
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Angga Hermawan | -- | 4420 | 2023-12-06 16:43:48 | | | |
| 2 | Lindsay Dong | + 1 word(s) | 4421 | 2023-12-08 01:51:02 | | |
Video Upload Options
MXenes are a class of 2D transition-metal carbides, nitrides, and carbonitrides with exceptional properties, including substantial electrical and thermal conductivities, outstanding mechanical strength, and a considerable surface area, rendering them an appealing choice for gas sensors.
1. Introduction
2. Synthesis and Properties of 2D MXenes
Generally, MXenes emerge through the selective elimination of A layers from MAX phases, giving rise to two-dimensional materials that are usually composed of three or more atomic layers. These 2D materials possess distinct properties when compared to their three-dimensional (3D) precursor counterparts [27]. In their early synthesis, the primary etching agents predominantly comprised fluorine-containing compounds, such as HF, LiF+HCl, bifluoride salts, and molten salts containing fluorine. These etchants dictated the surface terminations of MXenes, yielding three primary variations: -F, -OH, and -O. However, in 2017, alternative non-fluorine etching methods, including electrochemical etching and concentrated alkaline hydrothermal etching, were introduced, resulting in the production of fluorine-free MXenes. More recently, a non-aqueous molten salt etching approach, employing Lewis acidic
In contrast to the stacked configuration of MXenes, single-layer MXene nanosheets exhibit superior chemical properties, such as a notable increase in specific surface area, favourable hydrophilicity, and a wealth of surface chemistry. In fact, the initial report on MXenes employed ultrasonic treatment to disassemble accordion-like MXenes into layers, albeit with limited success due to the robust bonding between these layers, resulting in low yields and impractical outcomes [28]. The process of obtaining single-layer nanosheets from accordion-like MXenes can be accomplished through appropriate delamination techniques, with the ease of this process directly influenced by the composition of surface functional groups. Furthermore, increasing the interlayer spacing of MXene flakes through ion intercalation is a common strategy for delaminating multilayered MXenes [28].
Following this, depending on the synthetic route, the produced MXenes can have significantly different properties because these properties rely on the functional groups, defects, interlayer structures, etc. MXenes are versatile materials that fulfil the essential requirements for fully functional gas-sensing devices [29].
MXenes offer high transparency and light absorbance, with Ti3C2Tx MXenes in the range of 1–2 nm thickness achieving up to 91.2% optical transparency and excellent light-to-heat conversion efficiency. Different terminations impact their optical properties, with -F and -OH groups reducing visible light absorption and reflectivity but enhancing reflectivity in the UV region. They demonstrate excellent thermal stability, with Ti3C2Tx (T = F, OH) remaining stable up to 500 °C, or 800 °C in argon atmosphere. Surface functionalisation with functional groups helps mitigate surface oxidation.
3. Fundamental Sensing Mechanisms in 2D MXenes and MXene Heterostructures
3.1. Sensing Mechanism in Pristine 2D MXenes
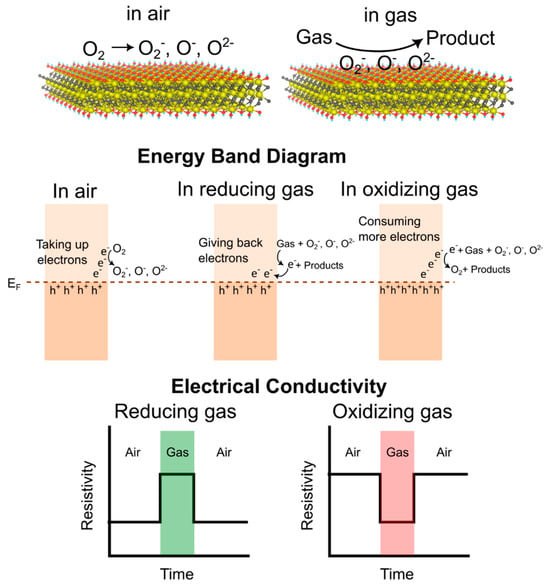
3.2. Sensing Mechanism in 2D MXene Heterostructures
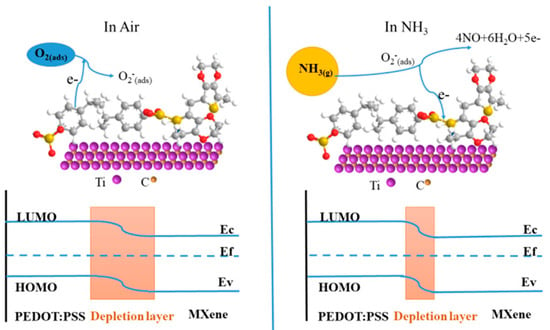
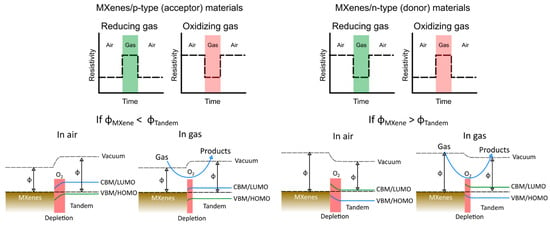
4. Engineering Approach to Enhance 2D MXene Sensing Performances
4.1. Surface Functionalisation
4.2. Layering Structures
5. Two-Dimensional MXene-Based Heterostructures as High-Performance Gas-Sensing Materials
5.1. MXene/Metal-Oxide Heterostructures
5.2. MXene/Polymer Heterostructures
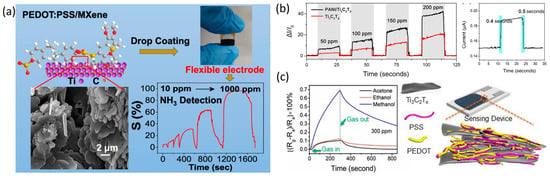
5.3. MXene/Carbonaceous Heterostructures
5.4. MXene/Noble-Metal Heterostructures
5.5. MXene/Metal Chalcogenide Heterostructures
6. Conclusions
In conclusion, MXene-based heterostructures hold great promise for gas-sensing applications due to their unique properties. However, there are still several technical challenges and limitations that must be addressed, including improving the stability of MXenes in different gas environments and developing highly sensitive and selective gas sensors using these materials. In addition to technical challenges, the economic feasibility and environmental impact of MXene-based gas sensors must also be considered. The large-scale synthesis of MXene-based heterostructures requires cost-effective and environmentally friendly synthesis routes. Additionally, the disposal of MXenes after use must be handled in an environmentally sustainable manner. Therefore, researchers must work towards developing scalable and sustainable synthesis routes and explore environmentally friendly methods for the disposal of MXenes. Despite these challenges, the potential applications of MXene-based heterostructures in gas sensing are vast, ranging from environmental monitoring to medical diagnostics. The continued exploration and optimisation of these materials for gas-sensing applications can lead to the development of highly sensitive, selective, and reliable gas sensors, ultimately contributing to the advancement of various industries and the betterment of society as a whole.
References
- Gupta, A.; Sakthivel, T.; Seal, S. Recent Development in 2D Materials beyond Graphene. Prog. Mater. Sci. 2015, 73, 44–126.
- Amrillah, T.; Hermawan, A.; Alviani, V.N.; Seh, Z.W.; Yin, S. MXenes and Their Derivatives as Nitrogen Reduction Reaction Catalysts: Recent Progress and Perspectives. Mater. Today Energy 2021, 22, 100864.
- Amrillah, T.; Supandi, A.R.; Puspasari, V.; Hermawan, A.; Seh, Z.W. MXene-Based Photocatalysts and Electrocatalysts for CO2 Conversion to Chemicals. Trans. Tianjin Univ. 2022, 28, 307–322.
- Amrillah, T.; Hermawan, A.; Cristian, Y.B.; Oktafiani, A.; Dewi, D.M.M.; Amalina, I.; Darminto; Juang, J.-Y. Potential of MXenes as a Novel Material for Spintronic Devices: A Review. Phys. Chem. Chem. Phys. 2023, 25, 18584–18608.
- Amrillah, T.; Abdullah, C.; Hermawan, A.; Sari, F.; Alviani, V. Towards Greener and More Sustainable Synthesis of MXenes: A Review. Nanomaterials 2022, 12, 4280.
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253.
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644.
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The World of Two-Dimensional Carbides and Nitrides (MXenes). Science 2021, 372, eabf1581.
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494.
- Wang, Z.; Wang, F.; Hermawan, A.; Zhu, J.; Yin, S. Surface Engineering of Ti3C2Tx MXene by Oxygen Plasma Irradiation as Room Temperature Ethanol Sensor. Funct. Mater. Lett. 2022, 15, 2251007.
- Bhardwaj, R.; Hazra, A. MXene-Based Gas Sensors. J. Mater. Chem. C 2021, 9, 15735–15754.
- Li, Q.; Li, Y.; Zeng, W. Preparation and Application of 2D MXene-Based Gas Sensors: A Review. Chemosensors 2021, 9, 225.
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831.
- Yamazoe, N. Toward Innovations of Gas Sensor Technology. Sens. Actuators B Chem. 2005, 108, 2–14.
- Yuan, W.; Shi, G. Graphene-Based Gas Sensors. J. Mater. Chem. A 2013, 1, 10078.
- Yamazoe, N.; Shimanoe, K. Theory of Power Laws for Semiconductor Gas Sensors. Sens. Actuators B Chem. 2008, 128, 566–573.
- Das, S.; Jayaraman, V. SnO2: A Comprehensive Review on Structures and Gas Sensors. Prog. Mater. Sci. 2014, 66, 112–255.
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106.
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217.
- Korotcenkov, G. Metal Oxides for Solid-State Gas Sensors: What Determines Our Choice? Mater. Sci. Eng. B 2007, 139, 1–23.
- Chachuli, S.A.M.; Hamidon, M.N.; Ertugrul, M.; Mamat, M.S.; Coban, O.; Tuzluca, F.N.; Yesilbag, Y.O.; Shamsudin, N.H. Effects of MWCNTs/Graphene Nanoflakes/MXene Addition to TiO2 Thick Film on Hydrogen Gas Sensing. J. Alloys Compd. 2021, 882, 160671.
- Zhang, Y.; Jiang, Y.; Duan, Z.; Huang, Q.; Wu, Y.; Liu, B.; Zhao, Q.; Wang, S.; Yuan, Z.; Tai, H. Highly Sensitive and Selective NO2 Sensor of Alkalized V2CTx MXene Driven by Interlayer Swelling. Sens. Actuators B Chem. 2021, 344, 130150.
- Zhang, H.-F.; Xuan, J.-Y.; Zhang, Q.; Sun, M.-L.; Jia, F.-C.; Wang, X.-M.; Yin, G.-C.; Lu, S.-Y. Strategies and Challenges for Enhancing Performance of MXene-Based Gas Sensors: A Review. Rare Met. 2022, 41, 3976–3999.
- Xia, Q.; Fan, Y.; Li, S.; Zhou, A.; Shinde, N.; Mane, R.S. MXene-Based Chemical Gas Sensors: Recent Developments and Challenges. Diam. Relat. Mater. 2023, 131, 109557.
- Nahirniak, S.; Saruhan, B. MXene Heterostructures as Perspective Materials for Gas Sensing Applications. Sensors 2022, 22, 972.
- Mehdi Aghaei, S.; Aasi, A.; Panchapakesan, B. Experimental and Theoretical Advances in MXene-Based Gas Sensors. ACS Omega 2021, 6, 2450–2461.
- Salim, O.; Mahmoud, K.A.; Pant, K.K.; Joshi, R.K. Introduction to MXenes: Synthesis and Characteristics. Mater. Today Chem. 2019, 14, 100191.
- Wei, Y.; Zhang, P.; Soomro, R.A.; Zhu, Q.; Xu, B. Advances in the Synthesis of 2D MXenes. Adv. Mater. 2021, 33, 2103148.
- Hermawan, A.; Amrillah, T.; Riapanitra, A.; Ong, W.; Yin, S. Prospects and Challenges of MXenes as Emerging Sensing Materials for Flexible and Wearable Breath-Based Biomarker Diagnosis. Adv. Healthc. Mater. 2021, 10, 202100970.
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993.
- Hermawan, A.; Zhang, B.; Taufik, A.; Asakura, Y.; Hasegawa, T.; Zhu, J.; Shi, P.; Yin, S. CuO Nanoparticles/Ti3C2Tx MXene Hybrid Nanocomposites for Detection of Toluene Gas. ACS Appl. Nano Mater. 2020, 3, 4755–4766.
- Lipatov, A.; Alhabeb, M.; Lukatskaya, M.R.; Boson, A.; Gogotsi, Y.; Sinitskii, A. Effect of Synthesis on Quality, Electronic Properties and Environmental Stability of Individual Monolayer Ti3C2 MXene Flakes. Adv. Electron. Mater. 2016, 2, 1600255.
- Liu, Y.-T.; Zhang, P.; Sun, N.; Anasori, B.; Zhu, Q.-Z.; Liu, H.; Gogotsi, Y.; Xu, B. Self-Assembly of Transition Metal Oxide Nanostructures on MXene Nanosheets for Fast and Stable Lithium Storage. Adv. Mater. 2018, 30, 1707334.
- Chen, W.Y.; Lai, S.-N.; Yen, C.-C.; Jiang, X.; Peroulis, D.; Stanciu, L.A. Surface Functionalization of Ti3C2Tx MXene with Highly Reliable Superhydrophobic Protection for Volatile Organic Compounds Sensing. ACS Nano 2020, 14, 11490–11501.
- Koh, H.-J.; Kim, S.J.; Maleski, K.; Cho, S.-Y.; Kim, Y.-J.; Ahn, C.W.; Gogotsi, Y.; Jung, H.-T. Enhanced Selectivity of MXene Gas Sensors through Metal Ion Intercalation: In Situ X-Ray Diffraction Study. ACS Sens. 2019, 4, 1365–1372.
- Shuvo, S.N.; Ulloa Gomez, A.M.; Mishra, A.; Chen, W.Y.; Dongare, A.M.; Stanciu, L.A. Sulfur-Doped Titanium Carbide MXenes for Room-Temperature Gas Sensing. ACS Sens. 2020, 5, 2915–2924.
- Li, N.; Jiang, Y.; Xiao, Y.; Meng, B.; Xing, C.; Zhang, H.; Peng, Z. A Fully Inkjet-Printed Transparent Humidity Sensor Based on a Ti3C2/Ag Hybrid for Touchless Sensing of Finger Motion. Nanoscale 2019, 11, 21522–21531.
- Pei, Y.; Zhang, X.; Hui, Z.; Zhou, J.; Huang, X.; Sun, G.; Huang, W. Ti3C2Tx-MXene for Sensing Applications: Recent Progress, Design Principles, and Future Perspectives. ACS Nano 2021, 15, 3996–4017.
- Xin, M.; Li, J.; Ma, Z.; Pan, L.; Shi, Y. MXenes and Their Applications in Wearable Sensors. Front. Chem. 2020, 8, 297.
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-Based Composites: Synthesis, Properties and Environment-Related Applications. Nanoscale Horiz. 2020, 5, 235–258.
- Yazdanparast, S.; Soltanmohammad, S.; Fash-White, A.; Tucker, G.J.; Brennecka, G.L. Synthesis and Surface Chemistry of 2D TiVC Solid-Solution MXenes. ACS Appl. Mater. Interfaces 2020, 12, 20129–20137.
- Naguib, M.; Unocic, R.R.; Armstrong, B.L.; Nanda, J. Large-Scale Delamination of Multi-Layers Transition Metal Carbides and Carbonitrides “MXenes”. Dalt. Trans. 2015, 44, 9353–9358.
- Maleski, K.; Mochalin, V.N.; Gogotsi, Y. Dispersions of Two-Dimensional Titanium Carbide MXene in Organic Solvents. Chem. Mater. 2017, 29, 1632–1640.
- Mashtalir, O.; Lukatskaya, M.R.; Kolesnikov, A.I.; Raymundo-Piñero, E.; Naguib, M.; Barsoum, M.W.; Gogotsi, Y. The Effect of Hydrazine Intercalation on the Structure and Capacitance of 2D Titanium Carbide (MXene). Nanoscale 2016, 8, 9128–9133.
- John, R.A.B.; Shruthi, J.; Ramana Reddy, M.V.; Ruban Kumar, A. Manganese Doped Nickel Oxide as Room Temperature Gas Sensor for Formaldehyde Detection. Ceram. Int. 2022, 48, 17654–17667.
- John, R.A.B.; Ruban Kumar, A.; Shruthi, J.; Ramana Reddy, M.V. FeXZn1−XOy as Room Temperature Dual Sensor for Formaldehyde and Ammonia Gas Detection. Inorg. Chem. Commun. 2022, 141, 109506.
- John, R.A.B.; Kumar, A.R. Structural, Morphological and Optical Aspects of Novel Synthesized Pristine and Hafnium Doped Nickel Oxide Nanoparticles. J. Indian Chem. Soc. 2022, 99, 100415.
- John, R.A.B.; Ruban Kumar, A. A Review on Resistive-Based Gas Sensors for the Detection of Volatile Organic Compounds Using Metal-Oxide Nanostructures. Inorg. Chem. Commun. 2021, 133, 108893.
- He, T.; Liu, W.; Lv, T.; Ma, M.; Liu, Z.; Vasiliev, A.; Li, X. MXene/SnO2 Heterojunction Based Chemical Gas Sensors. Sens. Actuators B Chem. 2021, 329, 129275.
- Yang, Z.; Jiang, L.; Wang, J.; Liu, F.; He, J.; Liu, A.; Lv, S.; You, R.; Yan, X.; Sun, P.; et al. Flexible Resistive NO2 Gas Sensor of Three-Dimensional Crumpled MXene Ti3C2Tx/ZnO Spheres for Room Temperature Application. Sens. Actuators B Chem. 2021, 326, 128828.
- Zhang, D.; Mi, Q.; Wang, D.; Li, T. MXene/Co3O4 Composite Based Formaldehyde Sensor Driven by ZnO/MXene Nanowire Arrays Piezoelectric Nanogenerator. Sens. Actuators B Chem. 2021, 339, 129923.
- Tai, H.; Duan, Z.; He, Z.; Li, X.; Xu, J.; Liu, B.; Jiang, Y. Enhanced Ammonia Response of Ti3C2T Nanosheets Supported by TiO2 Nanoparticles at Room Temperature. Sens. Actuators B Chem. 2019, 298, 126874.
- Bhati, V.S.; Kumar, M.; Banerjee, R. Gas Sensing Performance of 2D Nanomaterials/Metal Oxide Nanocomposites: A Review. J. Mater. Chem. C 2021, 9, 8776–8808.
- Ranjbar, F.; Hajati, S.; Ghaedi, M.; Dashtian, K.; Naderi, H.; Toth, J. Highly Selective MXene/V2O5/CuWO4-Based Ultra-Sensitive Room Temperature Ammonia Sensor. J. Hazard. Mater. 2021, 416, 126196.
- Liang, D.; Song, P.; Liu, M.; Wang, Q. 2D/2D SnO2 Nanosheets/Ti3C2Tx MXene Nanocomposites for Detection of Triethylamine at Low Temperature. Ceram. Int. 2022, 48, 9059–9066.
- Liu, M.; Wang, Z.; Song, P.; Yang, Z.; Wang, Q. In2O3 Nanocubes/Ti3C2Tx MXene Composites for Enhanced Methanol Gas Sensing Properties at Room Temperature. Ceram. Int. 2021, 47, 23028–23037.
- Kuang, D.; Wang, L.; Guo, X.; She, Y.; Du, B.; Liang, C.; Qu, W.; Sun, X.; Wu, Z.; Hu, W.; et al. Facile Hydrothermal Synthesis of Ti3C2Tx-TiO2 Nanocomposites for Gaseous Volatile Organic Compounds Detection at Room Temperature. J. Hazard. Mater. 2021, 416, 126171.
- Zhao, L.; Wang, K.; Wei, W.; Wang, L.; Han, W. High-performance Flexible Sensing Devices Based on Polyaniline/MXene Nanocomposites. InfoMat 2019, 1, 407–416.
- Liu, J.; Cui, N.; Xu, Q.; Wang, Z.; Gu, L.; Dou, W. High-Performance PANI-Based Ammonia Gas Sensor Promoted by Surface Nanostructuralization. ECS J. Solid State Sci. Technol. 2021, 10, 027007.
- Wang, X.; Sun, K.; Li, K.; Li, X.; Gogotsi, Y. Ti3C2T/PEDOT:PSS Hybrid Materials for Room-Temperature Methanol Sensor. Chin. Chem. Lett. 2020, 31, 1018–1021.
- Jin, L.; Wu, C.; Wei, K.; He, L.; Gao, H.; Zhang, H.; Zhang, K.; Asiri, A.M.; Alamry, K.A.; Yang, L.; et al. Polymeric Ti3C2Tx MXene Composites for Room Temperature Ammonia Sensing. ACS Appl. Nano Mater. 2020, 3, 12071–12079.
- Li, X.; Xu, J.; Jiang, Y.; He, Z.; Liu, B.; Xie, H.; Li, H.; Li, Z.; Wang, Y.; Tai, H. Toward Agricultural Ammonia Volatilization Monitoring: A Flexible Polyaniline/Ti3C2T Hybrid Sensitive Films Based Gas Sensor. Sens. Actuators B Chem. 2020, 316, 128144.
- Liu, M.; Wang, Z.; Song, P.; Yang, Z.; Wang, Q. Flexible MXene/RGO/CuO Hybrid Aerogels for High Performance Acetone Sensing at Room Temperature. Sens. Actuators B Chem. 2021, 340, 129946.
- Chen, W.Y.; Sullivan, C.D.; Lai, S.-N.; Yen, C.-C.; Jiang, X.; Peroulis, D.; Stanciu, L.A. Noble-Nanoparticle-Decorated Ti3C2Tx MXenes for Highly Sensitive Volatile Organic Compound Detection. ACS Omega 2022, 7, 29195–29203.
- Chen, W.Y.; Jiang, X.; Lai, S.-N.; Peroulis, D.; Stanciu, L. Nanohybrids of a MXene and Transition Metal Dichalcogenide for Selective Detection of Volatile Organic Compounds. Nat. Commun. 2020, 11, 1302.




