Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Luis Carlos Ramos Aguila and Version 2 by Rita Xu.
Climate change raises a serious threat to global entomofauna—the foundation of many ecosystems—by threatening species preservation and the ecosystem services they provide.
- temperature
- development
- mismatch
- asynchrony
- altitude
1. Introduction
As greenhouse gas emissions increase and according to climate models, the world’s average temperature will rise by between 2.1 °C and 3.9 °C by the end of the 21st century [1][2][1,2]. Extreme heat waves, droughts, and rainfall events across regions and sectors are likely outcomes of global warming predictions [3][4][3,4], raising serious threats to global biodiversity [5][6][7][8][9][10][5,6,7,8,9,10].
Pollution, increased frequency of extreme events, as well as altered weather patterns are important drivers of insect populations [11][12][13][11,12,13], thus exposing them to unprecedented challenging stresses [14][15][14,15]. Increasing temperatures are the main result of global anthropogenic climate change and are disrupting interactions between herbivore–plant, predator–prey and, parasitoid–host, therefore affecting the dynamics and structure of populations and communities [16][17][18][16,17,18]. Additionally, the current urbanization rate and agricultural land use also threaten arthropod biodiversity and may reshape insect communities by favoring some lineages over others [19][20][21][19,20,21]; e.g., human landscape modification and land-use intensity (monoculture) affect host–parasitoid interactions [22] and distributions of specialist insects [23], while habitats containing patchy cropland, meadows, hedgerows, flower/grassland strips, and shelterbelts have been shown to provide greater parasitoid abundance, diversity, and parasitism rates than more simple landscape systems [24][25][26][27][28][29][30][24,25,26,27,28,29,30]. Furthermore, these habitats provide diverse microclimate, shelter, and structural vegetation variety that is also important for beneficial diversity and associated ecosystem services [31][32][31,32].
The presence and influence of arthropod species have significant and well-known benefits/values to human well-being in terms of the ecosystem services they provide (e.g., pollination, food security, biological control, maintenance of wider biodiversity, and ecosystem stability) and as well in achieving Sustainable Development Goals (SDGs) (e.g., crop pest and disease vectors) [33][34][35][33,34,35]. From the perspective of biodiversity and ecological impact, insect parasitoids are quantitatively important components of terrestrial ecosystems [36][37][36,37], because they perform a top-down control of many insect pests and consequently regulate the abundance and dynamics of their hosts [38][39][40][38,39,40].
Regarding host–parasitoid interactions, the life cycle of insect parasitoids consists of a larval stage (parasitic) living inside the host followed by an adult stage in which the parasitoid is free-living (Figure 1a). Parasitoids depend on other insect hosts in order to develop their offspring [41]. The adult female parasitoid deposits one egg (or more than one) inside (endoparasitoid) or attached to the host surface (ectoparasitoid); the eggs hatch into larvae, which develop by feeding on their hosts’ bodies and eventually die (Figure 1a) [42].
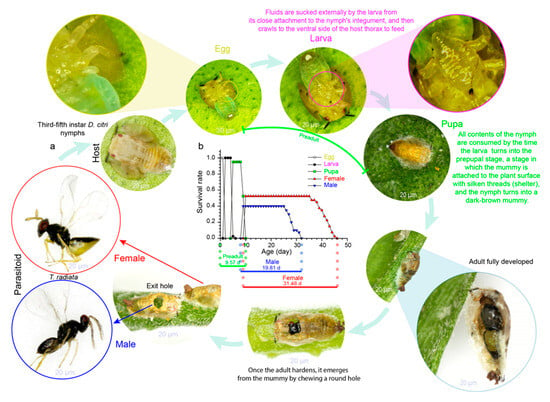
Figure 1. (a) Typical ectoparasitoid life cycle: Tamarixia radiata (Waterston) (Hymenoptera: Eulophidae) (credit: L.C.R.A., life cycle photos); (b) life-history events (phenology) of T. radiata reared at 27.5 °C.
The atmospheric temperature is intimately linked to the development and survival of parasitoid preadult/immature stages because their phenology, morphology, physiology, demography, and behavior have evolved and adapted in accordance with a specific range of thermal limits, enabling them to adapt to their surrounding environments [43][44]. However, it is likely that the newly predicted extreme climatic conditions will vary over time and space, thus challenging terrestrial arthropods’ life-history parameters/traits, due to the temperature-dependent nature of ectotherm activity and metabolism [44][45]. Nevertheless, in nature, climate changes occur over many years, decades, centuries, or longer and involve significant alterations in the averages of temperature, precipitation, wind, sunshine, etc. [45][46].
2. Arthropods’ Phenology and Climate Relationship
An organism’s phenology describes the timings of cyclical or seasonal biological events and how it progresses through its life cycle [46][47][47,48]: e.g., egg laying, the preadult developmental time (egg, larva, pupa), and adult longevity (female, male) (Figure 1b). In arthropod populations, the timing of life-history events is highly temperature-sensitive [48][49][49,50], and any change in temperature results in differential phenological shifts [50][51][51,52]. Currently, the ways in which these shifts might affect seasonal life cycles are increasingly being explored by ecologists [52][53]. So far, measures for climate change vulnerability have largely evaluated species’ responses to critical and lethal thermal limits [14][53][14,54]. This could be explained by its well-known direct effect on insect development [17][46][17,47], where warmer conditions accelerate preadult stages’ development [54][55][55,56]; conversely, low temperatures prolong arthropods’ developmental time [56][43]. Since insect metabolic rate is extremely dependent upon environmental temperature [57], any altered temperature regime is a critical factor influencing their population dynamics [58][59][58,59], mainly due to their limited capacity in maintaining body temperature through metabolic heat [60][61][62][60,61,62]; e.g., field experiments have demonstrated that high temperature has lethal impacts during the pupal stage releases of the parasitoid Telenomus podisi (Ashmead) (Hymenoptera: Platygastridae) throughout the soybean development cycle [63]. Nevertheless, in the case of parasitoids, if the ambient temperature is below the optimal temperature, increasing the temperature to close to the optimal temperature will accelerate their development [64][65][66][64,65,66]; however, for some species living in the tropics, the ambient temperature is near their optimal temperature (they are already living close to their thermal limits), and extreme heat waves will cause high preadult stage mortality and decrease parasitoids’ demography [43][44]. Furthermore, slightly warmer conditions may result in earlier adult emergence [67], benefiting some arthropod populations by increasing the number of generations per season [66], thus disrupting the relative timing of interacting species: e.g., a change in phenological synchrony between host–parasitoid interactions [5][38][68][69][70][71][5,38,68,69,70,71], affecting mismatched species’ fitness and abundance [6], disturbing ecosystem functioning [37][69][72][37,69,72], and ultimately leading to pest outbreaks [15][73][15,73]. For example, phenological mismatch among the cereal leaf beetle Oulema melanopus (Linnaeus) (Coleoptera: Chrysomelidae) and its associated parasitoid Tetrastichus julis (Walker) (Hymenoptera: Eulophidae) was attributed to changes in spring temperature over the years, where in warmer springs, larval phenology of O. melanopus was delayed relative to adult parasitoid activity and parasitism was reduced [74]. Also, increasing temperature reduces the window of the host Agrilus planipennis’s (Fairmaire) (Coleoptera: Buprestidae) susceptibility to Oobius agrili (Zhang and Huang) (Hymenoptera: Encyrtidae) parasitism [75]. In an experimental warming, development times of Euphydryas aurinia (Rottemburg) (Lepidoptera: Nymphalidae) were significantly affected, but not for its specialized parasitoid, Cotesia bignellii (Marshall) (Hymenoptera: Braconidae) [76]. Tropical ectotherms will be most adversely affected by climate change since their physiological optimum temperature is much closer to those at higher altitudes [77][78][79][77,78,79]. This implies that the sooner a certain degree of temperature is reached in this area, the higher the risk of extinction, since species will have less time to disperse naturally to track their physiological optimum climate. However, adaptive responses to new temperatures are also possible [80][81][80,81], since evidence of traits changing is strong; e.g., color variation of the body of the parasitoid Cirrospilus pictus (Nees) (Hymenoptera: Eulophidae) depends on the seasonal temperature (light individuals in spring–summer and dark individuals in autumn–winter), suggesting an ecological adaptation to climatic conditions [82]. But an explicit understanding of what underlies these changes, such as genetics or plasticity, is lacking [13]. Despite this, even within a landscape, populations and species may respond differently to climatic changes, making it difficult to identify general trends [83]. It is important to note, however, that species’ phenological shifts often do not occur at the same rate [84], and the same thermal stress can have different phenotypic and fitness effects during the various stages of an organism’s development [70][85][86][70,85,86]; these may consequently lead to unequal shifts in the seasonal timing [46][47]. For instance, recent field investigations have reported a mismatch in Torymus sinensis (Linnaeus) (Hymenoptera: Torymidae) emergence and a reduced biocontrol effectiveness of the Asian chestnut gall wasp Dryocosmus kuriphilus (Yasumatsu) (Hymenoptera: Cynipidae) as effects of warmer winter temperatures [39]. Warmer temperatures may therefore determine an earlier T. sinensis’s emergence, and by the time they emerge, fresh galls of the host are not available, resulting in a lower parasitism pressure and increasing the risk of host outbreaks [39]. In addition, climate-associated shifts in the phenology of wild bees have advanced by a mean of 10.4 ± 1.3 days and are associated with global temperature increases [87][88][87,88]. Also, climate change has been documented to be associated with shifts in autumn phenology toward later dates and spring phenology toward earlier dates [89][90][89,90]. Latitude has also been reported to alter the phenological responses between host and parasitoids [91], thereby affecting insect population abundance and range dynamics [54][55]. Insects have developed a seasonal timing system to measure day/night duration (photoperiod) and anticipate/coordinate their development and physiology [92][93][92,93]. This allows them to regulate their seasonal rhythms [94] and adapt their phenology to their local environment [51][95][52,95], in this manner allowing susceptible life stages to avoid unfavorable environmental conditions [96] and favoring the synchrony of insect populations with the resources they consume, which ultimately allows them to persist/survive [17][97][17,97]. However, new daylength regimes due to climate change are altering host–parasitoid interactions and community dynamics [98][99][98,99]. In addition, interactions within trophic networks have greatly influenced insect phenology [17]; in these interactions, organisms from a specific trophic level should regulate their life cycle to match those of their prey and hosts according to their level of trophic dependence [100]; otherwise, any phenological shifts have population-level consequences [101], therefore altering the already-established communities and systems function, and having an impact on the benefits and services provided by natural ecosystems.3. Host–Parasitoid Geographical Distribution under Climate Change
A temperature limit restricts the distribution of insects; however, as a result of climate change, more suitable areas have emerged allowing species’ upslope migration (Figure 2) [66][102][103][66,102,103], shifting their niches to escape warming and match their current thermal preferences [51][104][52,104]. However, according to Román-Palacios and Wiens [8], niche shifts in response to climate change can only potentially reduce less than 30% of species extinction, which sparks serious concerns for the future fate of biodiversity. Agricultural pests are most likely to benefit from present and future climate change with worldwide pest proliferation, especially in temperate zones (Figure 2) [105]; e.g., warm temperatures increase population growth of a nonnative defoliator Coleophora laricella (Hübner) (Lepidoptera: Coleophoridae) and inhibit demographic responses of two imported parasitoids, Agathis pumila (Ratzeburg) (Hymenoptera: Braconidae) and Chrysocharis laricinellae (Ratzeburg) (Hymenoptera: Eulophidae). The positive response of hosts to warming might have contributed to the outbreak of C. laricella in North America [106].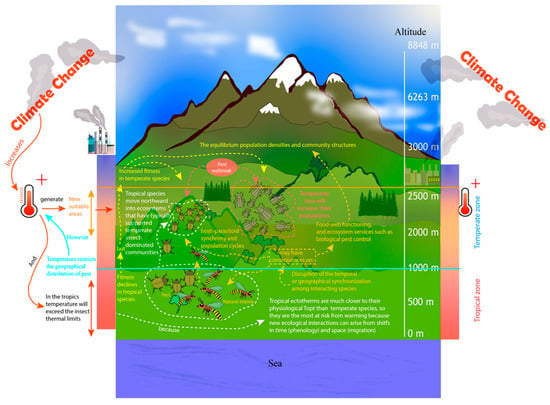
Figure 2. Effects of global rising temperatures on insect distribution, species interactions, and temperate taxa.
