Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yen-Ho Chu | -- | 2192 | 2023-12-06 11:56:12 | | | |
| 2 | Rita Xu | Meta information modification | 2192 | 2023-12-07 02:52:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, H.; Chu, Y. Small-Molecule Thermoresponsive Ionic Liquid Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/52436 (accessed on 07 February 2026).
Li H, Chu Y. Small-Molecule Thermoresponsive Ionic Liquid Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/52436. Accessed February 07, 2026.
Li, Hsin-Yi, Yen-Ho Chu. "Small-Molecule Thermoresponsive Ionic Liquid Materials" Encyclopedia, https://encyclopedia.pub/entry/52436 (accessed February 07, 2026).
Li, H., & Chu, Y. (2023, December 06). Small-Molecule Thermoresponsive Ionic Liquid Materials. In Encyclopedia. https://encyclopedia.pub/entry/52436
Li, Hsin-Yi and Yen-Ho Chu. "Small-Molecule Thermoresponsive Ionic Liquid Materials." Encyclopedia. Web. 06 December, 2023.
Copy Citation
Ionic liquids (ILs) are a class of low-melting molten salts (<100 °C) constituted entirely of ions, and their research has gained tremendous attention in line with their remarkably growing applications.
smart material
thermoresponsive ionic liquid
thermoresponsive zwitterionic liquid
1. Introduction
Ionic liquids (ILs) are advanced materials entirely made of anion and cation pairs. They possess unique characteristics of diversity and controllability as ionic solvents, setting them apart from conventional molecular solvents known for their high volatility. Figure 1 shows the common anion and cation structures. ILs exhibit a plethora of outstanding properties, including extremely low vapor pressure, a low melting point, high polarity, scarce inflammability, and high thermal and chemical stability [1][2]. Additionally, as attractive electrolytes for batteries, ILs exhibit high conductivity, tunable electrochemical windows, and a wide liquid temperature range. Due to these remarkable properties and their recyclability, ILs have the potential to replace commonly used volatile organic solvents [3][4]. Because ILs conform to the principles of green chemistry and have great controllability in structural design, they have been widely studied and experimented with in various fields [5].
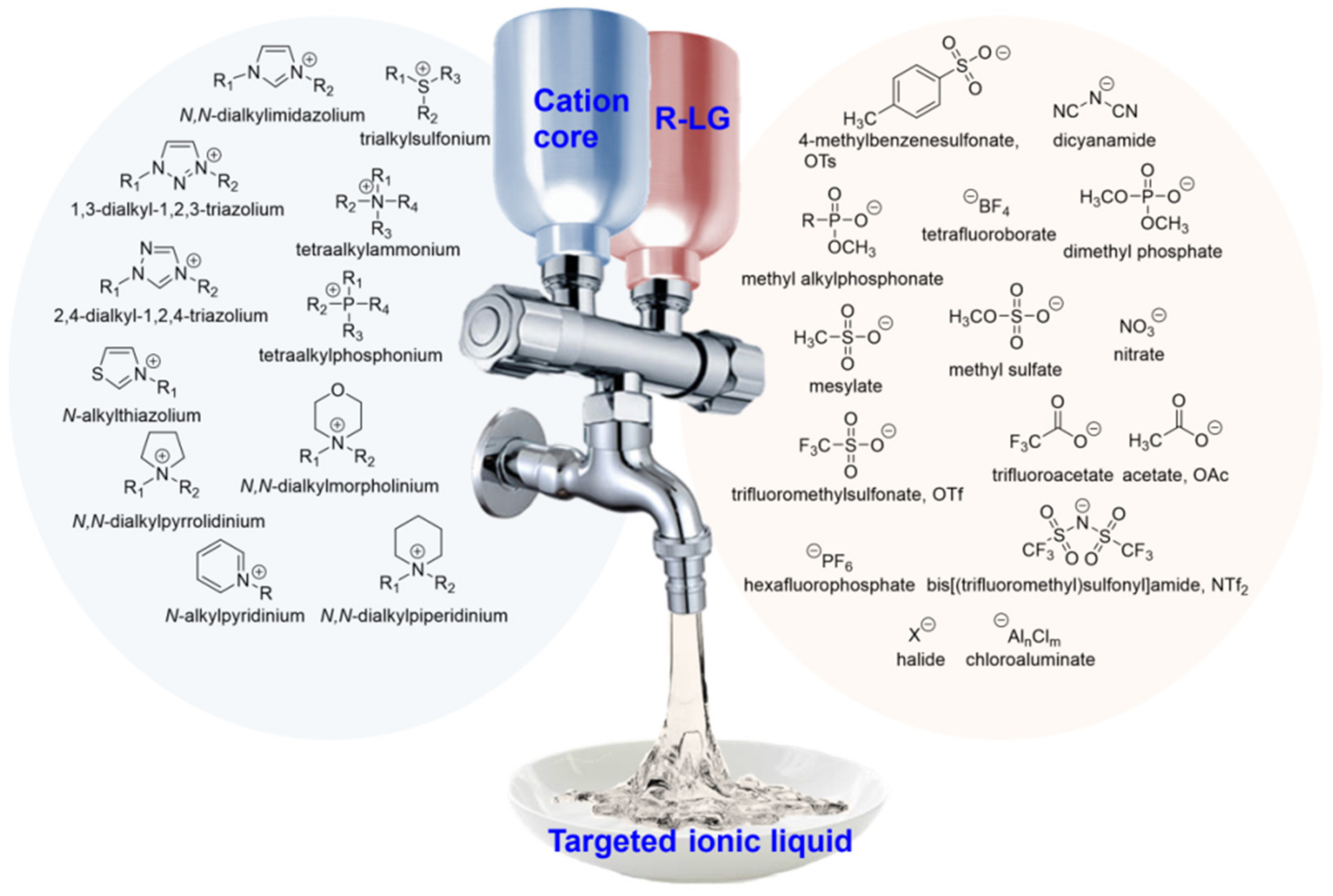
Figure 1. Ionic liquids are entirely composed of cations and anions.
Throughout the development of ILs, researchers can roughly classify them into four stages. The first generation of ILs was extremely sensitive to moisture and air, and their structures were organic cations combined with metal halide anions, which has attracted attention due to their physical properties and electrochemical redox potential. The drawback was that these ILs easily decomposed or deteriorated when exposed to water or air [6]. The second generation of ILs is room-temperature ILs with high conductivity, which are stable in water and air [7], allowing scientists from various research fields to extensively explore imidazolium-based ILs. The third generation of ILs has abandoned the existing structural framework, and scientists have designed and synthesized functional ionic liquids and applied them in targeted fields [8][9]. The fourth and newest generation of ILs is being developed with the aim of applying them in biology, ecology, and medicine, and the ‘bio-safer’ IL structures are synthesized and investigated [10][11].
2. Ionic Liquids Are Designable and Structurally Tunable
2.1. Ionic Liquids as Attractive Reaction Media
With the aforementioned advantages, ILs can likely replace conventional molecular solvents. However, most ILs have the disadvantages of high viscosity and laborious purification. This drawback may be overcome by using ILs in microemulsion systems. Bica-Schröder and coworkers showed that microemulsion systems can be divided into four types (Figure 2) [12]: Winsor I and II are oil-in-water (o/w) and water-in-oil (w/o) microemulsion surfactant biphase systems, respectively. The mesophase of the Winsor III excess surfactant is between the water and oil phases, forming coexistence. Winsor IV is a single-phase isotropic solution formed by more surfactants. Compared to traditional emulsions, microemulsions not only have a significantly small structural size (3–30 nm) but also have thermodynamic stability, which can be converted into the long-term stability of mixed-polarity/non-polarity systems.
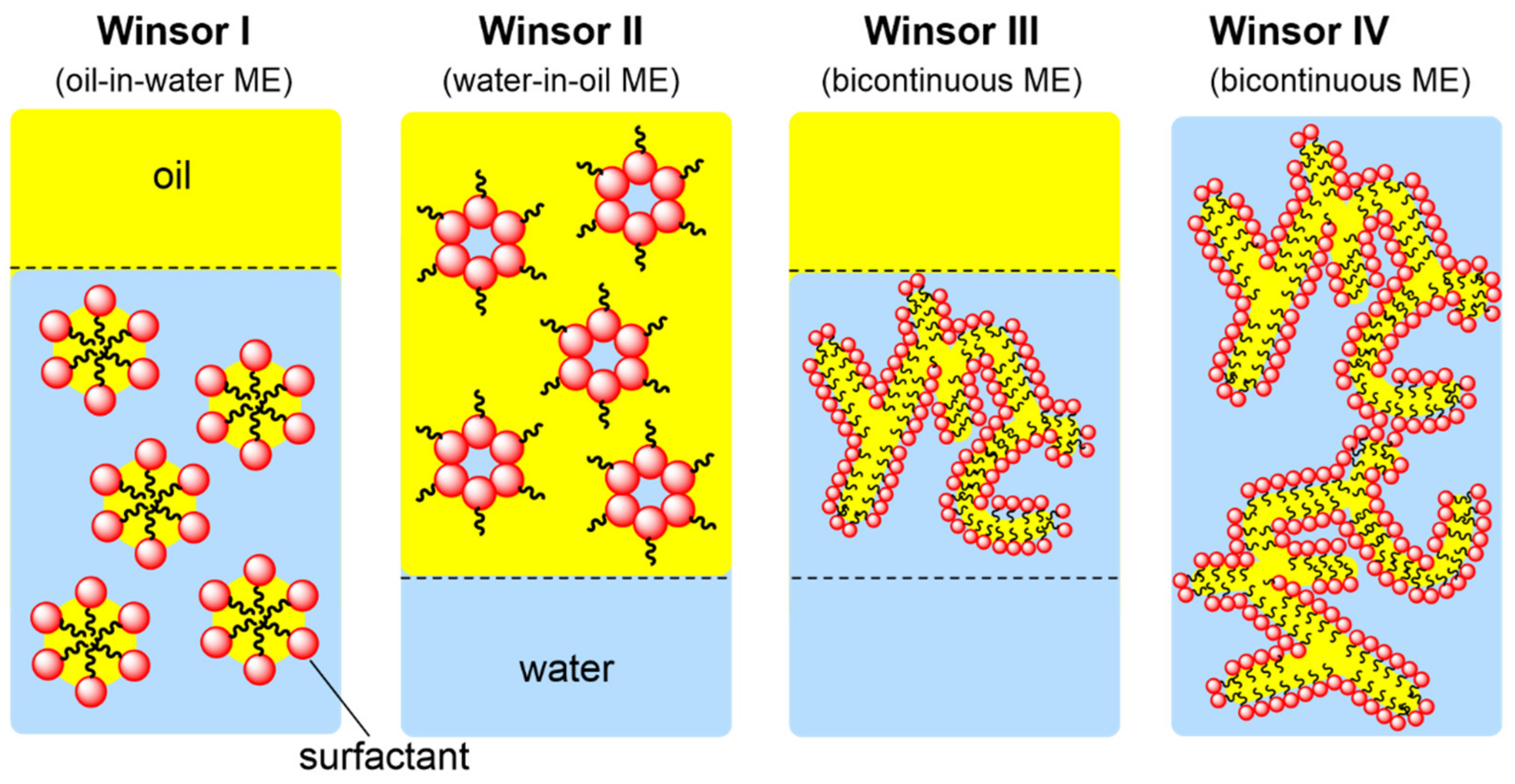
Figure 2. Winsor classification of microemulsions (ME).
In addition, ILs possess the advantages of ultra-low vapor pressure, excellent solvation power, and high chemical and thermal stabilities, making them more suitable as solvents for chemical synthesis. For example, many natural and unnatural products carry quinazoline rings. Also, a good number of quinazoline derivatives are currently in different stages of clinical practice, and some have been sold as drugs. Based on bicyclic imidazolium- and triazolium-based ILs in combination with microwave-assisted organic synthesis, researchers successfully synthesized tryptanthrin, batracylin, and rutaecarpine using the rapid, one-pot, and high-yielding method and investigated their biological activities when appropriate [13][14][15].
2.2. Chiral Ionic Liquids and Supported Ionic Liquids
In the past decade, more and more attention has been paid to the development of chiral ionic liquids (CILs) and their applications in various separation technologies. The molecular diversity of CILs can be large as long as the anion or cation carries a chiral center or even has a few ionic liquids that have cationic and anion chiral pairs at the same time. This allows for the design of multi-variable structures. With the characteristics of chiral recognition, CILs play an important role in enantiomeric separation [16], for which the application scope of CILs has been expanded. In 2020, Flieger and coworkers published a review article on CILs [17] and introduced CILs as a new type of chiral solid material that was used for chiral recognition of enantiomeric analytes. The applications of CILs include various extraction techniques and their use as catalysts in chemical reactions, such as the Diels–Alder and aza-Baylis–Hillman reactions, which enhance the purity of enantiomeric products. In 2009, Bonanni published a simple and direct synthesis method to obtain novel chiral pyrrolidinium ILs starting with tartaric acid [18].
In addition, supported ILs can be used to enhance the reaction rate. Salunkhe carried out Knoevenagel condensation in various ILs and found that the Brønsted acidic IL, [Hmim][Tfa], enhanced the reaction rate [19]. Bazureau used IL-supported benzaldehyde and underwent condensation reactions with alkylamines, followed by a 1,3-dipolar cycloaddition reaction regioselectively. Finally, the IL could be readily removed using NaOMe to obtain the desired product, and the initial IL was released [20].
2.3. Zwitterionic Ionic Liquids
Ohno reported for the first time the synthesis of zwitterionic imidazolium sulfonate ILs (ZILs) in 2001 [21]. ZILs refer to ILs in which cationic and anion pairs are tethered together within the same structure. ZILs often exhibit high melting points. However, charge delocalization in ZILs helps reduce the melting point and viscosity. ZILs have been employed as electrochemical ionophores [21], reaction catalysts [22], and attractive solvents for dissolving cellulose [23]. In general, ZILs have opened a new chapter in developing specific chemical applications.
2.4. Task-Specific Ionic Liquids for CO2 Capture
Carbon dioxide has a significant impact on the environment and is known to contribute to the greenhouse effect. Carbon capture and storage (CCS), or carbon capture and sequestration (CCS), is a widely recognized method for capturing carbon dioxide. Here, the carbon dioxide produced by petrochemical combustion is transported from its place of origin through pipelines or ships and finally stored deep underground in geological formations. However, the cost of CCS remains high, and it is difficult to compare it with that of renewable energy [24][25]. Therefore, it is critical to develop cost-effective capture technologies for carbon dioxide. ILs have been specifically applied to carbon dioxide capture, and this type of structural design generally aims to place basic groups at the end of side chains and make the target gas react with solutes in the liquid phase, which is the main mode of sequestration. For example, Davis and coworkers reported the use of ILs to capture carbon dioxide in a more convenient and safer manner compared to capture with conventional molecular solvents or alkaline substances [26]. They reported that the capture conversion rate of [AminoC3-b-im][BF4] to carbon dioxide could reach 49.6%, and the mass of carbon dioxide was increased by 7.4% at atmospheric pressure, which is far superior to that of [h-mim][PF6] (0.01%) [26]. In addition, Shukla and Mikkola (2019) summarized the technology of capturing carbon dioxide with ILs and the capture mechanism in detail. They discussed the feasibility of commercially capturing carbon dioxide with ILs [27].
2.5. Ionic Liquids as PCR Enhancers
Since its introduction in 1988, the polymerase chain reaction (PCR) has played a vital role in amplifying DNA fragments using thermophilic polymerases [28]. Today, PCR is widely and frequently used for medical and biological applications, such as gene duplication, disease diagnosis, and forensic identification [29]. Despite the considerable diagnostic potential of PCR, amplifying the GC-rich DNA sequences by PCR still poses a significant challenge. It is known experimentally that GC-rich DNAs are difficult to amplify using the standard PCR amplification procedures, which can be attributed to the secondary structure in DNA [30][31]. Although it is straightforward to enzymatically replicate GC-rich DNA in living organisms, improved technologies or new methods must be developed and implemented to replicate them in vitro. Literature reports have demonstrated that slowdown PCR or enhancers such as DMSO or betaine overcome such obstacles [30][32]. The laboratory previously revealed that ILs promoted PCR amplification of GC-rich DNAs by reducing their melting temperatures (Tm) [33].
2.6. Functionalized Ionic Liquids
Chemoselective detection of volatile organic compounds (VOCs) in the environment has always been considered an important research topic. Human naked eyes and noses cannot identify directly and sense remotely the presence of minute amounts of natural or man-made gaseous, potentially hazardous substances. If the target VOCs in the environment can be experimentally measured and accurately determined, an early warning on the potential exposure to dangerous and unhealthy environments could then be timely disclosed [34]. With their nature of negligible vapor pressure and diversity in structural tunability, ILs have the potential to be tailor-synthesized and thin-coated on sensorchips in the mass-sensitive piezoelectric quartz crystal microbalance (QCM) to detect chemoselectively the target VOCs. A pioneering work on the relationship between the mass deposited and resonance frequency shift of quartz crystal was published by Sauerbrey [35], which was later widely used to measure changes in nanoscale masses. The first research on the use of ILs as sensing materials with QCM was published by Dai in 2002 [36]. Dai adopted quartz chips with gold electrodes on their surface and coated them with IL to detect volatile gases. The quartz chips can be regenerated by washing with acetonitrile. Since then, ILs have been used for gas detection on the QCM platform for more than two decades. Related applications include disease diagnosis (i.e., bacteria, viruses, and protein adsorption study) [37]; manufacturing industry (i.e., flammable and toxic gases monitoring); environmental protection (i.e., vehicle gas emissions and greenhouse gases measurement); indoor air quality monitoring; and homeland security (i.e., chemical and biological warfare agents). More than 20 VOCs detected on QCM have been published in reputable journals [38]. Sensing ILs detect and identify volatile molecules in real-time on the QCM platform, during which ionic liquids show supersensitivity performance [39]. In the last decade, researchers have also actively engaged in the development and establishment of a QCM-based chemoselective gas detection platform in the laboratory and continuously improved its detection capability for various gases. The advantage of the technology is that it uses a minute amount of tailor-prepared sensing ILs to thin-coat it on the chip, which reduces the risk of irreversible damage caused by direct chemical modification on the chip and reuses the chip to greatly reduce the detection cost. This technical platform can be realized because ILs are able to make gases more readily permeable than solids and have low vapor pressure to ensure that they will not dry up on the QCM chip during detection. In addition, the high polarity, chemical stability, and excellent solubility of ILs make the envisaged detection reactions proceed smoothly. At present, the developed sensing ILs have been applied to the detection of various target gases chemoselectively, which are summarized in Figure 3 [40].
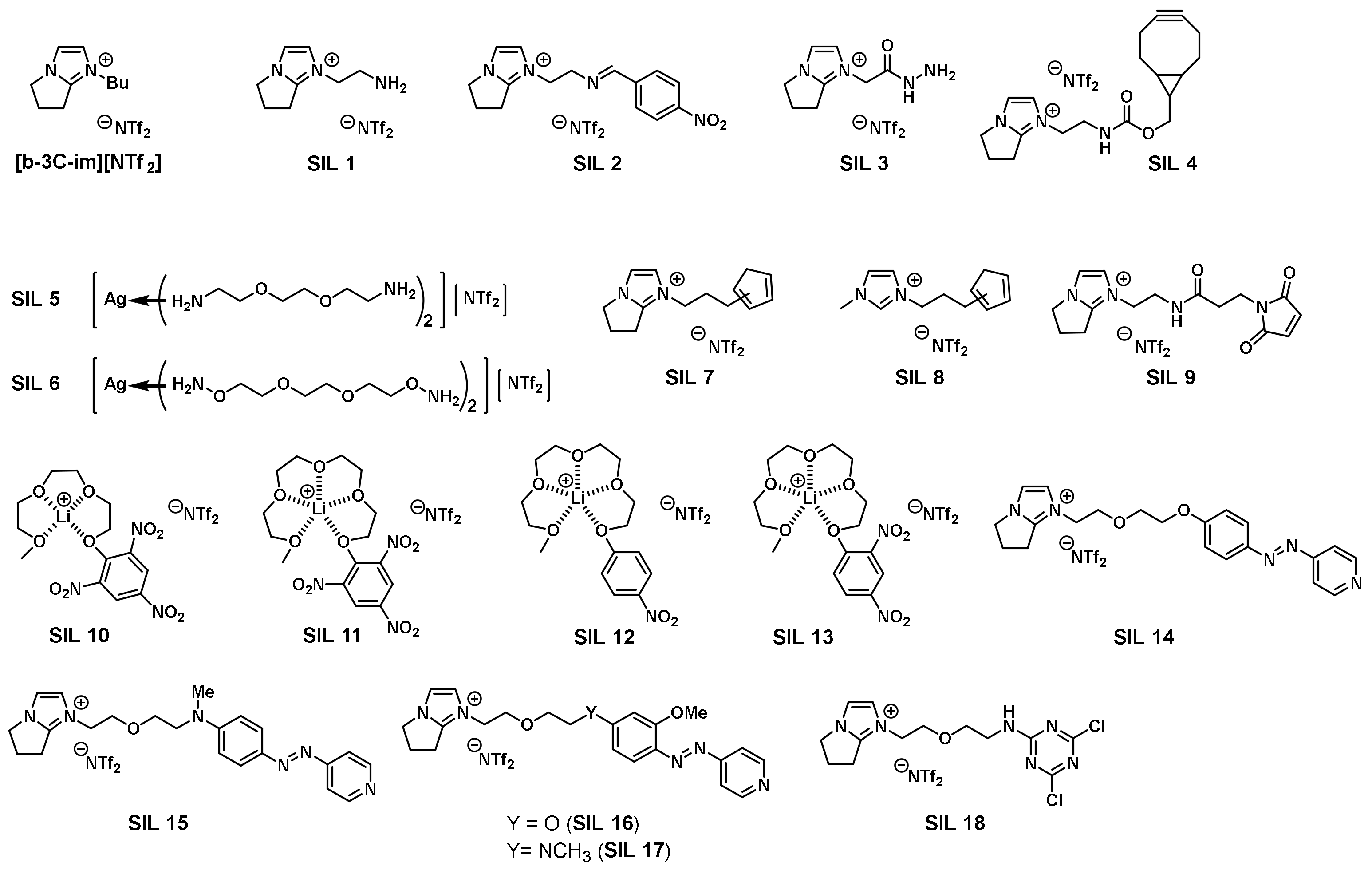
Figure 3. Structures for sensing ionic liquids were previously developed in the laboratory.
2.7. Affinity Ionic Liquids
Ionic liquids can also be combined with microextraction technology using small amounts of samples and solvents to reduce the excessive waste produced during the process; one such example is applying ILs in affinity extraction based on biomolecular recognition. Biomolecular recognition can be defined as the specific binding between receptors and ligands. There are two reasons for its selectivity: geometrical configuration and noncovalent bonding attraction. The former is the appropriate mutual recognition orientation between the receptor and ligand in geometric size and shape; the latter is the recognition of interaction between two molecules through noncovalent bonding such as hydrogen bonds or π–π interactions. ILs are designer solvents, as they are not solvents with a single function or form but can be modified and tailor-functionalized according to the requirements of the experiments. The laboratory reported an affinity ionic liquid (AIL) with molecular recognition function in 2009 [41] and developed a fast and highly efficient protein purification technology based on the affinity between biomolecules. This research uses fluorescent-tagged hexahistidine peptides FITC-(His)6-NH2 and His-tag green fluorescent protein (GFP) with AIL to establish separation and purification platforms, which can greatly improve the purification efficiency and shorten the purification duration.
Huang and coworkers introduced the structure of AIL with 1,4,7-triazacyclononane as the key affinity element, used for liquid–liquid extraction and purification of hexahistidine-tagged proteins [42]. This AIL could be readily regenerated using EDTA, followed by metal ion (Cu+2, Ni+2, and Zn+2) reincorporation. The M+2-AIL system showed high affinity toward the hexa(histidine)-tagged proteins so as to separate them from the protein mixture. In view of biological sample complexity, researchers reported the structure of crowned ILs (CILs) in 2016 [43]. Researchers synthesized a series of novel types of CILs based on the intramolecular Huisgen 1,3-dipolar [3+2] cycloaddition reaction [44]. In addition to the known high affinity of crown ether with potassium ions, 18-crown-6 can be associated with protonated organoammonium cations to form stable complexes in both the gas and liquid phases (Figure 4). Some amino acids with a primary amine on the branch chain, such as lysine, can combine with 18-crown-6 to form a stable complex [45][46]. Researchers introduced this concept into the CIL, mainly a functional IL with a bicyclic structure (Figure 4). The ring of triazolium provides this molecule with the special physical properties of IL, and crown ether ring sizes with different designs can control and influence the affinity with cations to achieve selective recognition and affinity bonding in the extraction of lysine- as well as arginine-rich peptides and proteins [43].
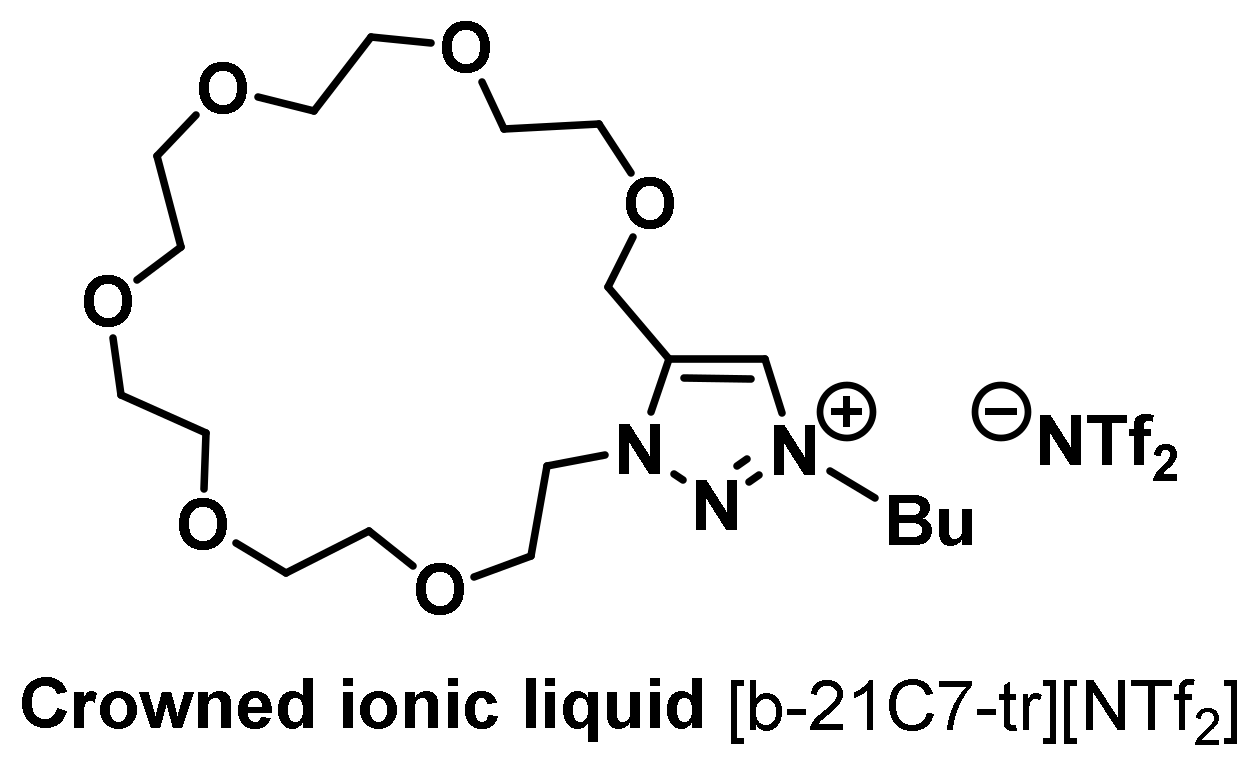
Figure 4. Structure of a crowned ionic liquid [b-21C7-tr][NTf2].
References
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556.
- Greer, A.J.; Johan Jacquemin, J.; Hardacre, C. Industrial applications of ionic liquids. Molecules 2020, 25, 5207.
- Wilkes, J.S. A short history of ionic liquids—From molten salts to neoteric solvents. Green Chem. 2002, 4, 73–80.
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706.
- Stephan Beil, S.; Markiewicz, M.; Pereira, C.S.; Stepnowski, P.; Thöming, J.; Stolte, S. Toward the proactive design of sustainable chemicals: Ionic liquids as a prime example. Chem. Rev. 2021, 121, 13132–13173.
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem. 1982, 21, 1263–1264.
- Bonhôte, P.; Dias, A.-P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg. Chem. 1996, 35, 1168–1178.
- Green, M.D.; Long, T.E. Designing imidazole-based Ionic liquids and ionic liquid monomers for emerging technologies. Polym. Rev. 2009, 49, 291–314.
- Raiguel, S.; Dehaen, W.; Binnemans, K. Stability of ionic liquids in Brønsted-basic media. Green Chem. 2020, 22, 5225–5252.
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189.
- Danush, S.; Dutta, A. Machine learning-based framework for predicting toxicity of ionic liquids. Mater. Today Proc. 2023, 72, 175–180.
- Hejazifar, M.; Lanaridi, O.; Bica-Schröder, K. Ionic liquid based microemulsions: A review. J. Mol. Liq. 2020, 303, 112264.
- Abonia, R.; Laali, K.K. Ionic liquid-mediated synthesis and functionalization of heterocyclic compounds. In Advances in Heterocyclic Chemistry; Scriven, E.F.V., Ramsden, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 128, pp. 333–431.
- Brehm, M.; Pulst, M.; Kressler, J.; Sebastiani, D. Triazolium-based ionic liquids: A novel class of cellulose solvents. J. Phys. Chem. B 2019, 123, 3994–4003.
- Nadaf, R.N.; Siddiqui, S.A.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. Room temperature ionic liquid promoted regioselective synthesis of 2-aryl benzimidazoles, benzoxazoles and benzthiazoles under ambient conditions. J. Mol. Catal. A Chem. 2004, 214, 155–160.
- Kaur, N.; Chopra, H.K. Synthesis and applications of carbohydrate based chiral ionic liquids as chiral recognition agents and organocatalysts. J. Mol. Liq. 2020, 298, 111994.
- Flieger, J.; Feder-Kubis, J.; Tatarczak-Michalewska, M. Chiral ionic liquids: Structural diversity, properties and applications in selected separation techniques. Int. J. Mol. Sci. 2020, 21, 4253.
- Bonanni, M.; Soldaini, G.; Faggi, C.; Goti, A.; Cardona, F. Novel L-tartaric acid derived pyrrolidinium cations for the synthesis of chiral ionic liquids. Synlett 2009, 5, 747–750.
- Sawant, A.D.; Raut, D.G.; Darvatkar, N.B.; Salunkhe, M.M. Recent developments of task-specific ionic liquids in organic synthesis. Green Chem. Lett. Rev. 2011, 4, 41–54.
- Fraga-Dubreuil, J.; Bazureau, J.P. Rate accelerations of 1,3-dipolar cycloaddition reactions in ionic liquids. Tetrahedron Lett. 2000, 41, 7351–7355.
- Yoshizawa, M.; Hirao, M.; Ito-Akita, K.; Ohno, H. Ion conduction in zwitterionic-type molten salts and their polymers. J. Mater. Chem. 2001, 11, 1057–1062.
- Cole, A.C.; Jensen, J.L.; Ntai, I.; Tran, K.L.T.; Weaver, K.J.; Forbes, D.C.; Davis, J.H., Jr. Novel brønsted acidic ionic liquids and their use as dual solvent-catalysts. J. Am. Chem. Soc. 2002, 124, 5962–5963.
- Liu, K.-L.; Chen, T.-U.; Cheng, T.-H.; Lin, Y.-D. Ionic Liquid and Viscose Composition. Patent TW I472545B, 11 February 2015.
- Huang, K.; Zhang, X.M.; Xu, Y.; Wu, Y.T.; Hu, X.B.; Xu, Y. Protic ionic liquids for the selective absorption of H2S from CO2: Thermodynamic analysis. AIChE J. 2014, 60, 4232–4240.
- George, G.; Bhoria, N.; AlHallaq, S.; Abdala, A.; Mittal, V. Polymer membranes for acid gas removal from natural gas. Sep. Purif. Technol. 2016, 158, 333–356.
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H., Jr. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927.
- Shukla, S.K.; Khokarale, S.G.; Bui, T.Q.; Mikkola, J.-P.T. Ionic liquids: Potential materials for carbon dioxide capture and utilization. Front. Mater. 2019, 6, 42.
- Saiki, R.K.; Gelfand, D.H.; Stoffel, S.; Scharf, S.J.; Higuchi, R.; Horn, G.T.; Mullis, K.B.; Erlich, H.A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988, 4839, 487–491.
- Zhu, H.; Zhang, H.; Xu, Y.; Laššáková, S.; Korabečná, M.; Neužil, P. PCR past, present and future. Biotechniques 2020, 69, 317–325.
- Frey, U.H.; Bachmann, H.S.; Peters, J.; Siffert, W. PCR-amplification of GC-rich regions: ‘Slowdown PCR’. Nat. Protoc. 2008, 3, 1312–1317.
- Green, M.R.; Sambrook, J. Polymerase chain reaction (PCR) amplification of GC-rich templates. Cold Spring Harb. Protoc. 2019, 2, 165–169.
- Chen, Z.; Zhang, Y. Dimethyl sulfoxide targets phage RNA polymerases to promote transcription. Biochem. Biophys. Res. Commun. 2005, 333, 664–670.
- Ventura, S.P.M.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Mara, G.; Freire, M.G.; Coutinho, J.A.P. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: Past, present, and future trends. Chem. Rev. 2017, 117, 6984–7052.
- Buljubasic, F.; Buchbauer, G. The scent of human diseases: A review on specific volatile organic compounds as diagnostic biomarkers. Flavour Fragr. J. 2015, 30, 5–25.
- Suerbrey, G. The use of quartz oscillators for weighing thin layers and for microweighing. Zeit. Phys. 1959, 155, 206–222.
- Liang, C.; Yuan, C.-Y.; Warmack, R.J.; Barnes, C.E.; Dai, S. Ionic liquids: A new class of sensing materials for detection of organic vapors based on the use of a quartz crystal microbalance. Anal. Chem. 2002, 74, 2172–2176.
- Buchatip, S.; Ananthanawat, C.; Sithigorngul, P.; Sangvanich, P.; Rengpipat, S.; Hoven, V.P. Detection of the shrimp pathogenic bacteria, Vibrio harveyi, by a quartz crystal microbalance-specific antibody based sensor. Sens. Actuators B Chem. 2010, 145, 259–264.
- Speight, R.E.; Cooper, M.A. A survey of the 2010 quartz crystal microbalance literature. J. Mol. Recognit. 2012, 25, 451–473.
- Xu, F.; Sun, L.; Huang, P.; Sun, Y.; Zheng, Q.; Zou, Y.; Chu, H.; Yan, E.; Zhang, H.; Wang, J.; et al. A pyridine vapor sensor based on metal-organic framework-modified quartz crystal microbalance. Sens. Actuators B Chem. 2018, 254, 872–877.
- Chang, A.; Li, H.-Y.; Chang, I.-N.; Chu, Y.-H. Affinity ionic liquids for chemoselective gas sensing. Molecules 2018, 23, 2380.
- Tseng, M.-C.; Tseng, M.-J.; Chu, Y.-H. Affinity ionic liquid. Chem. Commun. 2009, 48, 7503–7505.
- Ren, G.; Gong, X.; Wang, B.; Chen, Y.; Huang, J. Affinity ionic liquids for the rapid liquid–liquid extraction purification of hexahistidine tagged proteins. Sep. Purif. Technol. 2015, 146, 114–120.
- Tseng, M.-C.; Yuan, T.-C.; Li, Z.; Chu, Y.-H. Crowned ionic liquids for biomolecular interaction analysis. Anal. Chem. 2016, 88, 10811–10815.
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click chemistry for drug development and diverse chemical–biology applications. Chem. Rev. 2013, 113, 4905–4979.
- Pedersen, C.J. The discovery of crown ethers. Angew. Chem. Int. Ed. Engl. 1988, 27, 1021–1027.
- Rüdiger, V.; Schneider, H.-J.; Solov’ev, V.P.; Kazachenko, V.P.; Raevsky, O.A. Crown ether–ammonium complexes: Binding mechanisms and solvent effects. Eur. J. Org. Chem. 1999, 1999, 1847–1856.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
818
Revisions:
2 times
(View History)
Update Date:
07 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




