.; Palazzo, L.; Amaris, J.; Soldan, M.; Giostra, E.; Spahr, L.; Hadengue, A.; Fabre, M. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am. J. Gastroenterol. 2003, 98, 1516–1524.
- Abdelkader, A.; Hunt, B.; Hartley, C.P.; Panarelli, N.C.; Giorgadze, T. Cystic Lesions of the Pancreas: Differential Diagnosis and Cytologic-Histologic Correlation. Arch. Pathol. Lab. Med. 2020, 144, 47–61.
- Lilo, M.T.; VandenBussche, C.J.; Allison, D.B.; Lennon, A.M.; Younes, B.K.; Hruban, R.H.; Wolfgang, C.L.; Ali, S.Z. Serous Cystadenoma of the Pancreas: Potentials and Pitfalls of a Preoperative Cytopathologic Diagnosis. Acta Cytol. 2017, 61, 27–33.
- Huang, P.; Staerkel, G.; Sneige, N.; Gong, Y. Fine-needle aspiration of pancreatic serous cystadenoma: Cytologic features and diagnostic pitfalls. Cancer 2006, 108, 239–249.
- Nagula, S.; Kennedy, T.; Schattner, M.A.; Brennan, M.F.; Gerdes, H.; Markowitz, A.J.; Tang, L.; Allen, P.J. Evaluation of cyst fluid CEA analysis in the diagnosis of mucinous cysts of the pancreas. J. Gastrointest. Surg. 2010, 14, 1997–2003.
- Brugge, W.R.; Lewandrowski, K.; Lee-Lewandrowski, E.; Centeno, B.A.; Szydlo, T.; Regan, S.; del Castillo, C.F.; Warshaw, A.L. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology 2004, 126, 1330–1336.
- Simons-Linares, C.R.; Yadav, D.; Lopez, R.; Bhatt, A.; Jang, S.; El-Khider, F.; Sanaka, M.; Stevens, T.; Vargo, J.; Chahal, P. The utility of intracystic glucose levels in differentiating mucinous from non-mucinous pancreatic cysts. Pancreatology 2020, 20, 1386–1392.
- Guzman-Calderon, E.; Md, B.M.; Casellas, J.A.; Aparicio, J.R. Intracystic Glucose Levels Appear Useful for Diagnosis of Pancreatic Cystic Lesions: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2022, 67, 2562–2570.
- McCarty, T.R.; Garg, R.; Rustagi, T. Pancreatic cyst fluid glucose in differentiating mucinous from nonmucinous pancreatic cysts: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 94, 698–712.e6.
- Park, W.G.; Wu, M.; Bowen, R.; Zheng, M.; Fitch, W.L.; Pai, R.K.; Wodziak, D.; Visser, B.C.; Poultsides, G.A.; Norton, J.A.; et al. Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: Glucose and kynurenine. Gastrointest. Endosc. 2013, 78, 295–302.e2.
- Hawes, R.H.; Clancy, J.; Hasan, M.K. Endoscopic ultrasound-guided fine needle aspiration in cystic pancreatic lesions. Clin. Endosc. 2012, 45, 128–131.
- Snozek, C.L.H.; Mascarenhas, R.C.; O’Kane, D.J. Use of cyst fluid CEA, CA19-9, and amylase for evaluation of pancreatic lesions. Clin. Biochem. 2009, 42, 1585–1588.
- Springer, S.; Wang, Y.; Dal Molin, M.; Masica, D.L.; Jiao, Y.; Kinde, I.; Blackford, A.; Raman, S.P.; Wolfgang, C.L.; Tomita, T.; et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015, 149, 1501–1510.
- Paniccia, A.; Polanco, P.M.; Boone, B.A.; Wald, A.I.; McGrath, K.; Brand, R.E.; Khalid, A.; Kubiliun, N.; O’Broin-Lennon, A.M.; Park, W.G.; et al. Prospective, Multi-Institutional, Real-Time Next-Generation Sequencing of Pancreatic Cyst Fluid Reveals Diverse Genomic Alterations That Improve the Clinical Management of Pancreatic Cysts. Gastroenterology 2023, 164, 117–133.e7.
- Reid, M.D.; Choi, H.; Balci, S.; Akkas, G.; Adsay, V. Serous cystic neoplasms of the pancreas: Clinicopathologic and molecular characteristics. Semin. Diagn. Pathol. 2014, 31, 475–483.
- Lonser, R.R.; Glenn, G.M.; Walther, M.; Chew, E.Y.; Libutti, S.K.; Linehan, W.M.; Oldfield, E.H. von Hippel-Lindau disease. Lancet 2003, 361, 2059–2067.
- Napoleon, B.; Lemaistre, A.; Pujol, B.; Caillol, F.; Lucidarme, D.; Bourdariat, R.; Morellon-Mialhe, B.; Fumex, F.; Lefort, C.; Lepilliez, V.; et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: Needle-based confocal laser endomicroscopy. Endoscopy 2015, 47, 26–32.
- Konjeti, V.R.; McCarty, T.R.; Rustagi, T. Needle-based Confocal Laser Endomicroscopy (nCLE) for Evaluation of Pancreatic Cystic Lesions: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2022, 56, 72–80.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
07 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




