Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luis Carlos Ramos Aguila | -- | 2153 | 2023-12-05 02:14:13 | | | |
| 2 | Rita Xu | Meta information modification | 2153 | 2023-12-05 02:27:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ramos Aguila, L.C.; Li, X.; Akutse, K.S.; Bamisile, B.S.; Sánchez Moreano, J.P.; Lie, Z.; Liu, J. Host–Parasitoid Phenology under Climate Change. Encyclopedia. Available online: https://encyclopedia.pub/entry/52349 (accessed on 07 February 2026).
Ramos Aguila LC, Li X, Akutse KS, Bamisile BS, Sánchez Moreano JP, Lie Z, et al. Host–Parasitoid Phenology under Climate Change. Encyclopedia. Available at: https://encyclopedia.pub/entry/52349. Accessed February 07, 2026.
Ramos Aguila, Luis Carlos, Xu Li, Komivi Senyo Akutse, Bamisope Steve Bamisile, Jessica Paola Sánchez Moreano, Zhiyang Lie, Juxiu Liu. "Host–Parasitoid Phenology under Climate Change" Encyclopedia, https://encyclopedia.pub/entry/52349 (accessed February 07, 2026).
Ramos Aguila, L.C., Li, X., Akutse, K.S., Bamisile, B.S., Sánchez Moreano, J.P., Lie, Z., & Liu, J. (2023, December 05). Host–Parasitoid Phenology under Climate Change. In Encyclopedia. https://encyclopedia.pub/entry/52349
Ramos Aguila, Luis Carlos, et al. "Host–Parasitoid Phenology under Climate Change." Encyclopedia. Web. 05 December, 2023.
Copy Citation
Climate change raises a serious threat to global entomofauna—the foundation of many ecosystems—by threatening species preservation and the ecosystem services they provide.
temperature
development
mismatch
asynchrony
altitude
1. Introduction
As greenhouse gas emissions increase and according to climate models, the world’s average temperature will rise by between 2.1 °C and 3.9 °C by the end of the 21st century [1][2]. Extreme heat waves, droughts, and rainfall events across regions and sectors are likely outcomes of global warming predictions [3][4], raising serious threats to global biodiversity [5][6][7][8][9][10].
Pollution, increased frequency of extreme events, as well as altered weather patterns are important drivers of insect populations [11][12][13], thus exposing them to unprecedented challenging stresses [14][15]. Increasing temperatures are the main result of global anthropogenic climate change and are disrupting interactions between herbivore–plant, predator–prey and, parasitoid–host, therefore affecting the dynamics and structure of populations and communities [16][17][18]. Additionally, the current urbanization rate and agricultural land use also threaten arthropod biodiversity and may reshape insect communities by favoring some lineages over others [19][20][21]; e.g., human landscape modification and land-use intensity (monoculture) affect host–parasitoid interactions [22] and distributions of specialist insects [23], while habitats containing patchy cropland, meadows, hedgerows, flower/grassland strips, and shelterbelts have been shown to provide greater parasitoid abundance, diversity, and parasitism rates than more simple landscape systems [24][25][26][27][28][29][30]. Furthermore, these habitats provide diverse microclimate, shelter, and structural vegetation variety that is also important for beneficial diversity and associated ecosystem services [31][32].
The presence and influence of arthropod species have significant and well-known benefits/values to human well-being in terms of the ecosystem services they provide (e.g., pollination, food security, biological control, maintenance of wider biodiversity, and ecosystem stability) and as well in achieving Sustainable Development Goals (SDGs) (e.g., crop pest and disease vectors) [33][34][35]. From the perspective of biodiversity and ecological impact, insect parasitoids are quantitatively important components of terrestrial ecosystems [36][37], because they perform a top-down control of many insect pests and consequently regulate the abundance and dynamics of their hosts [38][39][40].
Regarding host–parasitoid interactions, the life cycle of insect parasitoids consists of a larval stage (parasitic) living inside the host followed by an adult stage in which the parasitoid is free-living (Figure 1a). Parasitoids depend on other insect hosts in order to develop their offspring [41]. The adult female parasitoid deposits one egg (or more than one) inside (endoparasitoid) or attached to the host surface (ectoparasitoid); the eggs hatch into larvae, which develop by feeding on their hosts’ bodies and eventually die (Figure 1a) [42].
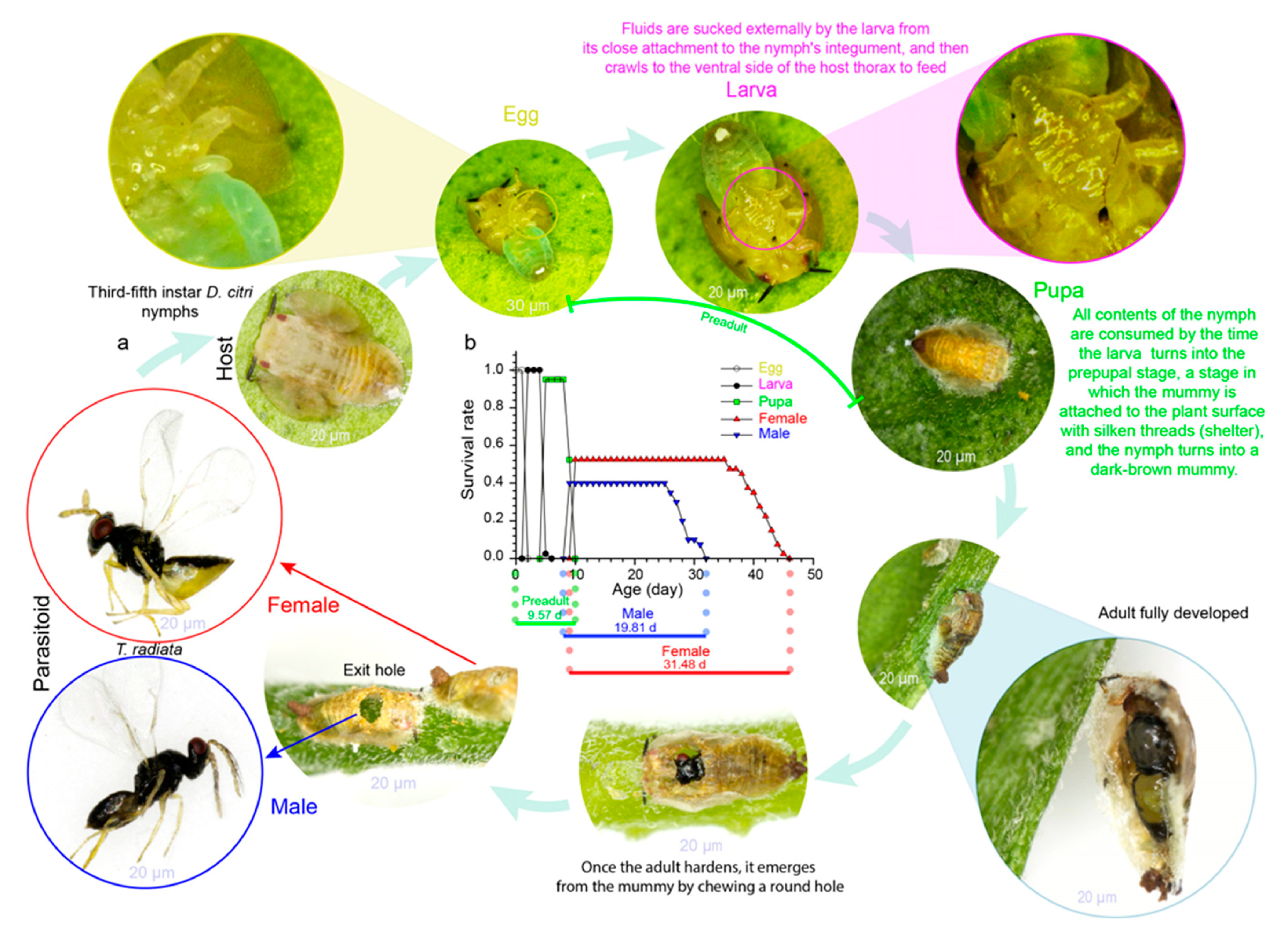
Figure 1. (a) Typical ectoparasitoid life cycle: Tamarixia radiata (Waterston) (Hymenoptera: Eulophidae) (credit: L.C.R.A., life cycle photos); (b) life-history events (phenology) of T. radiata reared at 27.5 °C.
The atmospheric temperature is intimately linked to the development and survival of parasitoid preadult/immature stages because their phenology, morphology, physiology, demography, and behavior have evolved and adapted in accordance with a specific range of thermal limits, enabling them to adapt to their surrounding environments [43]. However, it is likely that the newly predicted extreme climatic conditions will vary over time and space, thus challenging terrestrial arthropods’ life-history parameters/traits, due to the temperature-dependent nature of ectotherm activity and metabolism [44]. Nevertheless, in nature, climate changes occur over many years, decades, centuries, or longer and involve significant alterations in the averages of temperature, precipitation, wind, sunshine, etc. [45].
2. Arthropods’ Phenology and Climate Relationship
An organism’s phenology describes the timings of cyclical or seasonal biological events and how it progresses through its life cycle [46][47]: e.g., egg laying, the preadult developmental time (egg, larva, pupa), and adult longevity (female, male) (Figure 1b). In arthropod populations, the timing of life-history events is highly temperature-sensitive [48][49], and any change in temperature results in differential phenological shifts [50][51]. Currently, the ways in which these shifts might affect seasonal life cycles are increasingly being explored by ecologists [52]. So far, measures for climate change vulnerability have largely evaluated species’ responses to critical and lethal thermal limits [14][53]. This could be explained by its well-known direct effect on insect development [17][46], where warmer conditions accelerate preadult stages’ development [54][55]; conversely, low temperatures prolong arthropods’ developmental time [56].
Since insect metabolic rate is extremely dependent upon environmental temperature [57], any altered temperature regime is a critical factor influencing their population dynamics [58][59], mainly due to their limited capacity in maintaining body temperature through metabolic heat [60][61][62]; e.g., field experiments have demonstrated that high temperature has lethal impacts during the pupal stage releases of the parasitoid Telenomus podisi (Ashmead) (Hymenoptera: Platygastridae) throughout the soybean development cycle [63].
Nevertheless, in the case of parasitoids, if the ambient temperature is below the optimal temperature, increasing the temperature to close to the optimal temperature will accelerate their development [64][65][66]; however, for some species living in the tropics, the ambient temperature is near their optimal temperature (they are already living close to their thermal limits), and extreme heat waves will cause high preadult stage mortality and decrease parasitoids’ demography [43]. Furthermore, slightly warmer conditions may result in earlier adult emergence [67], benefiting some arthropod populations by increasing the number of generations per season [66], thus disrupting the relative timing of interacting species: e.g., a change in phenological synchrony between host–parasitoid interactions [5][38][68][69][70][71], affecting mismatched species’ fitness and abundance [6], disturbing ecosystem functioning [37][69][72], and ultimately leading to pest outbreaks [15][73]. For example, phenological mismatch among the cereal leaf beetle Oulema melanopus (Linnaeus) (Coleoptera: Chrysomelidae) and its associated parasitoid Tetrastichus julis (Walker) (Hymenoptera: Eulophidae) was attributed to changes in spring temperature over the years, where in warmer springs, larval phenology of O. melanopus was delayed relative to adult parasitoid activity and parasitism was reduced [74]. Also, increasing temperature reduces the window of the host Agrilus planipennis’s (Fairmaire) (Coleoptera: Buprestidae) susceptibility to Oobius agrili (Zhang and Huang) (Hymenoptera: Encyrtidae) parasitism [75]. In an experimental warming, development times of Euphydryas aurinia (Rottemburg) (Lepidoptera: Nymphalidae) were significantly affected, but not for its specialized parasitoid, Cotesia bignellii (Marshall) (Hymenoptera: Braconidae) [76].
Tropical ectotherms will be most adversely affected by climate change since their physiological optimum temperature is much closer to those at higher altitudes [77][78][79]. This implies that the sooner a certain degree of temperature is reached in this area, the higher the risk of extinction, since species will have less time to disperse naturally to track their physiological optimum climate. However, adaptive responses to new temperatures are also possible [80][81], since evidence of traits changing is strong; e.g., color variation of the body of the parasitoid Cirrospilus pictus (Nees) (Hymenoptera: Eulophidae) depends on the seasonal temperature (light individuals in spring–summer and dark individuals in autumn–winter), suggesting an ecological adaptation to climatic conditions [82]. But an explicit understanding of what underlies these changes, such as genetics or plasticity, is lacking [13]. Despite this, even within a landscape, populations and species may respond differently to climatic changes, making it difficult to identify general trends [83].
It is important to note, however, that species’ phenological shifts often do not occur at the same rate [84], and the same thermal stress can have different phenotypic and fitness effects during the various stages of an organism’s development [70][85][86]; these may consequently lead to unequal shifts in the seasonal timing [46]. For instance, recent field investigations have reported a mismatch in Torymus sinensis (Linnaeus) (Hymenoptera: Torymidae) emergence and a reduced biocontrol effectiveness of the Asian chestnut gall wasp Dryocosmus kuriphilus (Yasumatsu) (Hymenoptera: Cynipidae) as effects of warmer winter temperatures [39]. Warmer temperatures may therefore determine an earlier T. sinensis’s emergence, and by the time they emerge, fresh galls of the host are not available, resulting in a lower parasitism pressure and increasing the risk of host outbreaks [39]. In addition, climate-associated shifts in the phenology of wild bees have advanced by a mean of 10.4 ± 1.3 days and are associated with global temperature increases [87][88]. Also, climate change has been documented to be associated with shifts in autumn phenology toward later dates and spring phenology toward earlier dates [89][90]. Latitude has also been reported to alter the phenological responses between host and parasitoids [91], thereby affecting insect population abundance and range dynamics [54].
Insects have developed a seasonal timing system to measure day/night duration (photoperiod) and anticipate/coordinate their development and physiology [92][93]. This allows them to regulate their seasonal rhythms [94] and adapt their phenology to their local environment [51][95], in this manner allowing susceptible life stages to avoid unfavorable environmental conditions [96] and favoring the synchrony of insect populations with the resources they consume, which ultimately allows them to persist/survive [17][97]. However, new daylength regimes due to climate change are altering host–parasitoid interactions and community dynamics [98][99].
In addition, interactions within trophic networks have greatly influenced insect phenology [17]; in these interactions, organisms from a specific trophic level should regulate their life cycle to match those of their prey and hosts according to their level of trophic dependence [100]; otherwise, any phenological shifts have population-level consequences [101], therefore altering the already-established communities and systems function, and having an impact on the benefits and services provided by natural ecosystems.
3. Host–Parasitoid Geographical Distribution under Climate Change
A temperature limit restricts the distribution of insects; however, as a result of climate change, more suitable areas have emerged allowing species’ upslope migration (Figure 2) [66][102][103], shifting their niches to escape warming and match their current thermal preferences [51][104]. However, according to Román-Palacios and Wiens [8], niche shifts in response to climate change can only potentially reduce less than 30% of species extinction, which sparks serious concerns for the future fate of biodiversity. Agricultural pests are most likely to benefit from present and future climate change with worldwide pest proliferation, especially in temperate zones (Figure 2) [105]; e.g., warm temperatures increase population growth of a nonnative defoliator Coleophora laricella (Hübner) (Lepidoptera: Coleophoridae) and inhibit demographic responses of two imported parasitoids, Agathis pumila (Ratzeburg) (Hymenoptera: Braconidae) and Chrysocharis laricinellae (Ratzeburg) (Hymenoptera: Eulophidae). The positive response of hosts to warming might have contributed to the outbreak of C. laricella in North America [106].

Figure 2. Effects of global rising temperatures on insect distribution, species interactions, and temperate taxa.
Correlative species distribution modelling is a widely used approach for predicting the impacts of climate change on biodiversity, e.g., assessing extinction rates, estimating species distribution changes, and setting up conservation priorities [107][108]. Already, several insect taxa have shifted their distribution ranges towards higher altitudes [109][110]. However, regarding host–parasitoids, there is limited evidence of such geographical shifts and adaptations to these new climatic changes. For instance, D. citri in China has expanded significantly northward, and prediction studies revealed that this pest will move even further as a result of climate change [111]; however, using the Climate Change Experiment (CLIMEX) model, Souza et al. [112] and Aidoo et al. [113] reported that its associated natural enemy T. radiata will also move beyond its presently known native and non-native areas. Additionally, using climate change simulations, Li et al. [114] reported that three aphid species including Schizaphis graminum (Rondani), Rhopalosiphum padi (Linnaeus), and Sitobion avenae (Fabricius) (Hemiptera: Aphididae) and their associated natural enemies Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae), Episyrphus balteatus (De Geer) (Diptera: Syrphidae), and Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) will move toward higher altitudes in most regions, and as the climate warms, ladybug H. axyridis will become more effective at suppressing aphid populations. On the contrary, warming will weaken parasitoid A. gifuensis and hoverfly E. balteatus performance and survival. Also, Zhang et al. [115] reported a northward range shift of Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae) and its associated natural enemies Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae) and Dendrocopos major (Linnaeus) (Piciformes: Picidae). According to a model studied by Furlong and Zalucki [116] on the interaction between the diamondback moth Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) and its parasitoid Diadegma semiclausum (Hellén) (Hymenoptera: Ichneumonidae), the predicted temperature increases will negatively affect the parasitoid’s distribution more than its host’s. These studies suggested that warming can favor generalist predators over specialist (Hymenoptera) biocontrol agents.
A study carried out by Hódar et al. [117] reported that elevation decreased in both probability of occurrence and parasitism rate of the two main parasitoid species Ooencyrtus pityocampae (Mercet) (Hymenoptera: Encyrtidae) and Baryscapus servadeii (Domenichini) (Hymenoptera: Eulophidae) of the pine processionary moth Thaumetopoea pityocampa (Denis & Schiffermüller) (Lepidoptera: Notodontidae). Also, it is very important to consider that new host–parasitoid interactions (alternative resource species) will occur and adaptation to novel hosts is likely to increase [118][119][120][121]. In addition, with upslope range shifts, new host plants will also play a crucial role in shaping the assemblages between insect hosts and their natural enemies [122][123]. While some parasitoids are host-specific, e.g., T. radiata [124], others such as the case of parasitoids from the Eulophidae family (Hymenoptera: Chalcidoidea) parasitize alternative species living on wild vegetation during periods when their main host Phyllocnistis citrella (Stainton) (Lepidoptera: Gracillariidae) is unavailable [125]. Aphid parasitoids are also able to develop throughout the crop season on one or more host species [126]. Also, different parasitoid species can parasitize the same host; e.g., Kos et al. [127] recently reported 51 parasitoid species parasitizing the Asian chestnut gall wasp (ACGW). Other effects of global warming include (i) shifting fall migration timing in monarch butterflies Danaus plexippus (Linnaeus) (Lepidoptera: Nymphalidae) [128]; (ii) uphill shifts and warming altering mold body-size structures [129][130][131]; plant–pollinator mismatches [88][132]; and (iv) increasing herbivore consumption rates [133].
References
- Romshoo, S.A.; Bashir, J.; Rashid, I. Twenty-first century-end climate scenario of Jammu and Kashmir Himalaya, India, using ensemble climate models. Clim. Chang. 2020, 162, 1473–1491.
- Liu, P.R.; Raftery, A.E. Country-based rate of emissions reductions should increase by 80% beyond nationally determined contributions to meet the 2 °C target. Commun. Earth Environ. 2021, 2, 29.
- Schewe, J.; Gosling, S.N.; Reyer, C.; Zhao, F.; Ciais, P.; Elliott, J.; Francois, L.; Huber, V.; Lotze, H.K.; Seneviratne, S.I.; et al. State-of-the-art global models underestimate impacts from climate extremes. Nat. Commun. 2019, 10, 1005.
- Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R. Climate Change 2022: Impacts, Adaptation and Vulnerability; IPCC: Geneva, Switzerland, 2022.
- Abarca, M.; Spahn, R. Direct and indirect effects of altered temperature regimes and phenological mismatches on insect populations. Curr. Opin. Insect Sci. 2021, 47, 67–74.
- Yang, L.H.; Postema, E.G.; Hayes, T.E.; Lippey, M.K.; MacArthur-Waltz, D.J. The complexity of global change and its effects on insects. Curr. Opin. Insect Sci. 2021, 47, 90–102.
- Antão, L.H.; Bates, A.E.; Blowes, S.A.; Waldock, C.; Supp, S.R.; Magurran, A.E.; Dornelas, M.; Schipper, A.M. Temperature-related biodiversity change across temperate marine and terrestrial systems. Nat. Ecol. Evol. 2020, 4, 927–933.
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217.
- Manes, S.; Costello, M.J.; Beckett, H.; Debnath, A.; Devenish-Nelson, E.; Grey, K.-A.; Jenkins, R.; Khan, T.M.; Kiessling, W.; Krause, C.; et al. Endemism increases species’ climate change risk in areas of global biodiversity importance. Biol. Conserv. 2021, 257, 109070.
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782.
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27.
- Eggleton, P. The state of the world’s insects. Annu. Rev. Environ. Resour. 2020, 45, 61–82.
- Kellermann, V.; van Heerwaarden, B. Terrestrial insects and climate change: Adaptive responses in key traits. Physiol. Entomol. 2019, 44, 99–115.
- Pinsky, M.L.; Eikeset, A.M.; McCauley, D.J.; Payne, J.L.; Sunday, J.M. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 2019, 569, 108–111.
- Harvey, J.A.; Heinen, R.; Gols, R.; Thakur, M.P. Climate change-mediated temperature extremes and insects: From outbreaks to breakdowns. Glob. Chang. Biol. 2020, 26, 6685–6701.
- Schmitt, M.; Telusma, A.; Bigeard, E.; Guillou, L.; Alves-de-Souza, C. Temperature Affects the Biological Control of Dinoflagellates by the Generalist Parasitoid Parvilucifera rostrata. Microorganisms 2022, 10, 385.
- Damien, M.; Tougeron, K. Prey–predator phenological mismatch under climate change. Curr. Opin. Insect Sci. 2019, 35, 60–68.
- Holopainen, J.K.; Himanen, S.J.; Poppy, G.M. Climate Change and its Effects on the Chemical Ecology of Insect Parasitoids. In Chemical Ecology of Insect Parasitoids; WILEY: Hoboken, NJ, USA, 2013; pp. 168–190.
- Theodorou, P.; Radzevičiūtė, R.; Lentendu, G.; Kahnt, B.; Husemann, M.; Bleidorn, C.; Settele, J.; Schweiger, O.; Grosse, I.; Wubet, T.; et al. Urban areas as hotspots for bees and pollination but not a panacea for all insects. Nat. Commun. 2020, 11, 576.
- Grab, H.; Branstetter, M.G.; Amon, N.; Urban-Mead, K.R.; Park, M.G.; Gibbs, J.; Blitzer, E.J.; Poveda, K.; Loeb, G.; Danforth, B.N. Agriculturally dominated landscapes reduce bee phylogenetic diversity and pollination services. Science 2019, 363, 282–284.
- Ganuza, C.; Redlich, S.; Uhler, J.; Tobisch, C.; Rojas-Botero, S.; Peters, M.K.; Zhang, J.; Benjamin, C.S.; Englmeier, J.; Ewald, J. Interactive effects of climate and land use on pollinator diversity differ among taxa and scales. Sci. Adv. 2022, 8, eabm9359.
- Jonsson, M.; Buckley, H.L.; Case, B.S.; Wratten, S.D.; Hale, R.J.; Didham, R.K. Agricultural intensification drives landscape-context effects on host–parasitoid interactions in agroecosystems. J. Appl. Ecol. 2012, 49, 706–714.
- Nelson, A.E.; Forbes, A.A. Urban Land Use Decouples Plant-Herbivore-Parasitoid Interactions at Multiple Spatial Scales. PLoS ONE 2014, 9, e102127.
- Marino, P.C.; Landis, D.A. Effect of Landscape Structure on Parasitoid Diversity and Parasitism in Agroecosystems. Ecol. Appl. 1996, 6, 276–284.
- Ulina, E.S.; Rizali, A.; Manuwoto, S.; Pudjianto; Buchori, D. Does composition of tropical agricultural landscape affect parasitoid diversity and their host–parasitoid interactions? Agric. For. Entomol. 2019, 21, 318–325.
- Menalled, F.D.; Marino, P.C.; Gage, S.H.; Landis, D.A. Does agricultural landscape structure affect parasitism and parasitoid diversity? Ecol. Appl. 1999, 9, 634–641.
- Thies, C.; Tscharntke, T. Landscape Structure and Biological Control in Agroecosystems. Science 1999, 285, 893–895.
- Yang, Q.; Li, Z.; Ouyang, F.; Men, X.; Zhang, K.; Liu, M.; Guo, W.; Zhu, C.; Zhao, W.; Reddy, G.V.P.; et al. Flower strips promote natural enemies, provide efficient aphid biocontrol, and reduce insecticide requirement in cotton crops. Entomol. Gen. 2022, 43, 421–432.
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H.; et al. The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: A quantitative synthesis. Ecol. Lett. 2020, 23, 1488–1498.
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.; Jacot, K.A. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176.
- Fiedler, A.K.; Landis, D.A.; Wratten, S.D. Maximizing ecosystem services from conservation biological control: The role of habitat management. Biol. Control 2008, 45, 254–271.
- Jonsson, M.; Wratten, S.D.; Robinson, K.A.; Sam, S.A. The impact of floral resources and omnivory on a four trophic level food web. Bull. Entomol. Res. 2009, 99, 275–285.
- Dangles, O.; Casas, J. Ecosystem services provided by insects for achieving sustainable development goals. Ecosyst. Serv. 2019, 35, 109–115.
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229.
- Sharma, H.C. Climate Change Effects on Insects: Implications for Crop Protection and Food Security. J. Crop Improv. 2014, 28, 229–259.
- Forbes, A.A.; Bagley, R.K.; Beer, M.A.; Hippee, A.C.; Widmayer, H.A. Quantifying the unquantifiable: Why Hymenoptera, not Coleoptera, is the most speciose animal order. BMC Ecol. 2018, 18, 21.
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019, 5, eaax0121.
- Chidawanyika, F.; Mudavanhu, P.; Nyamukondiwa, C. Global climate change as a driver of bottom-up and top-down factors in agricultural landscapes and the fate of host-parasitoid interactions. Front. Ecol. Evol. 2019, 7, 80.
- Ferracini, C.; Pogolotti, C.; Alma, A. A mismatch in the emergence of Torymus sinensis may affect the effectiveness of this biocontrol agent? Biol. Control 2022, 174, 105029.
- Rosenheim, J.A. Higher-order predators and the regulation of insect herbivore populations. Annu. Rev. Entomol. 1998, 43, 421–447.
- Dicke, M.; Cusumano, A.; Poelman, E.H. Microbial symbionts of parasitoids. Annu. Rev. Entomol. 2020, 65, 171–190.
- Monticelli, L.S.; Bishop, J.; Desneux, N.; Gurr, G.M.; Jaworski, C.C.; McLean, A.H.C.; Thomine, E.; Vanbergen, A.J. Chapter Six —Multiple global change impacts on parasitism and biocontrol services in future agricultural landscapes. In Advances in Ecological Research; Bohan, D.A., Dumbrell, A.J., Vanbergen, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 65, pp. 245–304.
- Cañedo, V.; Dávila, W.; Carhuapoma, P.; Kroschel, J.; Kreuze, J. A temperature-dependent phenology model for Apanteles subandinus Blanchard, parasitoid of Phthorimaea operculella Zeller and Symmetrischema tangolias (Gyen). J. Appl. Entomol. 2022, 146, 424–439.
- Fitzgerald, J.L.; Stuble, K.L.; Nichols, L.M.; Diamond, S.E.; Wentworth, T.R.; Pelini, S.L.; Gotelli, N.J.; Sanders, N.J.; Dunn, R.R.; Penick, C.A. Abundance of spring-and winter-active arthropods declines with warming. Ecosphere 2021, 12, e03473.
- Zittis, G.; Almazroui, M.; Alpert, P.; Ciais, P.; Cramer, W.; Dahdal, Y.; Fnais, M.; Francis, D.; Hadjinicolaou, P.; Howari, F.; et al. Climate Change and Weather Extremes in the Eastern Mediterranean and Middle East. Rev. Geophys. 2022, 60, e2021RG000762.
- Visser, M.E.; Gienapp, P. Evolutionary and demographic consequences of phenological mismatches. Nat. Ecol. Evol. 2019, 3, 879–885.
- Cohen, J.M.; Lajeunesse, M.J.; Rohr, J.R. A global synthesis of animal phenological responses to climate change. Nat. Clim. Chang. 2018, 8, 224–228.
- Kong, J.D.; Hoffmann, A.A.; Kearney, M.R. Linking thermal adaptation and life-history theory explains latitudinal patterns of voltinism. Philos. Trans. R. Soc. B 2019, 374, 20180547.
- Mirth, C.K.; Saunders, T.E.; Amourda, C. Growing up in a changing world: Environmental regulation of development in insects. Annu. Rev. Entomol. 2021, 66, 81–99.
- Buckley, L.B. Temperature-sensitive development shapes insect phenological responses to climate change. Curr. Opin. Insect Sci. 2022, 52, 100897.
- Shah, A.A.; Dillon, M.E.; Hotaling, S.; Woods, H.A. High elevation insect communities face shifting ecological and evolutionary landscapes. Curr. Opin. Insect Sci. 2020, 41, 1–6.
- Yang, L.H. Toward a more temporally explicit framework for community ecology. Ecol. Res. 2020, 35, 445–462.
- Nanga, S.N.; Kekeunou, S.; Kuate, A.F.; Fiaboe, K.K.M.; Kenfak, M.A.D.; Tonnang, H.E.; Gnanvossou, D.; Djiéto-Lordon, C.; Hanna, R. Temperature-dependent phenology of the parasitoid Fopius arisanus on the host Bactrocera dorsalis. J. Therm. Biol. 2021, 100, 103031.
- Macgregor, C.J.; Thomas, C.D.; Roy, D.B.; Beaumont, M.A.; Bell, J.R.; Brereton, T.; Bridle, J.R.; Dytham, C.; Fox, R.; Gotthard, K.; et al. Climate-induced phenology shifts linked to range expansions in species with multiple reproductive cycles per year. Nat. Commun. 2019, 10, 4455.
- Teder, T. Phenological responses to climate warming in temperate moths and butterflies: Species traits predict future changes in voltinism. Oikos 2020, 129, 1051–1060.
- Ramos Aguila, L.C.; Hussain, M.; Huang, W.; Lei, L.; Bamisile, B.S.; Wang, F.; Chi, H.; Wang, L. Temperature-Dependent Demography and Population Projection of Tamarixia radiata (Hymenoptera: Eulophidea) reared on Diaphorina citri (Hemiptera: Liviidae). J. Econ. Entomol. 2020, 113, 55–63.
- Neven, L.G. Physiological responses of insects to heat. Postharvest Biol. Technol. 2000, 21, 103–111.
- Govindan, B.N.; Hutchison, W.D. Influence of Temperature on Age-Stage, Two-Sex Life Tables for a Minnesota-Acclimated Population of the Brown Marmorated Stink Bug (Halyomorpha halys). Insects 2020, 11, 108.
- Du Plessis, H.; Schlemmer, M.L.; Van den Berg, J. The Effect of Temperature on the Development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 228.
- Burraco, P.; Orizaola, G.; Monaghan, P.; Metcalfe, N.B. Climate change and ageing in ectotherms. Glob. Chang. Biol. 2020, 26, 5371–5381.
- Huey, R.B.; Kingsolver, J.G. Climate warming, resource availability, and the metabolic meltdown of ectotherms. Am. Nat. 2019, 194, E140–E150.
- Hill, S.J.; Silcocks, S.C.; Andrew, N.R. Impacts of temperature on metabolic rates of adult Extatosoma tiaratum reared on different host plant species. Physiol. Entomol. 2020, 45, 7–15.
- Braz, É.C.; Bueno, A.d.F.; Colombo, F.C.; de Queiroz, A.P. Temperature impact on Telenomus podisi emergence in field releases of unprotected and encapsulated parasitoid pupae. Neotrop. Entomol. 2021, 50, 462–469.
- Ramos Aguila, L.C.; Atlihan, R.; Ashraf, H.J.; Keppanan, R.; Lei, L.; Bamisile, B.S.; Cerda, H.; Wang, L. Temperature-Dependent Biological Control Effectiveness of Tamarixia radiata (Hymenoptera: Eulophidea) under Laboratory Conditions. J. Econ. Entomol. 2021, 114, 2009–2017.
- Zhang, Y.B.; Zhang, G.F.; Liu, W.X.; Wan, F.H. Variable temperatures across different stages have novel effects on behavioral response and population viability in a host-feeding parasitoid. Sci. Rep. 2019, 9, 2202.
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020, 18, 141–150.
- Gutiérrez, D.; Wilson, R.J. Intra-and interspecific variation in the responses of insect phenology to climate. J. Anim. Ecol. 2021, 90, 248–259.
- Kharouba, H.M.; Ehrlén, J.; Gelman, A.; Bolmgren, K.; Allen, J.M.; Travers, S.E.; Wolkovich, E.M. Global shifts in the phenological synchrony of species interactions over recent decades. Proc. Natl. Acad. Sci. USA 2018, 115, 5211–5216.
- Patterson, T.A.; Grundel, R.; Dzurisin, J.D.; Knutson, R.L.; Hellmann, J.J. Evidence of an extreme weather-induced phenological mismatch and a local extirpation of the endangered Karner blue butterfly. Conserv. Sci. Pract. 2020, 2, e147.
- Moore, M.E.; Hill, C.A.; Kingsolver, J.G. Developmental timing of extreme temperature events (heat waves) disrupts host–parasitoid interactions. Ecol. Evol. 2022, 12, e8618.
- Wetherington, M.T.; Jennings, D.E.; Shrewsbury, P.M.; Duan, J.J. Climate variation alters the synchrony of host–parasitoid interactions. Ecol. Evol. 2017, 7, 8578–8587.
- Garretson, A.C.; Feldsine, N.; Napoli, M.; Long, E.C.; Forkner, R.E. Networks of Phenological Synchrony Reveal a Highly Interconnected Ecosystem and Potential Vulnerability to Climate-Driven Mismatches. bioRxiv 2022.
- de Sassi, C.; Tylianakis, J.M. Climate Change Disproportionately Increases Herbivore over Plant or Parasitoid Biomass. PLoS ONE 2012, 7, e40557.
- Evans, E.W.; Carlile, N.R.; Innes, M.B.; Pitigala, N. Warm springs reduce parasitism of the cereal leaf beetle through phenological mismatch. J. Appl. Entomol. 2013, 137, 383–391.
- Duan, J.J.; Jennings, D.E.; Williams, D.C.; Larson, K.M. Patterns of parasitoid host utilization and development across a range of temperatures: Implications for biological control of an invasive forest pest. BioControl 2014, 59, 659–669.
- Klapwijk, M.J.; GrÖBler, B.C.; Ward, K.; Wheeler, D.; Lewis, O.T. Influence of experimental warming and shading on host–parasitoid synchrony. Glob. Chang. Biol. 2010, 16, 102–112.
- Johansson, F.; Orizaola, G.; Nilsson-Örtman, V. Temperate insects with narrow seasonal activity periods can be as vulnerable to climate change as tropical insect species. Sci. Rep. 2020, 10, 8822.
- Rajpurohit, S.; Solanki, P.S.; Mayekar, H.V.; Arya, H.; Aradhya, R.; Suravajhala, P.; Loeschcke, V. Tropical high-altitude insects show limited capacity to handle high temperatures. bioRxiv 2022.
- Parr, C.L.; Bishop, T.R. The response of ants to climate change. Glob. Chang. Biol. 2022, 28, 3188–3205.
- Both, C.; Van Asch, M.; Bijlsma, R.G.; Van Den Burg, A.B.; Visser, M.E. Climate change and unequal phenological changes across four trophic levels: Constraints or adaptations? J. Anim. Ecol. 2009, 78, 73–83.
- Thomson, L.J.; Macfadyen, S.; Hoffmann, A.A. Predicting the effects of climate change on natural enemies of agricultural pests. Biol. Control 2010, 52, 296–306.
- Lo Pinto, M.; Guarino, S.; Agrò, A. Evidence of Seasonal Variation in Body Color in Adults of the Parasitoid Cirrospilus pictus (Hymenoptera: Eulophidae) in Sicily, Italy. Insects 2023, 14, 90.
- Nice, C.C.; Forister, M.L.; Harrison, J.G.; Gompert, Z.; Fordyce, J.A.; Thorne, J.H.; Waetjen, D.P.; Shapiro, A.M. Extreme heterogeneity of population response to climatic variation and the limits of prediction. Glob. Chang. Biol. 2019, 25, 2127–2136.
- Nufio, C.R.; Buckley, L.B. Grasshopper phenological responses to climate gradients, variability, and change. Ecosphere 2019, 10, e02866.
- Moghadam, N.N.; Ketola, T.; Pertoldi, C.; Bahrndorff, S.; Kristensen, T.N. Heat hardening capacity in Drosophila melanogaster is life stage-specific and juveniles show the highest plasticity. Biol. Lett. 2019, 15, 20180628.
- Kingsolver, J.G.; Buckley, L.B. Ontogenetic variation in thermal sensitivity shapes insect ecological responses to climate change. Curr. Opin. Insect Sci. 2020, 41, 17–24.
- Bartomeus, I.; Ascher, J.S.; Wagner, D.; Danforth, B.N.; Colla, S.; Kornbluth, S.; Winfree, R. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20645–20649.
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-Pollinator Interactions over 120 Years: Loss of Species, Co-Occurrence, and Function. Science 2013, 339, 1611–1615.
- Radchuk, V.; Reed, T.; Teplitsky, C.; Van De Pol, M.; Charmantier, A.; Hassall, C.; Adamík, P.; Adriaensen, F.; Ahola, M.P.; Arcese, P. Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 2019, 10, 3109.
- Senior, V.L.; Evans, L.C.; Leather, S.R.; Oliver, T.H.; Evans, K.L. Phenological responses in a sycamore–aphid–parasitoid system and consequences for aphid population dynamics: A 20 year case study. Glob. Chang. Biol. 2020, 26, 2814–2828.
- Abarca, M.; Lill, J.T. Latitudinal variation in the phenological responses of eastern tent caterpillars and their egg parasitoids. Ecol. Entomol. 2019, 44, 50–61.
- Des Marteaux, L.; Xi, J.; Mano, G.; Goto, S.G. Circadian clock outputs regulating insect photoperiodism: A potential role for glutamate transporter. Biochem. Biophys. Res. Commun. 2022, 590, 186.
- Petrice, T.R.; Bauer, L.S.; Miller, D.L.; Poland, T.M.; Ravlin, F.W. A Phenology Model for Simulating Oobius agrili (Hymenoptera: Encyrtidae) Seasonal Voltinism and Synchrony with Emerald Ash Borer Oviposition. Environ. Entomol. 2020, 50, 280–292.
- Goto, S.G. Photoperiodic time measurement, photoreception, and circadian clocks in insect photoperiodism. Appl. Entomol. Zool. 2022, 57, 193–212.
- Kingsolver, J.G.; Buckley, L.B. How do phenology, plasticity, and evolution determine the fitness consequences of climate change for montane butterflies? Evol. Appl. 2018, 11, 1231–1244.
- Renner, S.S.; Zohner, C.M. Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 165–182.
- Schenk, M.; Krauss, J.; Holzschuh, A. Desynchronizations in bee–plant interactions cause severe fitness losses in solitary bees. J. Anim. Ecol. 2018, 87, 139–149.
- Kehoe, R.C.; Cruse, D.; Sanders, D.; Gaston, K.J.; van Veen, F.F. Shifting daylength regimes associated with range shifts alter aphid-parasitoid community dynamics. Ecol. Evol. 2018, 8, 8761–8769.
- Kehoe, R.; Sanders, D.; Cruse, D.; Silk, M.; Gaston, K.J.; Bridle, J.R.; van Veen, F. Longer photoperiods through range shifts and artificial light lead to a destabilizing increase in host–parasitoid interaction strength. J. Anim. Ecol. 2020, 89, 2508–2516.
- Tougeron, K.; Brodeur, J.; Le Lann, C.; van Baaren, J. How climate change affects the seasonal ecology of insect parasitoids. Ecol. Entomol. 2020, 45, 167–181.
- Iler, A.M.; CaraDonna, P.J.; Forrest, J.R.; Post, E. Demographic consequences of phenological shifts in response to climate change. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 221–245.
- McCain, C.M.; Garfinkel, C.F. Climate change and elevational range shifts in insects. Curr. Opin. Insect Sci. 2021, 47, 111–118.
- Vitasse, Y.; Ursenbacher, S.; Klein, G.; Bohnenstengel, T.; Chittaro, Y.; Delestrade, A.; Monnerat, C.; Rebetez, M.; Rixen, C.; Strebel, N. Phenological and elevational shifts of plants, animals and fungi under climate change in the European Alps. Biol. Rev. 2021, 96, 1816–1835.
- Jeffs, C.T.; Lewis, O.T. Effects of climate warming on host–parasitoid interactions. Ecol. Entomol. 2013, 38, 209–218.
- Schneider, L.; Rebetez, M.; Rasmann, S. The effect of climate change on invasive crop pests across biomes. Curr. Opin. Insect Sci. 2022, 50, 100895.
- Ward, S.F.; Aukema, B.H.; Fei, S.; Liebhold, A.M. Warm temperatures increase population growth of a nonnative defoliator and inhibit demographic responses by parasitoids. Ecology 2020, 101, e03156.
- Evans, T.G.; Diamond, S.E.; Kelly, M.W. Mechanistic species distribution modelling as a link between physiology and conservation. Conserv. Physiol. 2015, 3, cov056.
- Porfirio, L.L.; Harris, R.M.B.; Lefroy, E.C.; Hugh, S.; Gould, S.F.; Lee, G.; Bindoff, N.L.; Mackey, B. Improving the Use of Species Distribution Models in Conservation Planning and Management under Climate Change. PLoS ONE 2014, 9, e113749.
- Burke, J.L.; Bohlmann, J.; Carroll, A.L. Consequences of distributional asymmetry in a warming environment: Invasion of novel forests by the mountain pine beetle. Ecosphere 2017, 8, e01778.
- Keret, N.M.; Mutanen, M.J.; Orell, M.I.; Itämies, J.H.; Välimäki, P.M. Climate change-driven elevational changes among boreal nocturnal moths. Oecologia 2020, 192, 1085–1098.
- Wang, R.; Yang, H.; Wang, M.; Zhang, Z.; Huang, T.; Wen, G.; Li, Q. Predictions of potential geographical distribution of Diaphorina citri (Kuwayama) in China under climate change scenarios. Sci. Rep. 2020, 10, 9202.
- Souza, P.G.C.; Aidoo, O.F.; Farnezi, P.K.B.; Heve, W.K.; Júnior, P.A.S.; Picanço, M.C.; Ninsin, K.D.; Ablormeti, F.K.; Shah, M.A.; Siddiqui, S.A.; et al. Tamarixia radiata global distribution to current and future climate using the climate change experiment (CLIMEX) model. Sci. Rep. 2023, 13, 1823.
- Aidoo, O.F.; Souza, P.G.C.; Silva, R.S.; Júnior, P.A.S.; Picanço, M.C.; Heve, W.K.; Duker, R.Q.; Ablormeti, F.K.; Sétamou, M.; Borgemeister, C. Modeling climate change impacts on potential global distribution of Tamarixia radiata Waterston (Hymenoptera: Eulophidae). Sci. Total Environ. 2023, 864, 160962.
- Li, D.; Li, Z.; Liu, Z.; Yang, Y.; Khoso, A.G.; Wang, L.; Liu, D. Climate change simulations revealed potentially drastic shifts in insect community structure and crop yields in China’s farmland. J. Pest Sci. 2022, 96, 55–69.
- Zhang, Q.C.; Wang, J.G.; Lei, Y.H. Predicting Distribution of the Asian Longhorned Beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae) and Its Natural Enemies in China. Insects 2022, 13, 687.
- Furlong, M.J.; Zalucki, M.P. Climate change and biological control: The consequences of increasing temperatures on host–parasitoid interactions. Curr. Opin. Insect Sci. 2017, 20, 39–44.
- Hódar, J.A.; Cayuela, L.; Heras, D.; Pérez-Luque, A.J.; Torres-Muros, L. Expansion of elevational range in a forest pest: Can parasitoids track their hosts? Ecosphere 2021, 12, e03476.
- Fernandez Goya, L.; Lanteri, A.A.; Confalonieri, V.A.; Rodriguero, M.S. New host-parasitoid interactions in Naupactus cervinus (Coleoptera, Curculionidae) raise the question of Wolbachia horizontal transmission. Symbiosis 2022, 86, 325–336.
- Cornell, H.V.; Hawkins, B.A. Accumulation of Native Parasitoid Species on Introduced Herbivores: A Comparison of Hosts as Natives and Hosts as Invaders. Am. Nat. 1993, 141, 847–865.
- Godfray, H.C.J.; David, J.L.A.; Nash, D.R.; Lawton, J.H. The Recruitment of Parasitoid Species to Two Invading Herbivores. J. Anim. Ecol. 1995, 64, 393–402.
- Grabenweger, G.; Kehrli, P.; Zweimüller, I.; Augustin, S.; Avtzis, N.; Bacher, S.; Freise, J.; Girardoz, S.; Guichard, S.; Heitland, W.; et al. Temporal and spatial variations in the parasitoid complex of the horse chestnut leafminer during its invasion of Europe. Biol. Invasions 2010, 12, 2797–2813.
- Badillo-Montaño, R.; Amancio, G.; Falcón-Brindis, A.; León-Cortés, J.L.; Von Thaden, J.; Dzul-Cauich, F. Trophic host-parasitoid interactions of two Neotropical butterfly species in southeastern Mexico. Int. J. Trop. Insect Sci. 2022, 42, 1865–1875.
- Opedal, Ø.H.; Ovaskainen, O.; Saastamoinen, M.; Laine, A.L.; van Nouhuys, S. Host-plant availability drives the spatiotemporal dynamics of interacting metapopulations across a fragmented landscape. Ecology 2020, 101, e03186.
- Hoddle, M.S.; Hoddle, C.D. Classical biological control of Asian citrus psyllid with Tamarixia radiata in urban Southern California. Citrograph 2013, 4, 52–58.
- Massa, B.; Rizzo, M.; Caleca, V. Natural alternative hosts of Eulophidae (Hymenoptera Chalcidoidea) parasitoids of the Citrus Leafminer Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) in the Mediterranean Basin. J. Hym. Res. 2001, 10, 91–100.
- Lumbierres, B.; Starý, P.; Pons, X. Seasonal parasitism of cereal aphids in a Mediterranean arable crop system. J. Pest Sci. 2007, 80, 125–130.
- Kos, K.; Lacković, N.; Melika, G.; Matošević, D. Diversity and surge in abundance of native parasitoid communities prior to the onset of Torymus sinensis on the Asian chestnut gall wasp (Dryocosmus kuriphilus) in Slovenia, Croatia and Hungary. J. For. Res. 2021, 32, 1327–1336.
- Culbertson, K.A.; Garland, M.S.; Walton, R.K.; Zemaitis, L.; Pocius, V.M. Long-term monitoring indicates shifting fall migration timing in monarch butterflies (Danaus plexippus). Glob. Chang. Biol. 2022, 28, 727–738.
- Wu, C.H.; Holloway, J.D.; Hill, J.K.; Thomas, C.D.; Chen, I.; Ho, C.K. Reduced body sizes in climate-impacted Borneo moth assemblages are primarily explained by range shifts. Nat. Commun. 2019, 10, 4612.
- Gérard, M.; Martinet, B.; Maebe, K.; Marshall, L.; Smagghe, G.; Vereecken, N.J.; Vray, S.; Rasmont, P.; Michez, D. Shift in size of bumblebee queens over the last century. Glob. Chang. Biol. 2020, 26, 1185–1195.
- Tscholl, T.; Nachman, G.; Spangl, B.; Walzer, A. Heat waves affect prey and predators differently via developmental plasticity: Who may benefit most from global warming? Pest Manag. Sci. 2022, 78, 1099–1108.
- Gérard, M.; Vanderplanck, M.; Wood, T.; Michez, D. Global warming and plant–pollinator mismatches. Emerg. Top. Life Sci. 2020, 4, 77–86.
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate change alters plant–herbivore interactions. New Phytol. 2021, 229, 1894–1910.
More
Information
Subjects:
Biodiversity Conservation
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
804
Revisions:
2 times
(View History)
Update Date:
05 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




