Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aleksi Leikas | -- | 2565 | 2023-12-06 14:37:38 | | | |
| 2 | Rita Xu | -8 word(s) | 2557 | 2023-12-07 02:25:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Leikas, A.J.; Ylä-Herttuala, S.; Hartikainen, J.E.K. Adenoviral Gene Therapy Vectors. Encyclopedia. Available online: https://encyclopedia.pub/entry/52446 (accessed on 07 February 2026).
Leikas AJ, Ylä-Herttuala S, Hartikainen JEK. Adenoviral Gene Therapy Vectors. Encyclopedia. Available at: https://encyclopedia.pub/entry/52446. Accessed February 07, 2026.
Leikas, Aleksi J., Seppo Ylä-Herttuala, Juha E. K. Hartikainen. "Adenoviral Gene Therapy Vectors" Encyclopedia, https://encyclopedia.pub/entry/52446 (accessed February 07, 2026).
Leikas, A.J., Ylä-Herttuala, S., & Hartikainen, J.E.K. (2023, December 06). Adenoviral Gene Therapy Vectors. In Encyclopedia. https://encyclopedia.pub/entry/52446
Leikas, Aleksi J., et al. "Adenoviral Gene Therapy Vectors." Encyclopedia. Web. 06 December, 2023.
Copy Citation
Adenoviral vectors are commonly used in clinical gene therapy. Apart from oncolytic adenoviruses, vector replication is highly undesired as it may pose a safety risk for the treated patient. Thus, careful monitoring for the formation of replication-competent adenoviruses (RCA) during vector manufacturing is required.
adenovirus
gene therapy
HEK293
oncolytic adenoviruses
1. Introduction
Gene therapy holds great promise as a method for managing or even curing conditions previously deemed untreatable [1]. Examples of such diseases range from various types of cancer and β-thalassemia to retinal dystrophy, and the U.S. Food and Drug Administration (FDA) has already approved several gene therapy products for clinical use in these indications [2].
There are two fundamental categories of gene therapy, utilizing either ex vivo or in vivo transduction. In the former method, donor cells are first harvested from the patient or an individual without the disease. The cells are then transduced using a vector capable of changing their genotype and administered back as modified autologous or allogeneic cell products replacing the deficiently functioning host cells [3]. In in vivo transduction, gene transfer occurs within the patient, and there is a direct interaction between the vectors and the dysfunctional cells of the treated individual [4].
Regardless of the chosen principle, a reliable vector construct is necessary for the efficient transduction of target cells, which is the prerequisite for transgene expression and achieving the desired therapeutic effect. Adenoviruses are one of the most commonly used vectors in clinical trials until 2021 [5], although more recently, the focus of gene therapy vectors has shifted to other viruses, such as adeno-associated viruses and lentiviruses [1]. Adenoviruses offer higher titers and relatively easier large-scale manufacturing than other vectors. They also have several additional benefits, such as good transduction efficacy and retainment of the transgene as an episome without integration into the host genome, making them an attractive platform for transient in vivo gene therapy purposes [6]. Indeed, many recently approved vaccines developed for severe acute respiratory syndrome coronavirus 2 are based on immunization by spike-protein-encoding adenovirus vectors (AdVs) [7].
Despite being generally regarded as having a good safety profile, AdVs are characterized by their high immunogenicity via both innate and adaptive inflammatory responses against the adenoviral capsid structures [8]. In specific contexts, and depending on their magnitude, the host responses to AdVs may lead to fatal consequences, as evidenced by the death of an 18-year-old patient who developed systemic inflammatory response syndrome after receiving a high dose of intravascularly administered adenoviral gene therapy for the treatment of partial ornithine transcarbamylase deficiency [9]. However, it has been noted that there was a problem between the chosen dose and the immune status of the treated patient in this tragic event [10].
A concern that has received much less attention relates to the de novo emergence of replication-competent adenoviruses (RCA) during AdV production [11]. Naturally, RCA itself has been recognized as an essential safety aspect since the dawn of AdV development, and preclinical studies have demonstrated that the presence of RCA is associated with increased inflammation, cytotoxicity, and prolonged vector clearance [12][13][14]. Mirroring the potential safety hazards, the FDA requires the industry to monitor the RCA formation with the upper limit of 1 RCA in 3 × 1010 tested viral particles (vp) in the final drug product [15][16].
2. Adenoviruses
2.1. General Properties
Adenoviruses are double-stranded DNA viruses with a non-enveloped, icosahedral capsid of approximately 90 nm in diameter (Figure 1). Their prevalence in the population is high, and they are responsible for common diseases such as rhinitis, gastroenteritis, and conjunctivitis. The family of human adenoviruses is divided into species A–G, which are further classified into many different serotypes [17][18]. The majority of knowledge on adenovirus biology is based on the extensive research of HAdV-2 and HAdV-5, which both belong to subgroup C. In addition to humans, different adenoviruses have been isolated from various other vertebrates, such as reptiles, birds, and mammals. No adenovirus identified in other species has been shown to cause clinically significant disease in humans [18].
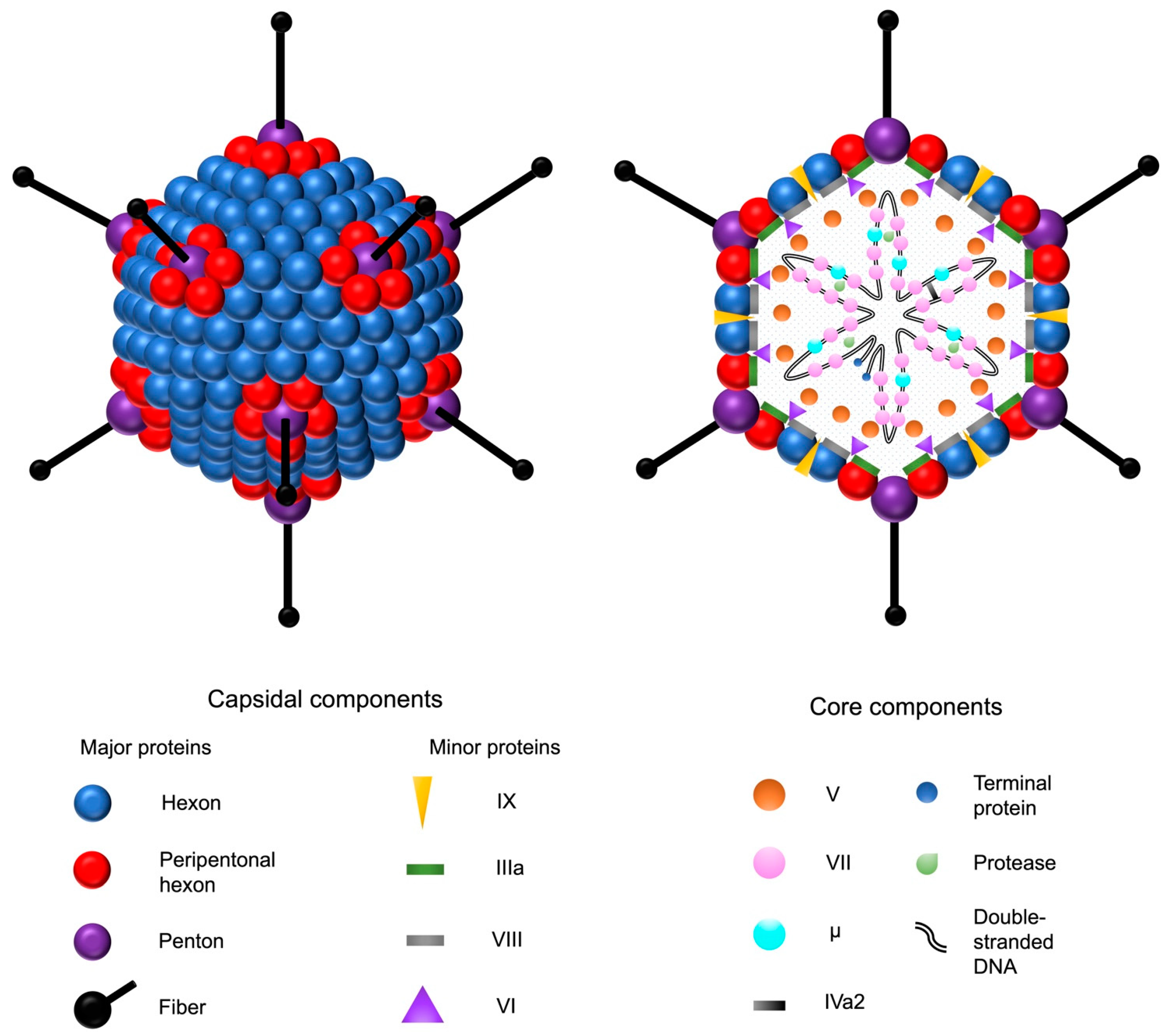
Figure 1. Adenovirus and its structural components. The double-stranded adenovirus DNA is encapsulated by an icosahedral capsid, formed by 252 major capsomers named hexons and pentons. The faces are formed by the hexons, whereas the pentons serve as the bases for fiber shafts that protrude from each vertex and culminate into tip structures called fiber knobs. The fiber proteins are responsible for the interactions between the adenovirus and the host cell receptors. In addition to the major proteins, four minor capsid-associated proteins stabilize the capsid structure. The five core proteins are associated with the adenoviral DNA. Adenovirus protease catalyzes the maturation of the precursor forms of the capsid proteins IIIa, VI, and VIII, as well as the DNA-associated proteins VII, μ, and terminal protein.
Adenovirus infection is typically self-resolving and usually causes only mild upper respiratory tract symptoms and fever in immunocompetent individuals. Up to half of all adenovirus infections are asymptomatic or cause only very mild symptoms [17][19]. Adenovirus infections account for approximately 5 to 10% of febrile infections in children and neonates, but their frequency is significantly lower in adults [19]. The incubation time for adenovirus infection is approximately six days [20]. Antibodies against HAdV-5 can be measured in nearly 85% of the population [21].
The most critical risk groups for adenovirus infections are neonates and immunocompromised individuals, in which the adenoviruses may cause severe pneumonia and diseases affecting organ systems other than the respiratory tract, depending on the level of immunocompetency [9][17][18]. In individuals with normal immune function, adenoviruses cause restricted focal infections, the location depending on the transmission route. There is variation in the tissue tropism between different adenovirus serotypes, and adenoviruses can theoretically replicate locally in several different organs [18]. Disseminated adenovirus infections are mainly seen in immunocompromised patients and are usually connected with other severe systemic symptoms [22].
2.2. Adenovirus Infection
The adenovirus infection can be mediated through a variety of receptors. Some adenovirus serotypes infect host cells by attaching to the coxsackie-adenovirus receptors (CARs) with their fiber knobs. The CARs are present in the tight junctions between epithelial cells in many organs. This primary interaction is followed by the binding of the pentons to the αvβ3 and αvβ5 integrins on a cell surface, which initiates the internalization of the virus via clathrin-dependent endocytosis. There are also serotypes that bind to the membrane proteins belonging to the cluster of the differentiation system and some other surface structures [18][22].
Cytolysis, which takes place at the end of the viral replication cycle, and the subsequent virus release from the host cell are thought to be the result of the viral hijacking of the host translational machinery and accumulation of mature viruses inside the host cell. The proteins encoded by the regions E1, E2, and E4 enable adenovirus replication in the host cell (Figure 2A). In addition, adenovirus death protein (ADP) encoded by the E3 region plays a crucial role in cytolysis, and its overexpression increases viral spread into adjacent cells [23].
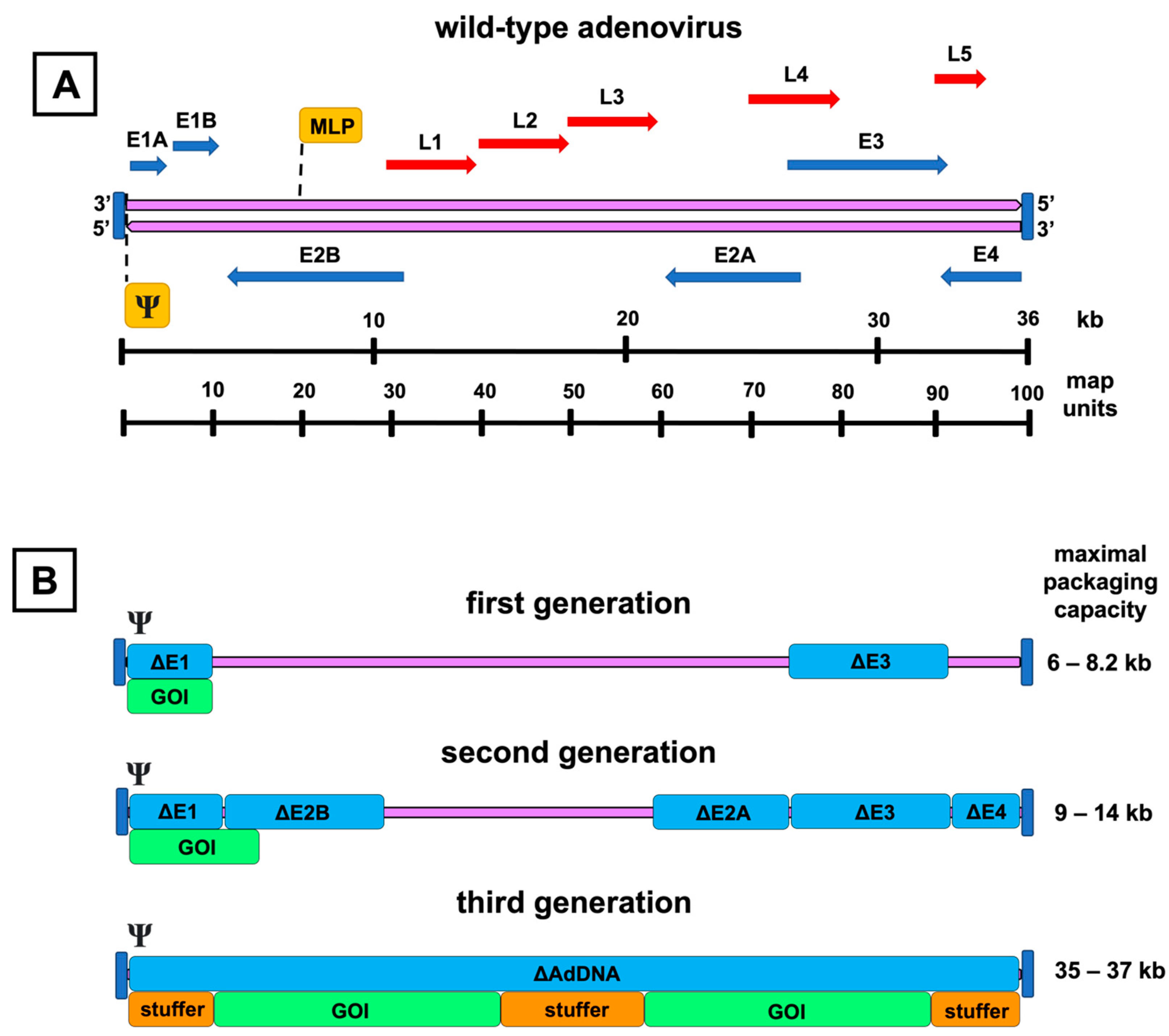
Figure 2. Genomic organization of the adenovirus and its vector derivatives. A schematic representation of the wild-type adenovirus genome and its transcription units is shown on top (A). The double-stranded adenoviral DNA (purple arrows) is flanked by inverted terminal repeats (vertical blue lines), and the packaging signal (Ψ) is at the left end of the genome. Early genes (E1–E4) are presented as blue arrows, and late genes (L1–L5) as red arrows with their respective transcription directions. Major late promoter (MLP) directs the transcription of the late genes. The picture below (B) presents the adenoviral vector generations with their respective genomic deletions (Δ), highlighted by the blue color over the adenovirus DNA (purple line). Under the deletion map, insertion spaces for the gene of interest (GOI) are shown. Inverted terminal repeats and packaging signals are present in each generation. Third-generation vectors are devoid of all coding adenoviral DNA (AdDNA). Additional stuffer DNA is inserted to reach the genomic size required for the encapsidation of the vector. The range for the maximal transgene packaging capacity (kb) is presented on the right.
The tropism of adenoviruses varies greatly depending on the adenovirus species. Viruses from species A, F, and G infect gastrointestinal epithelial cells. Species C, E, and part of species B have tropism to the respiratory tract, and the rest of species B to the urinary tract. In contrast, adenovirus species D infects the conjunctival surface of the eye. HAdV-5 belongs to the species C and has thus natural tropism to the respiratory epithelial cells, but also to the hepatocytes [18].
Intravenous administration of wild-type HAdV-5 at a dose of 2 × 1012 vp results in biodistribution mainly in the lungs and liver in a porcine model. In addition, minor biodistribution occurs in the kidney, heart, skeletal muscle, brain, and gonads, but the concentration of the viral DNA is many thousand-fold lower than in the lungs [24]. Using immunohistochemical and hybridization-based assays, it has been shown that infection by certain adenovirus serotypes can remain latent in the tonsils, adenoids, and lungs [25].
Wild-type adenovirus does not integrate into the host genome in replication-permissive cells, and the infection of the host cell leads to its inevitable death by cytolysis at the end of the adenoviral replication cycle [26]. Some adenovirus serotypes have been found to be oncogenic in rodent cells [27]. Adenovirus serotypes coexisting in the same individual can recombine and form hybrids causing new infections, especially in immunocompromised but also in healthy individuals [28][29].
2.3. Environmental Aspects
Adenoviral shedding from an infected person takes place via respiratory aerosols and feces. Horizontal transmission can occur, for example, if viruses enter the respiratory system or the conjunctiva via droplets. Transmission by the fecal-oral route is also possible, especially in children [18].
Adenoviruses of serotypes 5, 8, and 19 can survive for up to 49 days on environmental plastic and metal surfaces [30]. A study comparing twenty-one disinfectants concluded that mixtures containing at least 1900 ppm of chlorine, 65% ethanol, and 0.63% quaternary ammonium compounds or 79% ethanol and 0.1% quaternary ammonium compounds should be used to disinfect surfaces contaminated with serotype 8 adenoviruses [31].
Although the efficacy of some antiviral drugs that alter the function of DNA polymerase has already been tested in clinical trials, no antiviral drugs for adenoviral infections have yet been approved for clinical use [22]. On the other hand, enteric vaccines based on replication-competent wild-type adenoviruses have been used to prevent acute respiratory distress syndrome in some risk groups, for example, in military personnel. These vaccines have been proven to be safe and well-tolerated [32]. After administering an adenovirus vaccine for serotype 4, fecal shedding of adenovirus takes place for 2 to 3 weeks, but the transmission to unvaccinated individuals using the same facilities, such as toilets, has not been shown to take place [25][33].
3. Adenovirus Vectors
Based on the extent of their genetic modifications, the replication-deficient AdVs are categorized as first-, second-, or third-generation constructs. Additionally, conditionally replicating or oncolytic AdVs form another distinct and important AdV group.
3.1. First-Generation Adenovirus Vectors
First-generation AdVs are the simplest of all adenoviral constructs. They contain a genomic deletion of the E1 region and an expression cassette of the preferred transgene (Figure 2B). The E1 region consists of two separate E1A and E1B transcription units. These units encode proteins to bring the infected cell into the state that allows viral particles to be produced and evasion of the virus from the host immune response activated by the synthesis of adenovirus DNA.
Two of the most abundant proteins encoded by the E1A unit are E1A 12S and 13S. The proteins are identical in their amino acid sequences apart from a 46 amino acids long segment in the middle of the polypeptide 13S. 12S and 13S regulate viral replication by binding to multiple cellular proteins, thereby shifting the cell cycle from the G0 phase to the S phase [17]. The E1B unit mainly produces two proteins called E1B 19K and E1B 55K. E1B 55K inhibits the tumor protein p53-induced apoptosis and increases the degradation of p53 as a complex with the E4 open reading frame (ORF) 6 protein [34][35]. E1B 19K also contributes to the inhibition of apoptosis by preventing the induction of tumor necrosis factor (TNF) α and Fas-mediated cell death pathways [17]. E1-deleted AdVs cannot replicate in human cells because the genomic region encodes transcription factors essential for adenovirus replication. If some vectors regain the deleted E1 region by recombination, they can replicate nearly as efficiently as the wild-type adenoviruses [12].
In addition to the replication enabling E1 region, the E3 region can also be deleted to produce more space for genomic insertions. Although not necessary for viral replication, the seven proteins encoded by the E3 region contribute to the protection of the virus from the host immune responses, apart from E3 12.5K, whose function is still unknown, and ADP, which serves as a factor to aid the cytolysis and release of the mature virions from the cell during the late infection [36][37]. From the rest of the proteins, E3 gp19K prevents the introduction of antigens by a major histocompatibility complex 1 allowing the infected cell to escape from the cytotoxic T cells [38], E3 receptor internalization and degradation (RID) α and β proteins form a complex on the membrane of the infected cell that inhibits TNFα and Fas-mediated apoptosis [39], E3 6.7K forms a complex with RID β, inhibiting the apoptotic cascade induced by TNF-related apoptosis-inducing ligand [40], and E3 14.7K participates in the inhibition of apoptosis caused by TNFα and Fas [41][42].
3.2. Second-Generation Adenovirus Vectors
Second-generation AdVs have the genomic deletions of the first-generation AdVs and additional genomic deletions in the regions E2 or E4, intending to reduce immunogenicity and increase the transgene packaging capacity even further (Figure 2B). The three translation products of the E2 region, divided into the promoter-proximal region E2A and the distal E2B, participate in DNA replication and are denoted as DNA-binding protein, precursor terminal protein, and adenoviral DNA polymerase [43]. The E4 region encodes at least seven different ORFs. These proteins have diverse functions, ranging from transcriptional regulation to the augmentation of late protein synthesis [44][45].
Despite the elegant rationale behind the additional modifications of second-generation AdVs, it seems that in terms of immunogenicity and toxicity, the deletion of the regions E2 and E4 offers only a limited benefit compared to omitting only the E1 and E3 regions [46][47][48]. Moreover, the transgene expression seems to be unstable [48]. In fact, at least one study reported that E4 deletion in the first-generation AdV enhances endothelial apoptosis compared to the control vectors [49]. On top of having no apparent benefit over the first-generation AdVs concerning safety and efficacy, the production yields of the second-generation vectors remain lower [50].
3.3. Third-Generation Adenovirus Vectors
Finally, the third-generation AdVs, also called gutless, gutted, helper-dependent, or high-capacity AdVs, have been modified to be devoid of all adenovirus DNA, aside from the sequences mandatory for the packaging of the expression cassette (Figure 2B). This also includes the late-phase transcription regions L1–L5, which are all controlled by the same major late promoter and encode proteins forming the capsid and participating in the assembly, genomic packaging, and regulation of the maturing virions [51].
Third-generation AdVs are attractive vectors for gene therapy. Due to up to 35–37 kB of removed adenoviral DNA, they have room for a larger transgene cassette and, at least in theory, are also less immunogenic and capable of more extended transgene expression [52]. Compared to the first- and second-generation, the production line of the third-generation AdVs is more complex and, as the name “helper-dependent” implies, requires a separate helper virus to provide all the necessary proteins for vector propagation [50]. However, the additional effort might be worthwhile, as the current evidence supports the capability of the third-generation AdVs to produce substantially longer-lasting transgene expression than the first-generation AdVs with less inflammation [53][54][55].
References
- High, K.A.; Roncarolo, M.G. Gene Therapy. N. Engl. J. Med. 2019, 381, 455–464.
- Approved Cellular and Gene Therapy Products. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 22 September 2022).
- Naldini, L. Ex vivo gene transfer and correction for cell based therapies. Nat. Rev. Genet. 2011, 12, 301–315.
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 2021, 29, 464–488.
- Gene Therapy Clinical Trials Worldwide—Provided by the Journal of Gene Medicine. Available online: https://a873679.fmphost.com/fmi/webd/GTCT (accessed on 22 September 2022).
- Aldhamen, Y.A.; Amalfitano, A. Methods to Mitigate Immune Responses to Adenoviral Vectors. In Adenoviral Vectors for Gene Therapy, 2nd ed.; Curiel, D.T., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 391–422.
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines 2021, 6, 97.
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433.
- Raper, S.E.; Chirmule, N.; Lee, F.S.; Wivel, N.A.; Bagg, A.; Gao, G.P.; Wilson, J.M.; Batshaw, M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003, 80, 148–158.
- Rissanen, T.T.; Ylä-Herttuala, S. Current status of cardiovascular gene therapy. Mol. Ther. 2007, 15, 1233–1247.
- Fallaux, F.J.; van der Eb, A.J.; Hoeben, R.C. Who’s afraid of replication-competent adenoviruses? Gene Ther. 1999, 6, 709–712.
- Lochmüller, H.; Jani, A.; Huard, J.; Prescott, S.; Simoneau, M.; Massie, B.; Karpati, G.; Acsadi, G. Emergence of early region 1-containing replication-competent adenovirus in stocks of replication-defective adenovirus recombinants (ΔE1 + ΔE3) during multiple passages in 293 cells. Hum. Gene Ther. 1994, 5, 1485–1491.
- Hermens, W.T.; Verhaagen, J. Adenoviral vector-mediated gene expression in the nervous system of immunocompetent Wistar and T cell-deficient nude rats: Preferential survival of transduced astroglial cells in nude rats. Hum. Gene Ther. 1997, 8, 1049–1063.
- Imler, J.L.; Bout, A.; Dreyer, D.; Dieterlé, A.; Schultz, H.; Valerio, D.; Mehtali, M.; Pavirani, A. Trans-complementation of E1-deleted adenovirus: A new vector to reduce the possibility of codissemination of wild-type and recombinant adenoviruses. Hum. Gene Ther. 1995, 6, 711–721.
- U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy. Available online: https://www.fda.gov/media/72402/download (accessed on 24 October 2022).
- U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for Industry: Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs). Available online: https://www.fda.gov/media/113760/download (accessed on 24 October 2022).
- Berk, A.J. Adenoviridae: The viruses and their replication. In Fields Virology, 5th ed.; Fields, B.N., Knipe, D.M., Griffin, D.E., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 2355–2394.
- Rhee, G.E.; Barouch, D.H. Adenoviruses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, J., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 1787–1793.
- Fox, J.P.; Brandt, C.D.; Wassermann, F.E.; Hall, C.E.; Spigland, I.; Kogon, A.; Elveback, L.R. The virus watch program: A continuing surveillance of viral infections in metropolitan New York families: Observations of adenovirus infections: Virus excretion patterns, antibody response, efficiency of surveillance, patterns of infection, and relation to illness. Am. J. Epidemiol. 1969, 89, 25–50.
- Lessler, J.; Reich, N.G.; Brookmeyer, R.; Perl, T.M.; Nelson, K.E.; Cummings, D.A. Incubation periods of acute respiratory viral infections: A systematic review. Lancet Infect. Dis. 2009, 9, 291–300.
- Mast, T.C.; Kierstead, L.; Gupta, S.B.; Nikas, A.A.; Kallas, E.G.; Novitsky, V.; Mbewe, B.; Pitisuttithum, P.; Schechter, M.; Vardas, E.; et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: Correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 2010, 28, 950–957.
- Lenaerts, L.; De Clercq, E.; Naesens, L. Clinical features and treatment of adenovirus infections. Rev. Med. Virol. 2008, 18, 357–374.
- Subramanian, T.; Vijayalingam, S.; Chinnadurai, G. Genetic identification of adenovirus type 5 genes that influence viral spread. J. Virol. 2006, 80, 2000–2012.
- Jogler, C.; Hoffmann, D.; Theegarten, D.; Grunwald, T.; Uberla, K.; Wildner, O. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J. Virol. 2006, 80, 3549–3558.
- Lichtenstein, D.L.; Wold, W.S. Experimental infections of humans with wild-type adenoviruses and with replication-competent adenovirus vectors: Replication, safety, and transmission. Cancer Gene Ther. 2004, 11, 819–829.
- Harui, A.; Suzuki, S.; Kochanek, S.; Mitani, K. Frequency and stability of chromosomal integration of adenovirus vectors. J. Virol. 1999, 73, 6141–6146.
- McAllister, R.M.; Nicolson, M.O.; Reed, G.; Kern, J.; Gilden, R.V.; Huebner, R.J. Transformation of rodent cells by adenovirus 19 and other group D adenoviruses. J. Natl. Cancer Inst. 1969, 43, 917–923.
- de Jong, P.J.; Valderrama, G.; Spigland, I.; Horwitz, M.S. Adenovirus isolates from urine of patients with acquired immunodeficiency syndrome. Lancet 1983, 1, 1293–1296.
- Vora, G.J.; Lin, B.; Gratwick, K.; Meador, C.; Hansen, C.; Tibbetts, C.; Stenger, D.A.; Irvine, M.; Seto, D.; Purkayastha, A.; et al. Co-infections of adenovirus species in previously vaccinated patients. Emerg. Infect. Dis. 2006, 12, 921–930.
- Gordon, Y.J.; Gordon, R.Y.; Romanowski, E.; Araullo-Cruz, T.P. Prolonged recovery of desiccated adenoviral serotypes 5, 8, and 19 from plastic and metal surfaces in vitro. Ophthalmology 1993, 100, 1835–1840.
- Rutala, W.A.; Peacock, J.E.; Gergen, M.F.; Sobsey, M.D.; Weber, D.J. Efficacy of hospital germicides against adenovirus 8, a common cause of epidemic keratoconjunctivitis in health care facilities. Antimicrob. Agents Chemother. 2006, 50, 1419–1424.
- Gaydos, C.A.; Gaydos, J.C. Adenovirus vaccines in the U.S. military. Mil. Med. 1995, 160, 300–304.
- Rosenbaum, M.J.; De Berry, P.; Sullivan, E.J.; Edwards, E.A.; Pierce, W.E.; Muldoon, R.L.; Jackson, G.G.; Peckinpaugh, R.O. Characteristics of vaccine-induced and natural infection with adenovirus type 4 in naval recruits. Am. J. Epidemiol. 1968, 88, 45–54.
- Chinnadurai, G. Control of apoptosis by human adenovirus genes. Semin. Virol. 1998, 5, 399–408.
- Steegenga, W.T.; Riteco, N.; Jochemsen, A.G.; Fallaux, F.J.; Bos, J.L. The large E1B protein together with the E4orf6 protein target p53 for active degradation in adenovirus infected cells. Oncogene 1998, 16, 349–357.
- Lichtenstein, D.L.; Toth, K.; Doronin, K.; Tollefson, A.E.; Wold, W.S.M. Functions and mechanisms of action of the adenovirus E3 proteins. Int. Rev. Immunol. 2004, 23, 75–111.
- Tollefson, A.E.; Scaria, A.; Hermiston, T.W.; Ryerse, J.S.; Wold, L.J.; Wold, W.S. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J. Virol. 1996, 70, 2296–2306.
- Burgert, H.G.; Maryanski, J.L.; Kvist, S. “E3/19K” protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc. Natl. Acad. Sci. USA 1987, 84, 1356–1360.
- Gooding, L.R.; Ranheim, T.S.; Tollefson, A.E.; Aquino, L.; Duerksen-Hughes, P.; Horton, T.M.; Wold, W.S. The 10,400- and 14,500-dalton proteins encoded by region E3 of adenovirus function together to protect many but not all mouse cell lines against lysis by tumor necrosis factor. J. Virol. 1991, 65, 4114–4123.
- Benedict, C.A.; Norris, P.S.; Prigozy, T.I.; Bodmer, J.L.; Mahr, J.A.; Garnett, C.T.; Martinon, F.; Tschopp, J.; Gooding, L.R.; Ware, C.F. Three adenovirus E3 proteins cooperate to evade apoptosis by tumor necrosis factor-related apoptosis-inducing ligand receptor-1 and -2. J. Biol. Chem. 2001, 276, 3270–3278.
- Chen, P.; Tian, J.; Kovesdi, I.; Bruder, J.T. Interaction of the adenovirus 14.7kDa protein with FLICE inhibits Fas ligand-induced apoptosis. J. Biol. Chem. 1998, 273, 5815–5820.
- Krajcsi, P.; Dimitrov, T.; Hermiston, T.W.; Tollefson, A.E.; Ranheim, T.S.; Vande Pol, S.B.; Stephenson, A.H.; Wold, W.S. The adenovirus E3-14.7K protein and the E3-10.4K/14.5K complex of proteins, which independently inhibit tumor necrosis factor (TNF)-induced apoptosis, also independently inhibit TNF-induced release of arachidonic acid. J. Virol. 1996, 70, 4904–4913.
- Swaminathan, S.; Thimmapaya, B. Regulation of adenovirus E2 transcription unit. Curr. Top. Microbiol. Immunol. 1995, 199, 177–194.
- Huang, M.M.; Hearing, P. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J. Virol. 1989, 63, 2605.
- Bridge, E.; Ketner, G. Redundant control of adenovirus late gene expression by early region 4. J. Virol. 1989, 63, 631–638.
- Lusky, M.; Christ, M.; Rittner, K.; Dieterle, A.; Dreyer, D.; Mourot, B.; Schultz, H.; Stoeckel, F.; Pavirani, A.; Mehtali, M. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J. Virol. 1998, 72, 2022–2032.
- O’Neal, W.K.; Zhou, H.; Morral, N.; Aguilar-Cordova, E.; Pestaner, J.; Langston, C.; Mull, B.; Wang, Y.; Beaudet, A.L.; Lee, B. Toxicological comparison of E2a-deleted and first-generation adenoviral vectors expressing alpha1-antitrypsin after systemic delivery. Hum. Gene. Ther. 1998, 9, 1587–1598.
- Wen, S.; Schneider, D.B.; Driscoll, R.M.; Vassalli, G.; Sassani, A.B.; Dichek, D.A. Second-generation adenoviral vectors do not prevent rapid loss of transgene expression and vector DNA from the arterial wall. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1452–1458.
- Jornot, L.; Petersen, H.; Lusky, M.; Pavirani, A.; Moix, I.; Morris, M.A.; Rochat, T. Effects of first generation E1E3-deleted and second generation E1E3E4-deleted/modified adenovirus vectors on human endothelial cell death. Endothelium 2001, 8, 167–179.
- Kovesdi, I.; Hedley, S.J. Adenoviral producer cells. Viruses 2010, 2, 1681–1703.
- Guimet, A.; Hearin, P. Adenovirus Replication. In Adenoviral Vectors for Gene Therapy, 2nd ed.; Curiel, D.T., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 59–84.
- Segura, M.M.; Alba, R.; Bosch, A.; Chillón, M. Advances in helper-dependent adenoviral vector research. Curr. Gene Ther. 2008, 8, 222–235.
- Fleury, S.; Driscoll, R.; Simeoni, E.; Dudler, J.; von Segesser, L.K.; Kappenberger, L.; Vassalli, G. Helper-dependent adenovirus vectors devoid of all viral genes cause less myocardial inflammation compared with first-generation adenovirus vectors. Basic Res. Cardiol. 2004, 99, 247–256.
- Brunetti-Pierri, N.; Ng, T.; Iannitti, D.; Cioffi, W.; Stapleton, G.; Law, M.; Breinholt, J.; Palmer, D.; Grove, N.; Rice, K.; et al. Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors. Hum. Gene. Ther. 2013, 24, 761–765.
- Rastall, D.P.; Seregin, S.S.; Aldhamen, Y.A.; Kaiser, L.M.; Mullins, C.; Liou, A.; Ing, F.; Pereria-Hicks, C.; Godbehere-Roosa, S.; Palmer, D.; et al. Long-term, high-level hepatic secretion of acid α-glucosidase for Pompe disease achieved in non-human primates using helper-dependent adenovirus. Gene Ther. 2016, 23, 743–752.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
811
Revisions:
2 times
(View History)
Update Date:
07 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




