
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giovanna Scorrano | -- | 2316 | 2023-12-06 09:50:37 | | | |
| 2 | Catherine Yang | Meta information modification | 2316 | 2023-12-06 09:56:25 | | |
Video Upload Options
Cardiofaciocutaneous (CFC) syndrome is one of the rarest RASopathies characterized by multiple congenital ectodermal, cardiac and craniofacial abnormalities with a mild to severe ocular, gastrointestinal and neurological involvement. It is an autosomal dominant syndrome, with complete penetrance, caused by heterozygous pathogenic variants in the genes BRAF, MAP2K1/MEK1, MAP2K2/MEK2, KRAS or, rarely, YWHAZ, all part of the RAS-MAPK pathway. This pathway is a signal transduction cascade that plays a crucial role in normal cellular processes such as cell growth, proliferation, differentiation, survival, metabolism and migration. CFC syndrome overlaps with Noonan syndrome, Costello syndrome, neurofibromatosis type 1 and Legius syndrome, therefore making the diagnosis challenging.
1. Introduction
| Gene | OMIM Number | Prevalence | Inheritance | CFC Phenotypic Features |
|---|---|---|---|---|
| BRAF | *164757 | 75% |
|
Moderate to severe polymorphic seizures (+exons 11–16) 57% Moderate ID 89% Motor delay and hypotonia (unable to walk, needing support) 29% Cardiac disease 72% Greater risk of skin abnormalities Ocular hypertelorism, optic nerve hypoplasia Tumors (+melanoma, thyroid, colorectal, and ovarian cancers, benign nevi, premalignant colon polyps 8% Pulmonary stenosis 50% |
| MAP2K1 | *176872 | 25% | De novo missense heterozygous variants | Moderate to severe polymorphic seizures (+p.Y130C/H/N variant, exon 3) 61% Mild ID 84% Motor delay and hypotonia (unable to walk, needing support) 71% Cardiac disease (lower frequency, NA) Macrocephaly, high forehead, bitemporal constriction, hypoplasia of the supraorbital ridges, downslanting palpebral fissures Musculoskeletal abnormalities |
| MAP2K2 | *601263 | 25% |
|
Lower risk of seizure occurrence and less severe seizure types 30% Mild ID 25% Motor delay and hypotonia (unable to walk, needing support) 13% Cardiac disease 64% Macrocephaly, high forehead, bitemporal constriction, hypoplasia of the supraorbital ridges, downslanting palpebral fissures |
| KRAS | *190070 | 2% |
|
No epilepsy Neurodevelopmental delay Coarse face Cardiac defects |
| YWHAZ | *601288 | Rarely, NA | De novo missense heterozygous variants | Developmental delay, behavioural disorders ID Short stature Motor and speech delay Triangular facies, ptosis Seizures Feeding problems |
2. Neurological Findings
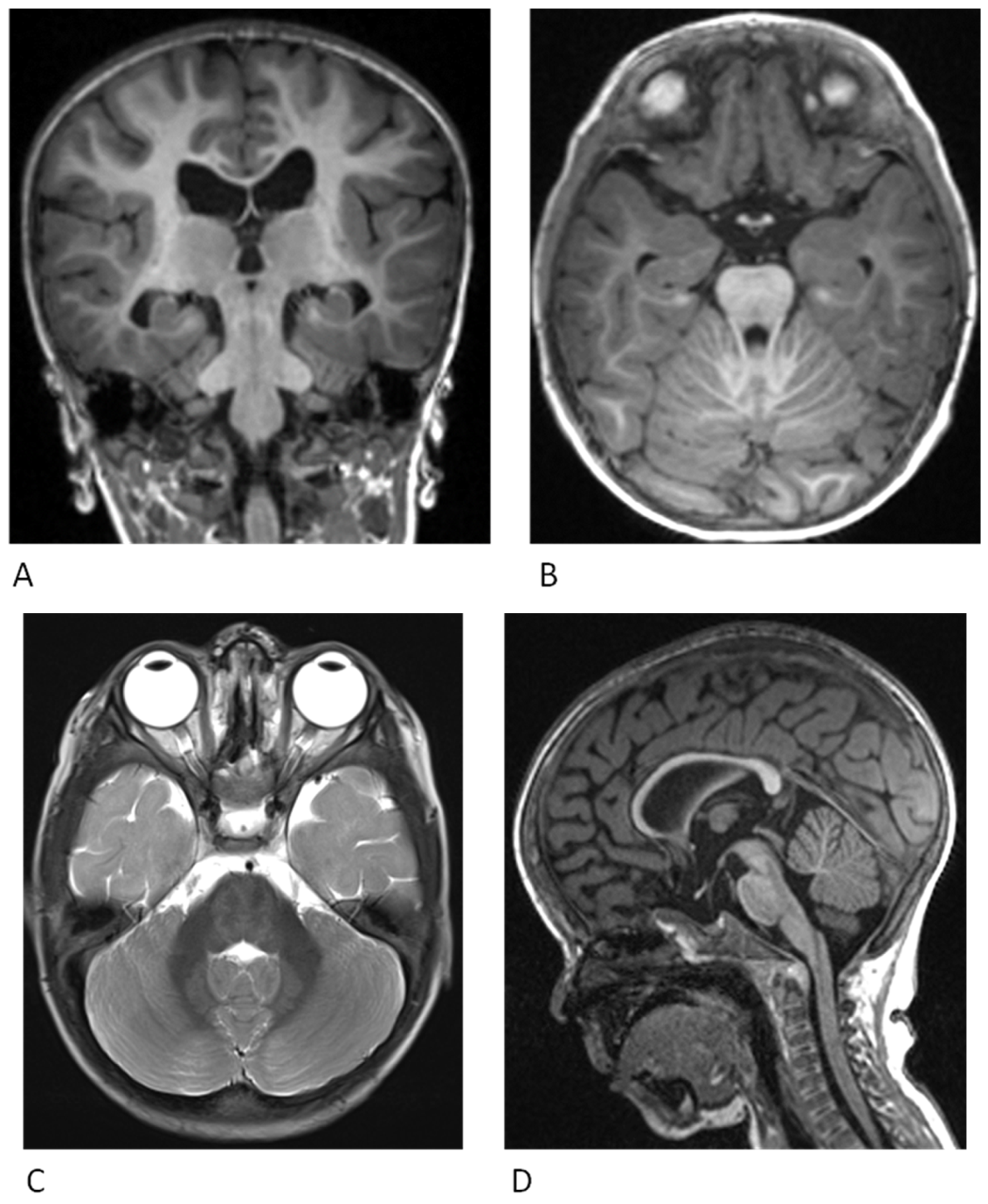
2.1. Cardiac Findings
2.2. Ectodermal Findings
2.3. Craniofacial Findings
2.4. Gastrointestinal and Growth Findings

2.5. Additional Features
References
- Hebron, K.E.; Hernandez, E.R.; Yohe, M.E. The RASopathies: From pathogenetics to therapeutics. Dis. Models Mech. 2022, 15, dmm049107.
- Abe, Y.; Aoki, Y.; Kuriyama, S.; Kawame, H.; Okamoto, N.; Kurosawa, K.; Ohashi, H.; Mizuno, S.; Ogata, T.; Kure, S.; et al. Prevalence and clinical features of Costello syndrome and cardio-facio-cutaneous syndrome in Japan: Findings from a nationwide epidemiological survey. Am. J. Med. Genet. Part A 2012, 158, 1083–1094.
- Lim, J.K.M.; Leprivier, G. The impact of oncogenic RAS on redox balance and implications for cancer development. Cell Death Dis. 2019, 10, 955.
- Zenker, M. Clinical overview on RASopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 414–424.
- Rauen, K.A. Cardiofaciocutaneous Syndrome. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993.
- Pierpont, E.I.; Kenney-Jung, D.L.; Shanley, R.; Zatkalik, A.L.; Whitmarsh, A.E.; Kroening, S.J.; Roberts, A.E.; Zenker, M. Neurologic and neurodevelopmental complications in cardiofaciocutaneous syndrome are associated with genotype: A multinational cohort study. Genet. Med. 2022, 24, 1556–1566.
- Kenney-Jung, D.L.; Rogers, D.J.; Kroening, S.J.; Zatkalik, A.L.; Whitmarsh, A.E.; Roberts, A.E.; Zenker, M.; Gambardella, M.L.; Contaldo, I.; Leoni, C.; et al. Infantile epileptic spasms syndrome in children with cardiofaciocutanous syndrome: Clinical presentation and associations with genotype. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 501–509.
- Popov, I.K.; Hiatt, S.M.; Whalen, S.; Keren, B.; Ruivenkamp, C.; van Haeringen, A.; Chen, M.-J.; Cooper, G.M.; Korf, B.R.; Chang, C. A YWHAZ Variant Associated With Cardiofaciocutaneous Syndrome Activates the RAF-ERK Pathway. Front. Physiol. 2019, 10, 388.
- Pierpont, M.E.M.; Magoulas, P.L.; Adi, S.; Kavamura, M.I.; Neri, G.; Noonan, J.; Pierpont, E.I.; Reinker, K.; Roberts, A.E.; Shankar, S.; et al. Cardio-Facio-Cutaneous Syndrome: Clinical Features, Diagnosis, and Management Guidelines. Pediatrics 2014, 134, e1149–e1162.
- Yoon, G.; Rosenberg, J.; Blaser, S.; Rauen, K.A. Neurological complications of cardio-facio-cutaneous syndrome. Dev. Med. Child Neurol. 2007, 49, 894–899.
- Aizaki, K.; Sugai, K.; Saito, Y.; Nakagawa, E.; Sasaki, M.; Aoki, Y.; Matsubara, Y. Cardio-facio-cutaneous syndrome with infantile spasms and delayed myelination. Brain Dev. 2011, 33, 166–169.
- Wakusawa, K.; Kobayashi, S.; Abe, Y.; Tanaka, S.; Endo, W.; Inui, T.; Iwaki, M.; Watanabe, S.; Togashi, N.; Nara, T.; et al. A girl with Cardio-facio-cutaneous syndrome complicated with status epilepticus and acute encephalopathy. Brain Dev. 2014, 36, 61–63.
- Adachi, M.; Abe, Y.; Aoki, Y.; Matsubara, Y. Epilepsy in RAS/MAPK syndrome: Two cases of cardio-facio-cutaneous syndrome with epileptic encephalopathy and a literature review. Seizure 2012, 21, 55–60.
- Battaglia, D.I.; Gambardella, M.L.; Veltri, S.; Contaldo, I.; Chillemi, G.; Veredice, C.; Quintiliani, M.; Leoni, C.; Onesimo, R.; Verdolotti, T.; et al. Epilepsy and BRAF Mutations: Phenotypes, Natural History and Genotype-Phenotype Correlations. Genes 2021, 12, 1316.
- Iacomino, M.; Baldassari, S.; Tochigi, Y.; Kośla, K.; Buffelli, F.; Torella, A.; Severino, M.; Paladini, D.; Mandarà, L.; Riva, A.; et al. Loss of Wwox Perturbs Neuronal Migration and Impairs Early Cortical Development. Front. Neurosci. 2020, 14, 644.
- Neuray, C.; Maroofian, R.; Scala, M.; Sultan, T.; Pai, G.S.; Mojarrad, M.; Khashab, H.E.; deHoll, L.; Yue, W.; Alsaif, H.S.; et al. Early-infantile onset epilepsy and developmental delay caused by bi-allelic GAD1 variants. Brain 2020, 143, 2388–2397.
- Manole, A.; Efthymiou, S.; O’Connor, E.; Mendes, M.I.; Jennings, M.; Maroofian, R.; Davagnanam, I.; Mankad, K.; Lopez, M.R.; Salpietro, V.; et al. De Novo and Bi-allelic Pathogenic Variants in NARS1 Cause Neurodevelopmental Delay Due to Toxic Gain-of-Function and Partial Loss-of-Function Effects. Am. J. Hum. Genet. 2020, 107, 311–324.
- Niestroj, L.-M.; Perez-Palma, E.; Howrigan, D.P.; Zhou, Y.; Cheng, F.; Saarentaus, E.; Nürnberg, P.; Stevelink, R.; Daly, M.J.; Palotie, A.; et al. Epilepsy subtype-specific copy number burden observed in a genome-wide study of 17 458 subjects. Brain 2020, 143, 2106–2118.
- Alfieri, P.; Piccini, G.; Caciolo, C.; Perrino, F.; Gambardella, M.L.; Mallardi, M.; Cesarini, L.; Leoni, C.; Leone, D.; Fossati, C.; et al. Behavioral profile in RASopathies. Am. J. Med. Genet. A 2014, 164, 934–942.
- Allanson, J.E.; Annerén, G.; Aoki, Y.; Armour, C.M.; Bondeson, M.-L.; Cave, H.; Gripp, K.W.; Kerr, B.; Nystrom, A.-M.; Sol-Church, K.; et al. Cardio-facio-cutaneous syndrome: Does genotype predict phenotype? Am. J. Med. Genet. 2011, 157, 129–135.
- Rauen, K.A.; Tidyman, W.E.; Estep, A.L.; Sampath, S.; Peltier, H.M.; Bale, S.J.; Lacassie, Y. Molecular and functional analysis of a novel MEK2 mutation in cardio-facio-cutaneous syndrome: Transmission through four generations. Am. J. Med. Genet. 2010, 152, 807–814.
- Steel, D.; Salpietro, V.; Phadke, R.; Pitt, M.; Gentile, G.; Massoud, A.; Batten, L.; Bashamboo, A.; Mcelreavey, K.; Saggar, A.; et al. Whole exome sequencing reveals a MLL de novo mutation associated with mild developmental delay and without “hairy elbows”: Expanding the phenotype of Wiedemann-Steiner syndrome. J. Genet. 2015, 94, 755–758.
- Efthymiou, S.; Salpietro, V.; Malintan, N.; Poncelet, M.; Kriouile, Y.; Fortuna, S.; De Zorzi, R.; Payne, K.; Henderson, L.B.; Cortese, A.; et al. Biallelic mutations in neurofascin cause neurodevelopmental impairment and peripheral demyelination. Brain 2019, 142, 2948–2964.
- Salpietro, V.; Efthymiou, S.; Manole, A.; Maurya, B.; Wiethoff, S.; Ashokkumar, B.; Cutrupi, M.C.; Dipasquale, V.; Manti, S.; Botia, J.A.; et al. A loss-of-function homozygous mutation in DDX59 implicates a conserved DEAD-box RNA helicase in nervous system development and function. Hum. Mutat. 2018, 39, 187–192.
- Johnson, B.; Goldberg-Strassler, D.; Gripp, K.; Thacker, M.; Leoni, C.; Stevenson, D. Function and disability in children with Costello syndrome and Cardiofaciocutaneous syndrome. Am. J. Med. Genet. A 2015, 167, 40–44.
- Papadopoulou, E.; Sifakis, S.; Sol-Church, K.; Klein-Zighelboim, E.; Stabley, D.L.; Raissaki, M.; Gripp, K.W.; Kalmanti, M. CNS imaging is a key diagnostic tool in the evaluation of patients with CFC syndrome: Two cases and literature review. Am. J. Med. Genet. A 2011, 155, 605–611.
- Weaver, K.N.; Gripp, K.W. Central nervous system involvement in individuals with RASopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 494–500.
- Delogu, A.B.; Limongelli, G.; Versacci, P.; Adorisio, R.; Kaski, J.P.; Blandino, R.; Maiolo, S.; Monda, E.; Putotto, C.; De Rosa, G.; et al. The heart in RASopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 440–451.
- Leoni, C.; Blandino, R.; Delogu, A.B.; De Rosa, G.; Onesimo, R.; Verusio, V.; Marino, M.V.; Lanza, G.A.; Rigante, D.; Tartaglia, M.; et al. Genotype-cardiac phenotype correlations in a large single-center cohort of patients affected by RASopathies: Clinical implications and literature review. Am. J. Med. Genet. A 2022, 188, 431–445.
- Hilal, N.; Chen, Z.; Chen, M.H.; Choudhury, S. RASopathies and cardiac manifestations. Front. Cardiovasc. Med. 2023, 10, 1176828.
- Dentici, M.L.; Sarkozy, A.; Pantaleoni, F.; Carta, C.; Lepri, F.; Ferese, R.; Cordeddu, V.; Martinelli, S.; Briuglia, S.; Digilio, M.C.; et al. Spectrum of MEK1 and MEK2 gene mutations in cardio-facio-cutaneous syndrome and genotype–phenotype correlations. Eur. J. Hum. Genet. 2009, 17, 733–740.
- Moss, C. RASopathies and the skin. Br. J. Dermatol. 2019, 180, 21.
- Kavamura, M.I.; Leoni, C.; Neri, G. Dermatological manifestations, management, and care in RASopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 452–458.
- Bessis, D.; Morice-Picard, F.; Bourrat, E.; Abadie, C.; Aouinti, S.; Baumann, C.; Best, M.; Bursztejn, A.-C.; Capri, Y.; Chiaverini, C.; et al. Dermatological manifestations in cardiofaciocutaneous syndrome: A prospective multicentric study of 45 mutation-positive patients. Br. J. Dermatol. 2019, 180, 172–180.
- Roberts, A.; Allanson, J.; Jadico, S.K.; Kavamura, M.I.; Noonan, J.; Opitz, J.M.; Young, T.; Neri, G. The cardiofaciocutaneous syndrome. J. Med. Genet. 2006, 43, 833–842.
- Kiuru, M.; Urban, J.; Zhu, G.; Rybak, I.; Terrell, J.R.; Qi, L.; McPherson, J.D.; Marghoob, A.A.; Rauen, K.A. RAS pathway influences the number of melanocytic nevi in cardiofaciocutaneous and Costello syndromes. J. Am. Acad. Dermatol. 2020, 82, 1091–1093.
- Leoni, C.; Guerriero, C.; Onesimo, R.; Coco, V.; Di Ruscio, C.; Acampora, A.; Esposito, I.; Romano, A.; Tartaglia, M.; Genuardi, M.; et al. Melanocytic nevi in RASopathies: Insights on dermatological diagnostic handles. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e83–e85.
- Siegel, D.H.; McKenzie, J.; Frieden, I.J.; Rauen, K.A. Dermatological findings in 61 mutation-positive individuals with cardiofaciocutaneous syndrome. Br. J. Dermatol. 2011, 164, 521–529.
- Onesimo, R.; Sforza, E.; Giorgio, V.; Viscogliosi, G.; Kuczynska, E.M.; Margiotta, G.; Perri, L.; Limongelli, D.; Proli, F.; De Rose, C.; et al. The “FEEDS (FEeding Eating Deglutition Skills)” over Time Study in Cardiofaciocutaneous Syndrome. Genes 2023, 14, 1338.
- Onesimo, R.; Giorgio, V.; Viscogliosi, G.; Sforza, E.; Kuczynska, E.; Margiotta, G.; Iademarco, M.; Proli, F.; Rigante, D.; Zampino, G.; et al. Management of nutritional and gastrointestinal issues in RASopathies: A narrative review. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 478–493.
- Leoni, C.; Romeo, D.M.; Pelliccioni, M.; Di Già, M.; Onesimo, R.; Giorgio, V.; Flex, E.; Tedesco, M.; Tartaglia, M.; Rigante, D.; et al. Musculo-skeletal phenotype of Costello syndrome and cardio-facio-cutaneous syndrome: Insights on the functional assessment status. Orphanet J. Rare Dis. 2021, 16, 43.
- Leoni, C.; Viscogliosi, G.; Onesimo, R.; Bisanti, C.; Massese, M.; Giorgio, V.; Corbo, F.; Tedesco, M.; Acampora, A.; Cipolla, C.; et al. Characterization of bone homeostasis in individuals affected by cardio-facio-cutaneous syndrome. Am. J. Med. Genet. A 2022, 188, 414–421.
- Siano, M.A.; Pivonello, R.; Salerno, M.; Falco, M.; Mauro, C.; De Brasi, D.; Klain, A.; Sestito, S.; De Luca, A.; Pinna, V.; et al. Endocrine system involvement in patients with RASopathies: A case series. Front. Endocrinol. 2022, 13, 1030398.
- Crincoli, E.; Leoni, C.; Viscogliosi, G.; Onesimo, R.; Mattei, R.; Tartaglia, M.; Catania, F.; Rizzo, S.; Zampino, G.; Salerni, A. Systematic ophthalmologic evaluation in cardio-facio-cutaneous syndrome: A genotype-endophenotype correlation. Am. J. Med. Genet. A 2023, 191, 2783–2792.
- Leoni, C.; Triumbari, E.K.A.; Vollono, C.; Onesimo, R.; Podagrosi, M.; Giorgio, V.; Kuczynska, E.; Veltri, S.; Tartaglia, M.; Zampino, G. Pain in individuals with RASopathies: Prevalence and clinical characterization in a sample of 80 affected patients. Am. J. Med. Genet. A 2019, 179, 940–947.
- Makita, Y.; Narumi, Y.; Yoshida, M.; Niihori, T.; Kure, S.; Fujieda, K.; Matsubara, Y.; Aoki, Y. Leukemia in Cardio-facio-cutaneous (CFC) syndrome: A patient with a germline mutation in BRAF proto-oncogene. J. Pediatr. Hematol. Oncol. 2007, 29, 287–290.
- Kratz, C.P.; Franke, L.; Peters, H.; Kohlschmidt, N.; Kazmierczak, B.; Finckh, U.; Bier, A.; Eichhorn, B.; Blank, C.; Kraus, C.; et al. Cancer spectrum and frequency among children with Noonan, Costello, and cardio-facio-cutaneous syndromes. Br. J. Cancer 2015, 112, 1392–1397.
- Niihori, T.; Aoki, Y.; Narumi, Y.; Neri, G.; Cavé, H.; Verloes, A.; Okamoto, N.; Hennekam, R.C.M.; Gillessen-Kaesbach, G.; Wieczorek, D.; et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat. Genet. 2006, 38, 294–296.
- Al-Rahawan, M.M.; Chute, D.J.; Sol-Church, K.; Gripp, K.W.; Stabley, D.L.; McDaniel, N.L.; Wilson, W.G.; Waldron, P.E. Hepatoblastoma and heart transplantation in a patient with cardio-facio-cutaneous syndrome. Am. J. Med. Genet. A 2007, 143, 1481–1488.
- Ney, G.; Gross, A.; Livinski, A.; Kratz, C.P.; Stewart, D.R. Cancer incidence and surveillance strategies in individuals with RASopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 530–540.




