Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Helena Vieira | -- | 2894 | 2023-12-04 18:09:01 | | | |
| 2 | Fanny Huang | Meta information modification | 2894 | 2023-12-06 03:43:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vieira, H.; Lestre, G.M.; Solstad, R.G.; Cabral, A.E.; Botelho, A.; Helbig, C.; Coppola, D.; De Pascale, D.; Robbens, J.; Raes, K.; et al. Marine Chitin/Chitosan and Collagen Value Chains. Encyclopedia. Available online: https://encyclopedia.pub/entry/52340 (accessed on 07 February 2026).
Vieira H, Lestre GM, Solstad RG, Cabral AE, Botelho A, Helbig C, et al. Marine Chitin/Chitosan and Collagen Value Chains. Encyclopedia. Available at: https://encyclopedia.pub/entry/52340. Accessed February 07, 2026.
Vieira, Helena, Gonçalo Moura Lestre, Runar Gjerp Solstad, Ana Elisa Cabral, Anabela Botelho, Carlos Helbig, Daniela Coppola, Donatella De Pascale, Johan Robbens, Katleen Raes, et al. "Marine Chitin/Chitosan and Collagen Value Chains" Encyclopedia, https://encyclopedia.pub/entry/52340 (accessed February 07, 2026).
Vieira, H., Lestre, G.M., Solstad, R.G., Cabral, A.E., Botelho, A., Helbig, C., Coppola, D., De Pascale, D., Robbens, J., Raes, K., Lian, K., Tsirtsidou, K., Leal, M.C., Scheers, N., Calado, R., Corticeiro, S., Rasche, S., Altintzoglou, T., Zou, Y., ...Lillebø, A.I.. (2023, December 04). Marine Chitin/Chitosan and Collagen Value Chains. In Encyclopedia. https://encyclopedia.pub/entry/52340
Vieira, Helena, et al. "Marine Chitin/Chitosan and Collagen Value Chains." Encyclopedia. Web. 04 December, 2023.
Copy Citation
Chitin/chitosan and collagen are two of the most important bioactive compounds, with applications in the pharmaceutical, veterinary, nutraceutical, cosmetic, biomaterials, and other industries. When extracted from non-edible parts of fish and shellfish, by-catches, and invasive species, their use contributes to a more sustainable and circular economy.
collagen
chitin
chitosan
sustainability
value chain
market
trends
1. Introduction
The ocean represents ca. 95% of the biosphere and is crucial for the planet and humankind, as it provides a plethora of important resources and services [1][2]. Although currently recognized as a common provider of social, environmental, and economic benefits [3], the ocean has faced, and continues to face, several natural and anthropogenic threats. Some of the major threats are related to the overexploitation of marine resources, climate change, pollution, ocean acidification, habitat damage, and management failure [4]. To mitigate these major threats, it is critical to maintain the balance between the exploitation of marine resources and the ecosystem resilience to such exploitation. This balance should be evident to all, as well as coordinated with and integrated into public policies, governance, finance, and management of global supply chains where ocean resources play a role [3]. To achieve this goal, sustainable and circular business models, as well as integrated policies that protect marine ecosystem functions and regulate all major activities occurring in the ocean, must be implemented or improved across the globe.
The blue economy reached a Gross Value Added (GVA) of EUR 129.1 billion and a turnover of EUR 523 billion in 2020 across seven different sectors (living resources, non-living resources, marine energy, port activities, shipbuilding and repair, maritime transport, and coastal tourism) [5]. The marine living resources sector comprises the harvesting and farming of biological resources, as well as their conversion and distribution, and that sector alone generated more than EUR 19.4 billion in GVA and EUR 119 billion in turnover in 2020. Despite this GVA, it may still be underestimating the value of the EU blue bioeconomy as a whole, as this does not encompass sectors such as blue biotechnology. Fisheries and aquaculture, two ocean-related major economic activities, have grown throughout the years, in part due to the increasing demand for food by an expanding human population [6]. If exploited sustainably, ocean resources potentially have the capacity to regenerate and feed a large proportion of the world’s population. According to the Food and Agriculture Organization of the United Nations (FAO), aquaculture accounted for 122.6 million tonnes of the unprecedented total of 214 million tonnes produced by fisheries and aquaculture in 2020 [6]. In this year, the number of people employed in primary fisheries and aquaculture exceeded 58 million [6], indicating the importance of these activities in the economic development of multiple countries. Moreover, as marine organisms have evolved for thousands of years to be able to thrive in complex habitats and are exposed to extreme conditions, they produce a wide variety of specific and potent bioactive substances [7]. Hence, the ocean is a rich and natural source of many bioactive compounds that cannot be found elsewhere. Thousands of marine bioactive compounds have been extracted, identified, and characterized in recent decades [8]. Indeed, ~7000 of these molecules are already in use or being validated for several purposes, ranging from medicine to industrial applications [9]. For instance, in 2020 and 2021, 1407 and 1425 new bioactive compounds were reported from marine organisms [10]. However, the increased extraction and use of such compounds has been exerting even more pressure on the limited natural resources of the marine realm.
Environmental and economic concerns have been increasingly driving the use of eco-friendly alternatives to exploit marine natural resources. In the age of sustainability, where development models are changing towards circularity and zero waste, the fisheries and aquaculture sectors, alongside many of the other sectors they connect with (like fish and seafood transformation industries), are key players in supplying new by- and co-products that work as raw materials for other industries. Examples include the once considered “waste streams” of fish by-catches, the shells and non-edible parts of shellfish and crustaceans, and invasive species such as crabs and starfish, which can serve as raw materials for different bio-based products. Many industries, including the pharmaceutical, veterinary, nutraceutical, cosmetic, biomaterials, and others, benefit from the development of products and/or processes using these marine resources [11]. Such products may take the form of pharmaceutical drugs, livestock feed formulas, pet food products, specialty foods and nutritional supplements for several human conditions, medical biocomponents, beauty supplements, functional textiles or new fibres, biomaterials used in construction or nature-based building solutions, and additives or enzymes used in manufacturing and industrial processes, just to name a few, to improve productivity with lower environmental impacts [12][13][14][15]. These approaches promote the development of sustainable products, circular (bio)economy models, zero-waste strategies, and reduce environmental pollution.
Chitin, its derivative chitosan, and collagen, are highly relevant marine bioactive compounds to the biomedical, nutraceutical, cosmetic, feed, and wastewater treatment industries, among others [12][16][17][18][19]. Both chitin and collagen represent unified templates for biomineralization and skeletogenesis in many organisms and are essential elements for their structural life support functions [20]. In fact, both biopolymers represent examples of the “scaffolding strategy”, a modern trend of using naturally occurring 3D scaffolds made of chitin and collagen (i.e., in sponges) for tissue engineering and technology derived thereof [21][22][23]. These naturally occurring compounds, or derivatives, are also used in applications such as preservative food coatings due to their thermal stability and antimicrobial qualities [24] but also in a wide range of different biomaterials, some even in the framework of extreme biomimetics inspiration [25][26].
Chitin is one of the most abundant biopolymers in nature [27]. It can be extracted from the exoskeletons of crustaceans, molluscs, insects, and fungi. It can also be obtained from some Porifera, like sponges [28]. Chitin is classified in three different groups: α-chitin, usually extracted from the exoskeleton of crustaceans such as shrimps and crabs; β-chitin, extracted from squid pens; and γ-chitin, obtained from fungi and yeasts [29]. Chitin and chitosan properties are highly variable depending on their source, as well as on the deacetylation, protein concentration, and extraction procedures [30]. The conventional way of making chitin and chitosan include demineralisation, deproteinisation (+deacetylation for chitosan), or electrochemical methods [31]. Both chitin and chitosan undergo modifications (e.g., deacetylation, quaternization, oxidation) to enhance their physical properties [32]. Although chitin has poor solubility, its derivative chitosan is a soluble biopolymer in aqueous acidic conditions [33]. Therefore, chitin is often chemically modified by deacetylation to obtain chitosan.
Collagen has at least 28 types (I-XXVIII) described. The most abundant types are in mammals, fibrillar collagen types I–III, predominantly sourced from commercialized porcine, bovine, ovine, and chicken tissues [34]. It can also be obtained from marine sponges [35][36], jellyfish, squids, and fishes [37]. The skin, bones, fins, head, and scales of fish are rich in collagen and account for approximately 75% of the fish wet weight [38]. Collagen has multiple sources, but an increase in marine-derived collagen is being seen [39][40] and its usages range from cosmetic and nutraceutical preparations to tissue engineering, medical or pharmaceutical high-value products [41][42], and even several manufacturing biomaterials applications [43][44]. In fact, collagen from marine organisms utilised for biomedical applications has been recognised as a convenient and safe source, and some advantages have been pointed out when compared to collagen from mammalian origin, including (1) less significant religious and ethical constraints; (2) greater absorption due to low molecular weight; (3) low inflammatory response; (4) and minor regulatory and quality control problem [45]. Even more, it represents an option towards the valorisation of marine by-products and the development of the circular economy concept, as providing new solutions for the reuse of materials is highly targeted on the EU policy making agenda [46].
As chitin and collagen can be extracted from sources that would otherwise be considered as waste (e.g., non-edible parts of fish and shellfish, fisheries’ by-catch, and invasive species), the use of these compounds represents an opportunity to reinforce circular business models and to reuse and reduce the waste streams derived from marine fisheries, aquaculture, and food processing industries. Chitin and collagen markets currently represent USD ~7900 million and USD 4700 million, respectively [47], meaning they both have substantial commercial interest. The application and transformation of what was once considered waste has therefore led to new valorisation strategies, creating opportunities to capitalize these co-products and side streams in market segments not yet explored [12][48], building novel business models in new value networks for the marine-derived chitin/chitosan and collagen.
2. Marine Chitin/Chitosan and Collagen Value Chains
2.1. Trends in the Distribution and Number of Publications per Value Chain
The number of peer-reviewed publications (hereafter referred to as publications) related to the chitin/chitosan value chain was almost twice that of publications related to the collagen value chain (138 vs. 84). Four publications contained information relevant for both value chains. Approximately half of the analysed publications were published in top tier (i.e., Q1) journals. For the chitin/chitosan value chain, 49% of the publications analysed (n = 67) were published in journals in Q1 and 31% (n = 43) in Q2. For the collagen value chain, 50% of the publications (n = 42) were published in journals in Q1 and 35% (n = 29) in Q2. Globally, for both value chains, publications were distributed as follows: Q1, 48% (n = 106); Q2, 32% (n = 71); Q3, 12% (n = 26); and Q4, 7% (n = 16).
As for the evolution of the number of publications related to each value chain (Figure 1), the first scientific publication approaching the chitin/chitosan value chain was published in 1990, a second in 1992, and a third in 1993 (Figure 1a). After a 7-year gap, a fourth publication was published in 2000; after a period of intermittent publication from 2001 to 2009, publications related to the chitin/chitosan value chain have been published yearly, with an increasing trend being recorded over the years (Figure 1a). The maximum number of publications (n = 28) was observed in 2022, with 20 being published in Q1 journals.
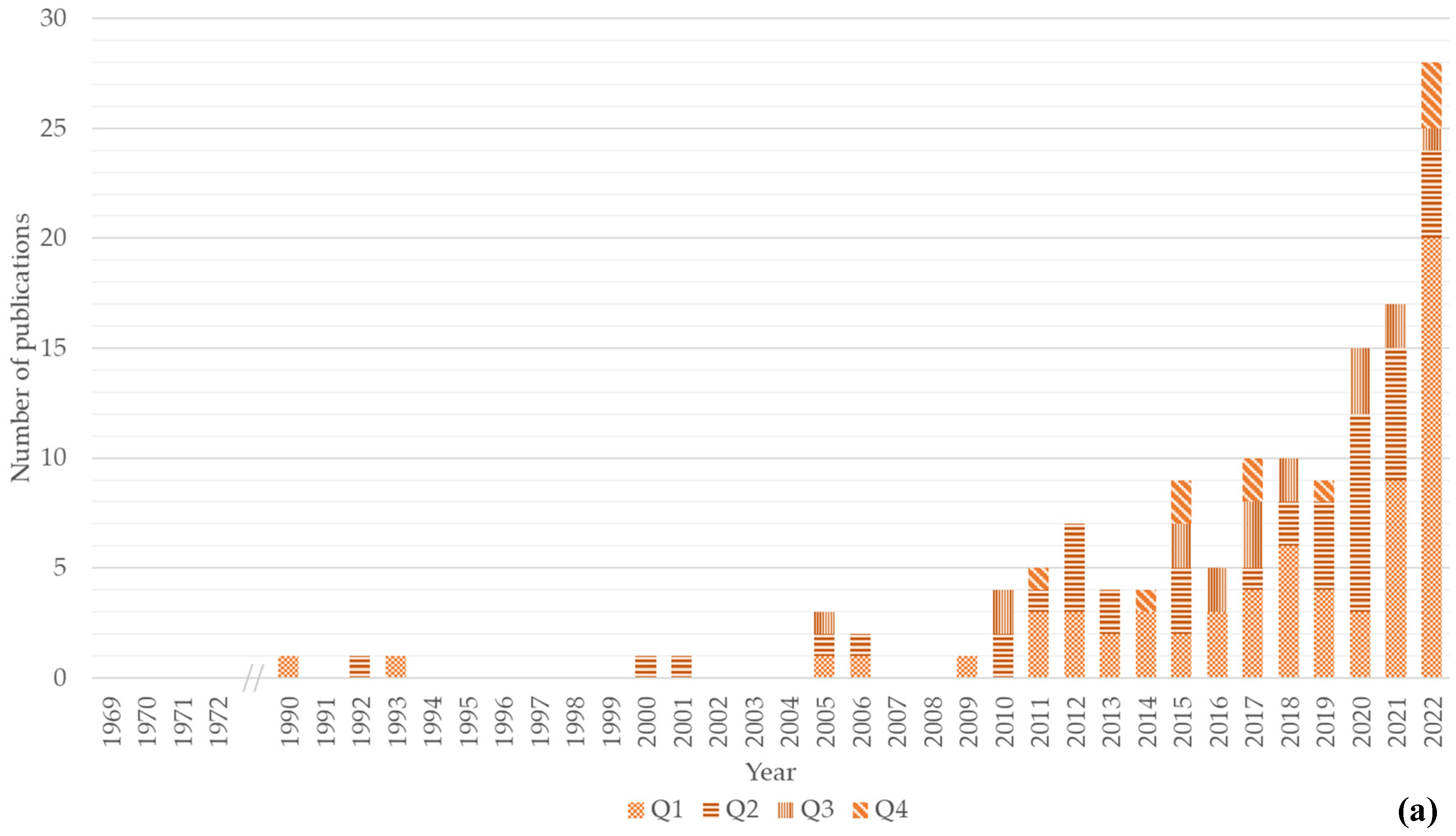
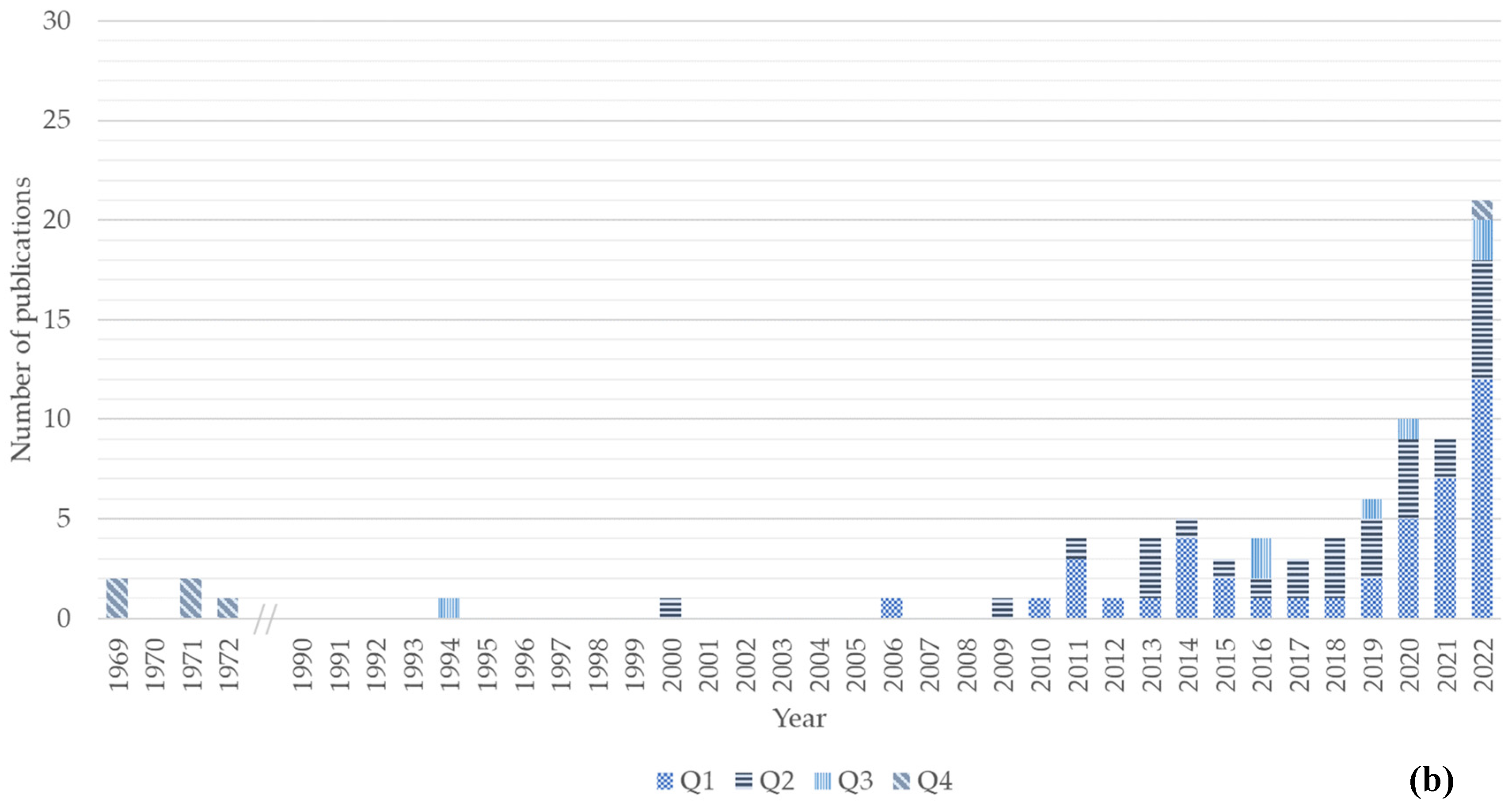
Figure 1. Number of publications per year and quartile (Q1–Q4). (a) Number of publications for the chitin/chitosan value chain; (b) number of publications for the collagen value chain. Quartile classification according to SCImago.
The first scientific publication approaching the collagen value chain was published in 1969, followed by a second and third publication in 1971 and 1972, respectively, and a fourth and fifth in 1994 and 2000 (Figure 1b). After a 5-year gap, a publication was published in 2006, but only since 2009 have publications been published on this topic on a yearly basis. An increasing trend has been observed since 2009 (Figure 1b), with the maximum number of publications in 2022. In this year, 12 of the 21 publications were published in Q1 journals.
2.2. Trends in the Geographical Origin of Publications per Value Chain
The scientific publications related to each value chain were differently distributed based on the country of the corresponding author(s). Publications related to the chitin/chitosan value chain originated from 43 countries (Figure 2), whereas those related to the collagen value- chain originated from 25 countries (Figure 3). Most publications related to the chitin/chitosan value chain were from India (n = 20), while most publications related to the collagen value chain originated from China (n = 13), closely followed by India (n = 12). Asia was the most relevant region, with 43% and 57% of the corresponding authors of publications related to the chitin/chitosan and collagen value chains, respectively, being based in Asian countries.
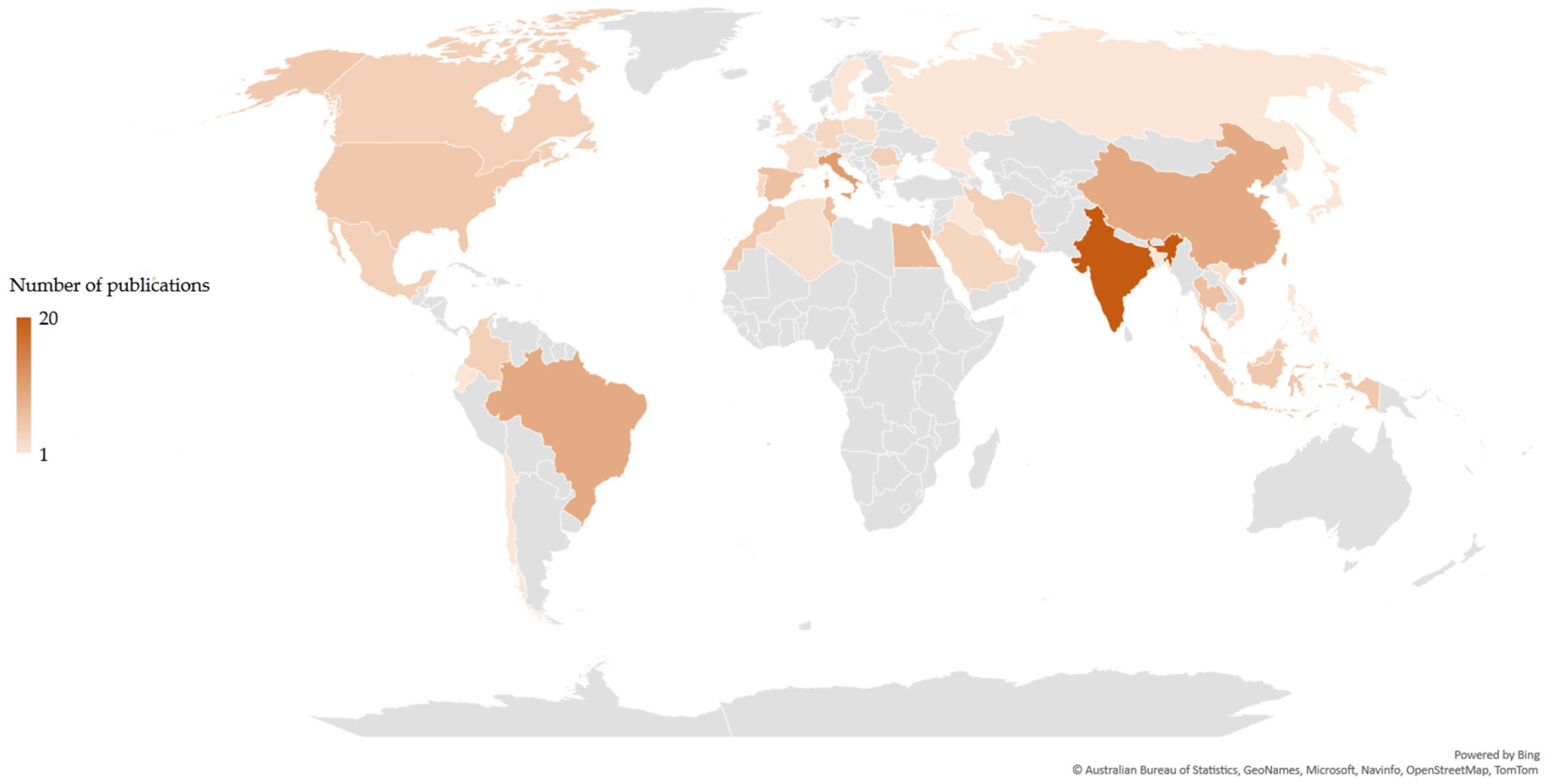
Figure 2. Geographic distribution of the chitin/chitosan value chain-related publications based on the country of the corresponding author(s).
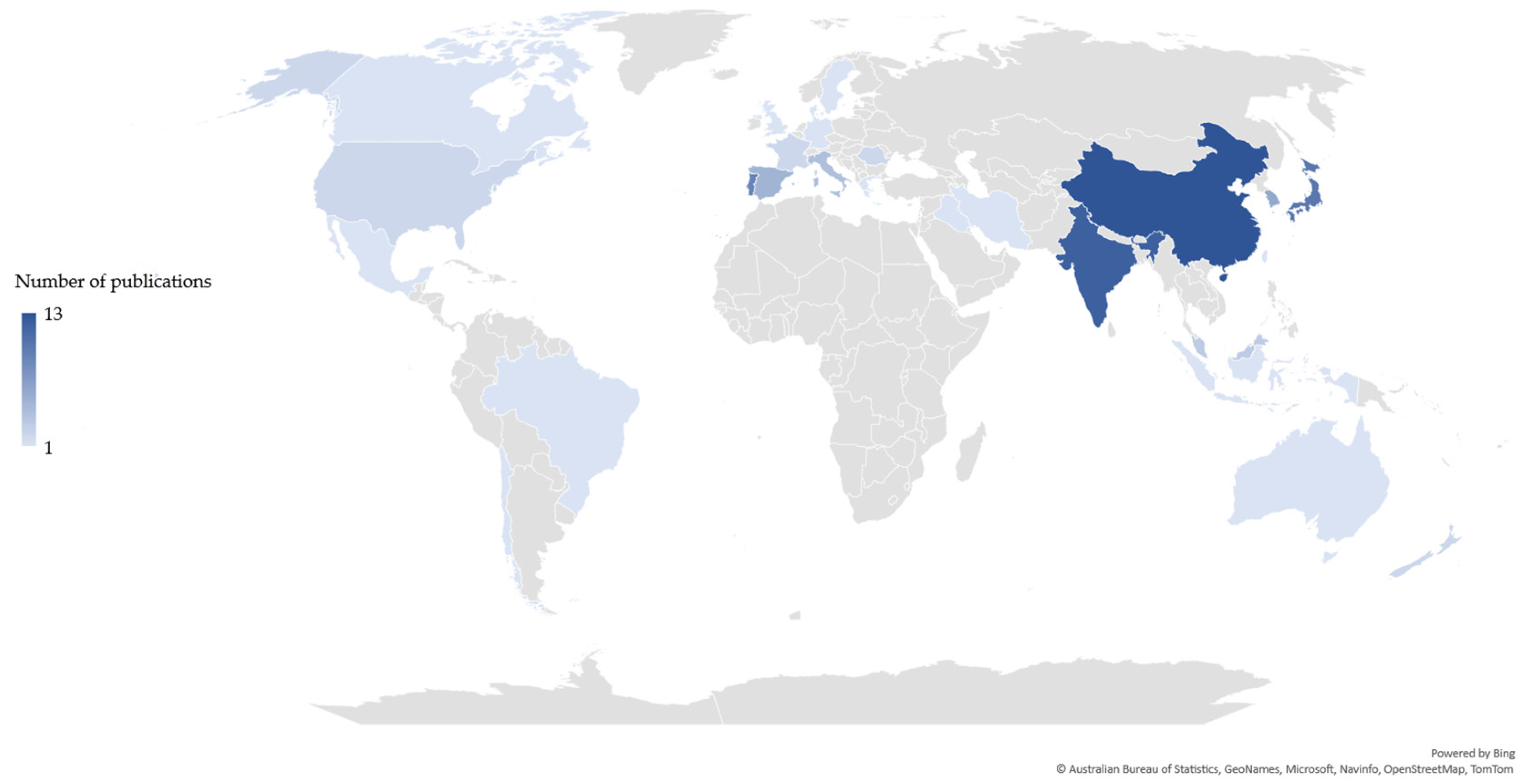
Figure 3. Geographic distribution of the collagen value chain-related publications based on the country of the corresponding author(s).
2.3. Trends in the Origin of the Marine Raw Materials and Feedstock per Value Chain
The origin of the raw material(s) used differed considerably between the two value chains, based on the information provided by the analysed publications (Figure 4). For the chitin/chitosan value chain, the “food processing industry” and “fisheries” were the most frequent sources of raw materials used in publications (34% and 31%, respectively) (Figure 4a). The source “aquaculture” showed a low value (6%), considering the rising interest in this sector related to the aquaculture production of species that may be a source of chitin and its derivatives, such as chitosan (i.e., crustaceans) [6]. For the collagen value chain, most publications used raw materials from “fisheries” (52%) followed by the “food processing industry” (22%) (Figure 4b). Although “aquaculture” was also the least frequent source of raw materials in collagen value chain publications, its relative contribution was twice that calculated for the chitin/chitosan value chain (12% vs. 6%, respectively). Globally, “fisheries” have been the most relevant source of raw materials for both value chains. It is worth noting that “undisclosed” was the third most common source on both value chains; furthermore, in the chitin/chitosan value chain, its value (29%) was similar to that of the two most common sources (Figure 4a).
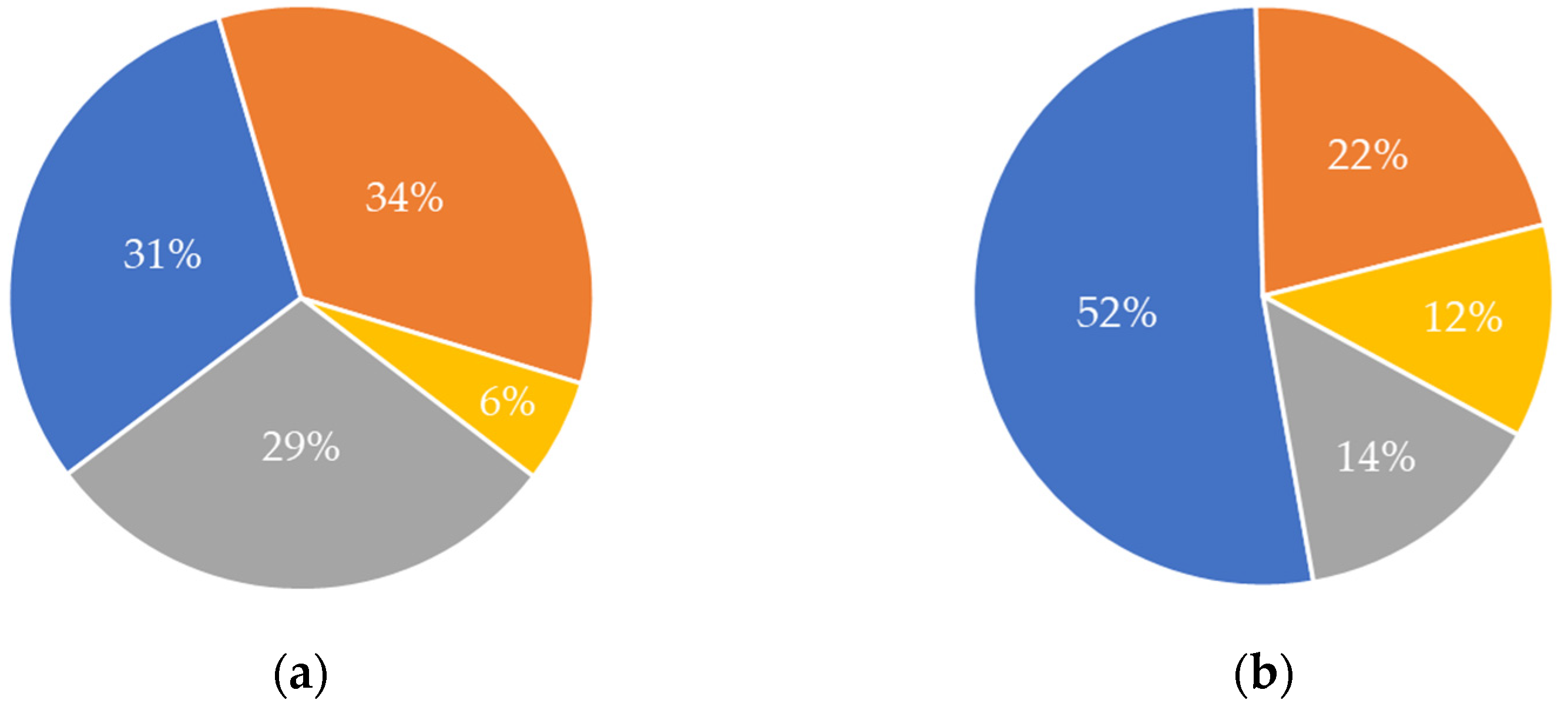
Figure 4. Origin of the raw materials as their frequency of occurrence in the publications analysed for each value chain. (a) Chitin/chitosan value chain; (b) collagen value chain. Blue, fisheries; orange, food processing industry; yellow, aquaculture; grey, undisclosed.
For the chitin/chitosan value chain, “crustacean waste” was the most used feedstock in the studies analysed (71%), especially “shrimp waste” (35%) (Figure 5a). The percentage obtained for “algae and seagrasses” (15%) resulted from a single publication that mentioned endophytic fungi isolated from 19 different species of algae and 10 different species of seagrasses [49]. Regarding the collagen value chain, “fish scales, skin, and bones” were the feedstock used in 62% of the analysed publications (Figure 5b). Globally, fish and crustacean wastes were the most used feedstock in the studies related to both value chains.
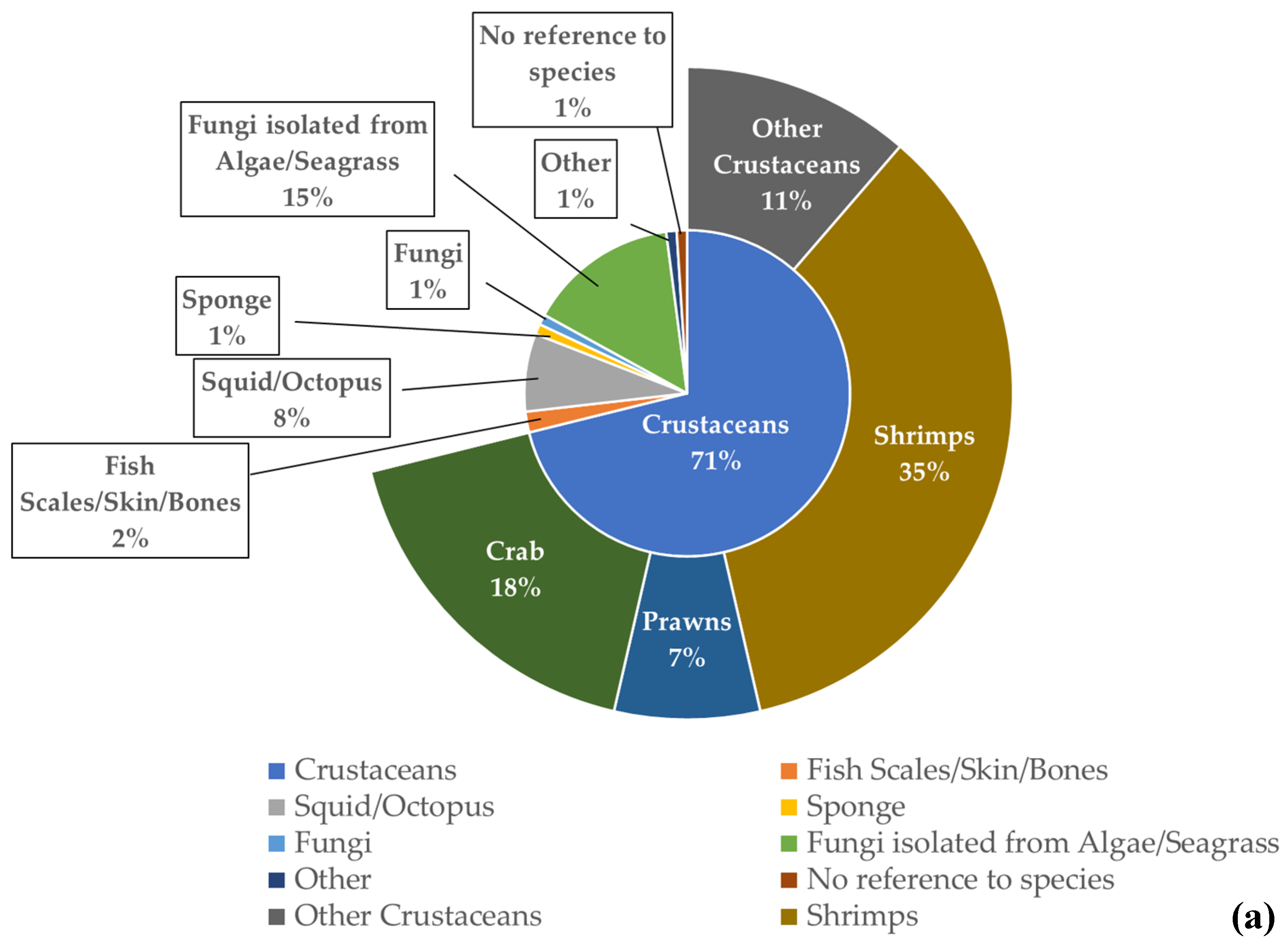
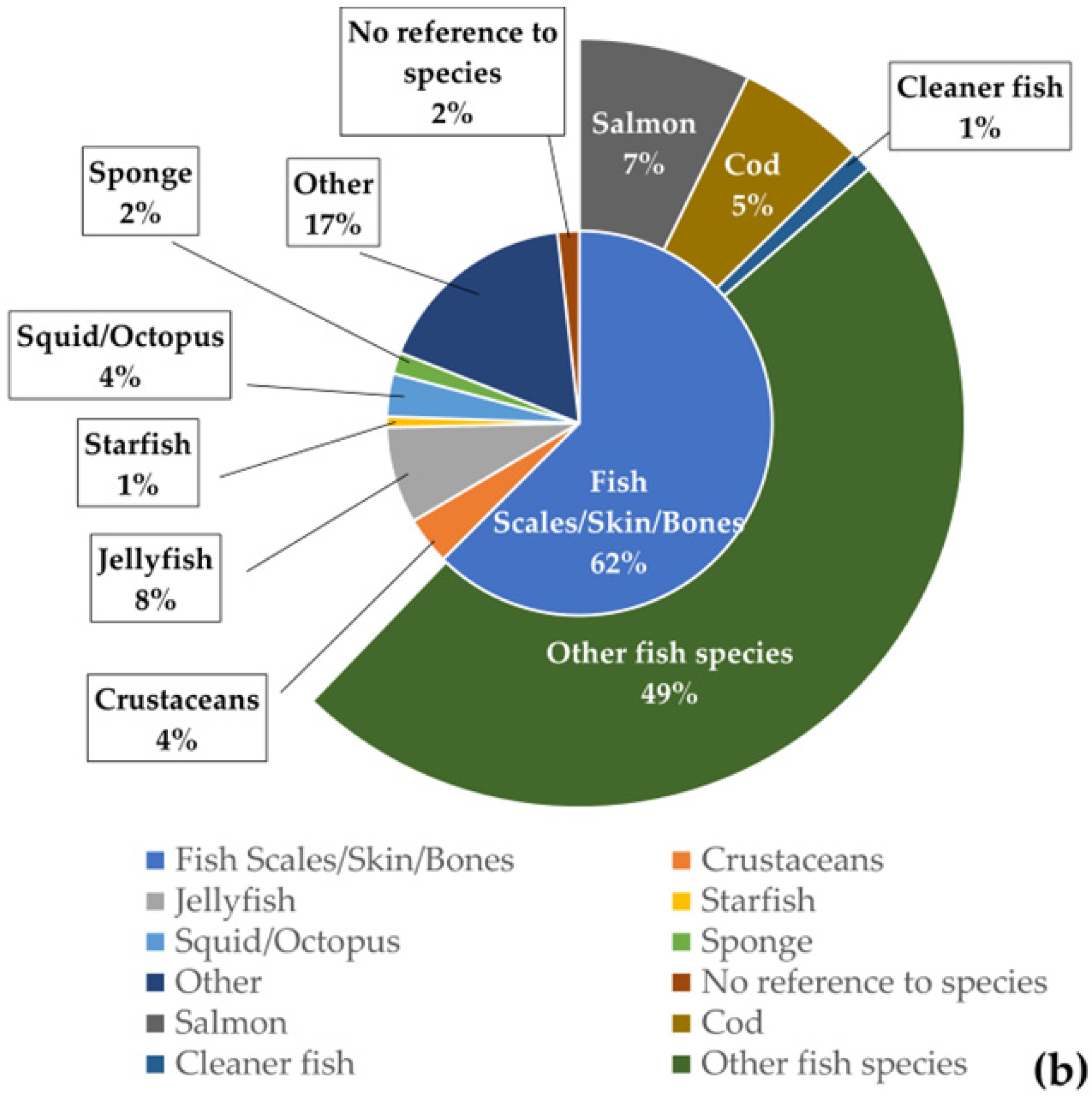
Figure 5. Feedstock used as source of extraction and their frequency of occurrence in the publications analysed for each value chain. (a) Chitin/chitosan value chain; (b) collagen value chain.
2.4. Trends in the Perception of Sustainability for Chitin/Chitosan and Collagen Value Chains
The sustainability, as expressed in the scientific publications, for each value chain was categorized into economic, environmental, and social. Economic sustainability is mostly related to the improved cost efficiency of the extraction methods, especially regarding them being cheaper than previously established methods or capable of achieving a higher quality or higher quantity of compounds. Environmental sustainability is related to environmentally friendly methods of compound extraction and to waste reduction and reuse. Social sustainability refers to practices that may improve society well-being and reduce inequalities, such as those related to consumer cultural or dietary needs.
Overall, more economic, environmental, and social sustainability practices have been applied in the chitin/chitosan value chain than in the collagen value chain, particularly environmental and economic sustainability practices (Figure 6). Environmental practices are the most referred to in publications related to both value chains, such as environmentally friendly methods of extraction [50][51], reduce/reuse of waste [52][53][54], or reduction in environmental harm [55], followed by economical practices, such as cheaper consumables [50][56], cheaper methodologies [57][58][59][60], more cost-efficient processes [61][62][63][64][65], and new potential products [19][66][67].
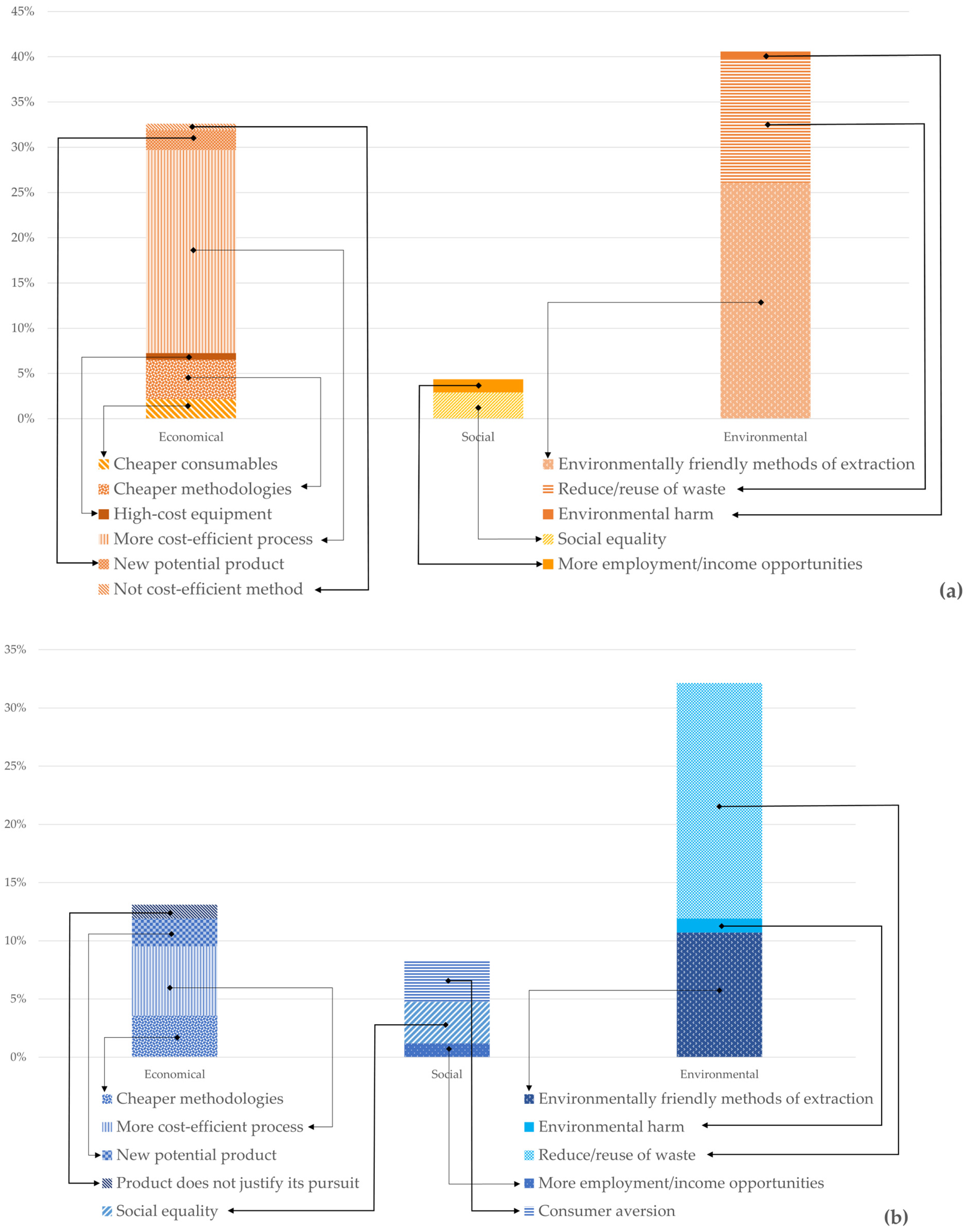
Figure 6. Percentage of publications referring to each of the three categories of sustainable practices per value chain. (a) Chitin/chitosan value chain; (b) collagen value chain. Economical mentions refer to cheaper consumables; cheaper methodologies; high cost equipment; more cost-efficient process; new potential product; not cost-efficient method; product that does not justify its use. Sustainability mentions refer to consumer aversion; more employment/income opportunities; social equality. Environmental mentions refer to environmentally friendly methods of extraction; environmental harm; reduce/reuse of waste.
2.5. Trends in Market Applications for Each Value Chain
Regarding the market applications of chitin/chitosan and collagen, several different sectors were mentioned as both present and future applications. Overall, collagen products are currently less used than chitin/chitosan products (Figure 7), and an increased use of both types of products is expected, as described by the authors of the screened publications. Chitin/chitosan products are mostly used in the industrial sector, newly derived and purified compounds, food applications, and wastewater treatment (Figure 7) [49][64][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87]. An increased use in these sectors, as well as in biomedical applications [88][89][90][91], is envisioned. However, in the analysed scientific publication, the authors state that more time is needed to assess how the use of chitin/chitosan products in biomedical applications will evolve [92][93]. Collagen products are mostly used in biomedical applications [61][94] and as purified compounds (Figure 7) [61][95], and a substantial rise in biomedical, cosmetic, and pharmaceutical applications is suggested by the authors in many of the analysed publications [37][54][55][96][97].
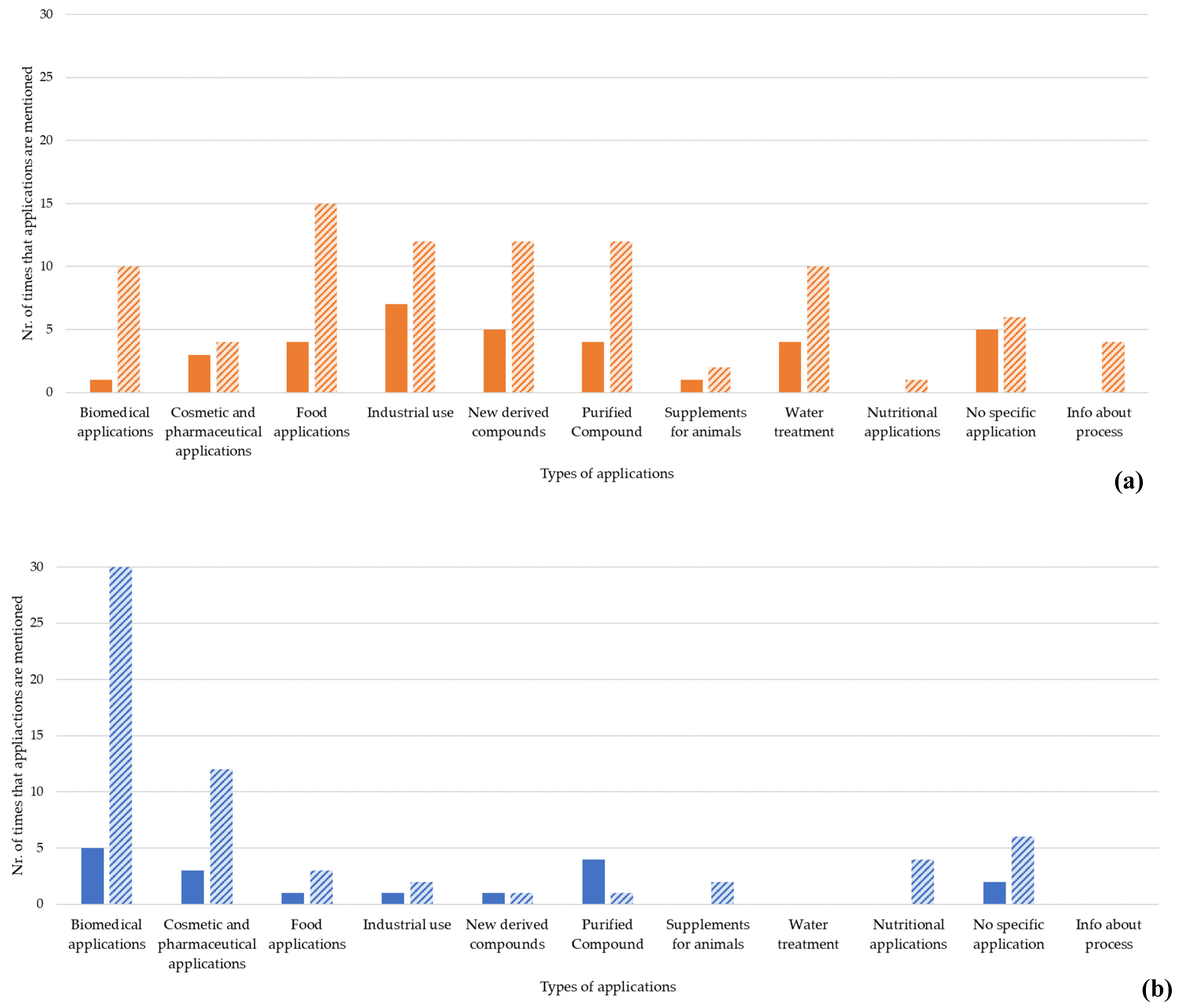
Figure 7. Number of current (block colour) and future (striped pattern) applications reported by sectors for chitin/chitosan (a) and collagen (b) products. The resulting bars correspond to the exact number of each application field mentioned as current or future applications in the analysed publications dataset.
2.6. Trends in Data Distribution per Category of Information per Value Chain
There is a high degree of information discrepancy between the different categories of information presented in Figure 8, with more information presented in the categories relating to raw material origin, feedstock, pre-processing, and processing. While the sources and processes for obtaining chitin/chitosan or collagen were documented in >70% of the publications related to each value chain, market information related to the current applicability of both products and their derivatives was scarce (~23% in the case of the chitin/chitosan value chain and ~20% for the collagen value chain) (Figure 8). Moreover, the applicability of these products is generally documented as a possibility rather than a reality, and very few publications have mentioned patents, profitability, or marketability. Even when considering future perspectives, ~60% of the publications refer to products but only ~5% refer to market growth or profitability.
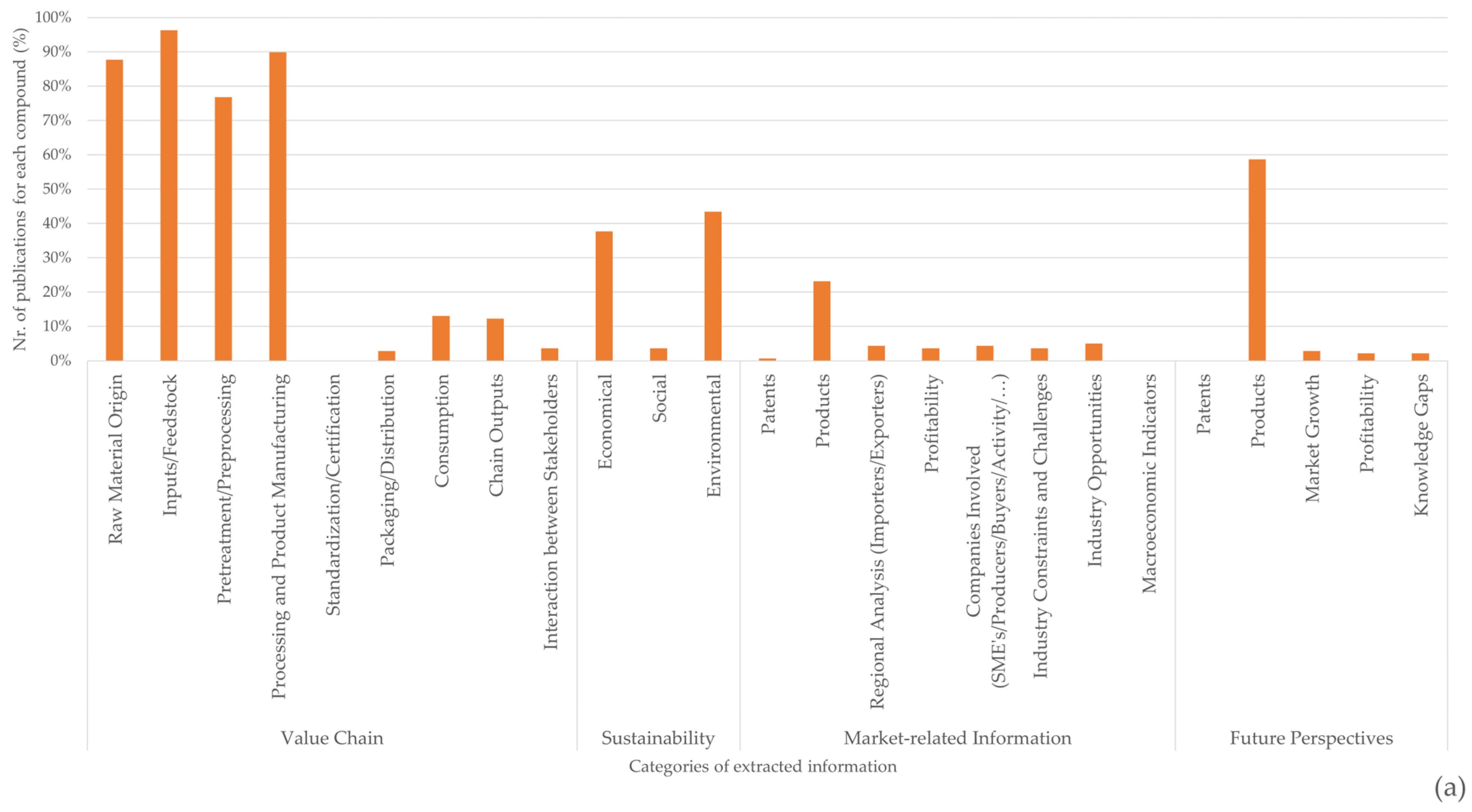
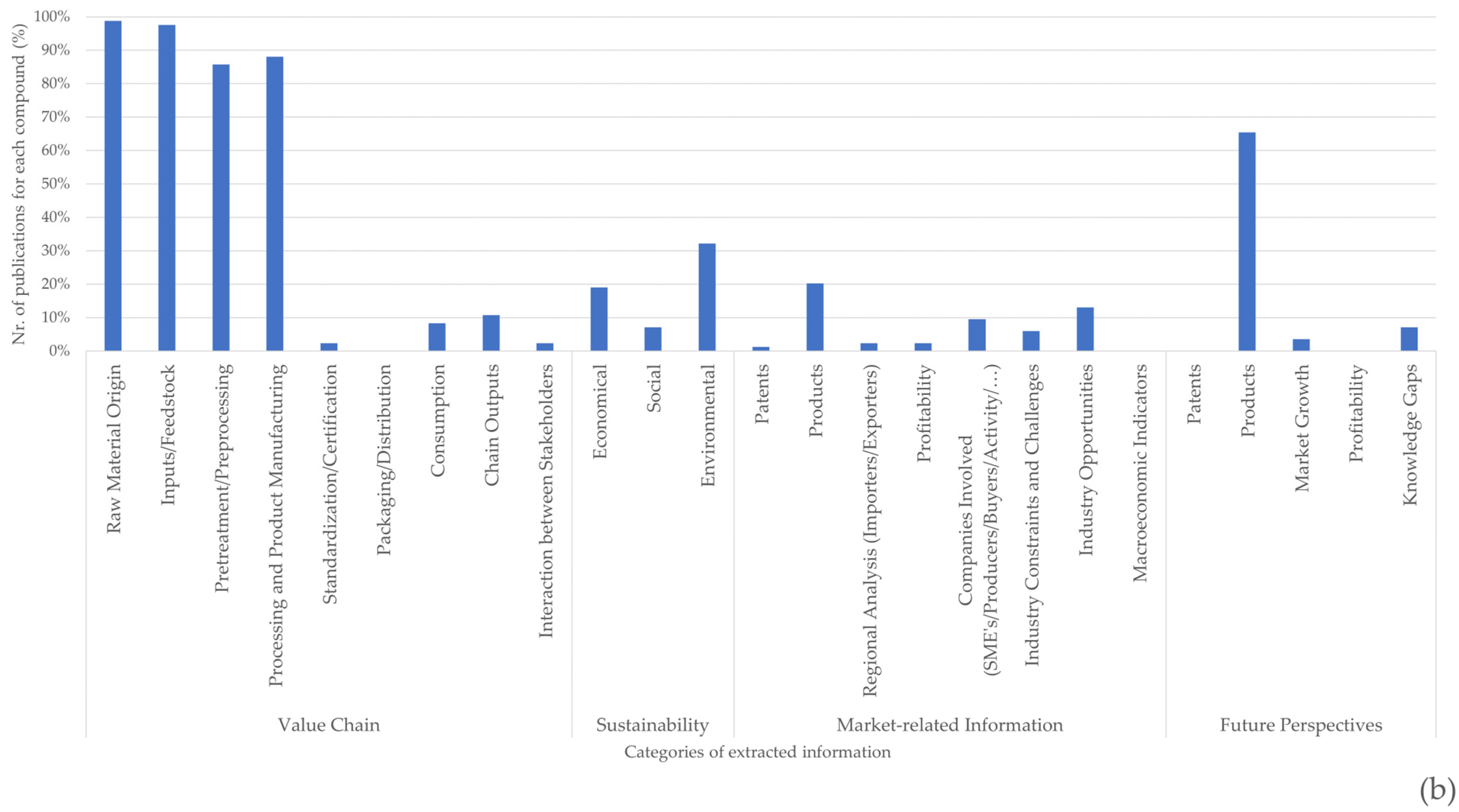
Figure 8. Percentage of publications referring to each category of extracted information for chitin/chitosan (a) and collagen (b) value chains.
Although the economic and environmental sustainability of the chitin/chitosan value chain has been addressed in ~40% of the analysed publications, this value was much higher than that found for the collagen value chain (~30% for environmental sustainability and ~20% for economic sustainability) (Figure 8). Social sustainability was only seldom referred to for both value chains (<10% of publications).
References
- Estes, M.; Anderson, C.; Appeltans, W.; Bax, N.; Bednaršek, N.; Canonico, G.; Djavidnia, S.; Escobar, E.; Fietzek, P.; Gregoire, M.; et al. Enhanced Monitoring of Life in the Sea Is a Critical Component of Conservation Management and Sustainable Economic Growth. Mar. Policy 2021, 132, 104699.
- CBD Oceans Contain a Wealth of Biodiversity. Available online: www.cbd.int (accessed on 24 October 2023).
- Golden, J.S.; Virdin, J.; Nowacek, D.; Halpin, P.; Bennear, L.; Patil, P.G. Making Sure the Blue Economy Is Green. Nat. Ecol. Evol. 2017, 1, 17.
- Kvamsdal, S.; Hopland, A.O.; Li, Y.; Selle, S. Expert Opinions on Threats and Impacts in the Marine Environment. Mar. Policy 2023, 147, 105382.
- The EU Blue Economy Report 2023; Publications Office of the European Union: Luxembourg, 2023; ISBN 9789268033456.
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Roma, Italy, 2022.
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465.
- Romano, G.; Almeida, M.; Varela Coelho, A.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219.
- Ambrosino, L.; Tangherlini, M.; Colantuono, C.; Esposito, A.; Sangiovanni, M.; Miralto, M.; Sansone, C.; Chiusano, M.L. Bioinformatics for Marine Products: An Overview of Resources, Bottlenecks, and Perspectives. Mar. Drugs 2019, 17, 576.
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2023, 40, 275–325.
- Vasconcelos, V.; Moreira-Silva, J.; Moreira, S. Portugal Blue Bioeconomy Roadmap—BLUEandGREEN; CIIMAR: Matosinhos, Portugal, 2019.
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffey, J.; Manning, L.; Moosavi Basri, S.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and Chitosan Derived from Crustacean Waste Valorization Streams Can Support Food Systems and the UN Sustainable Development Goals. Nat. Food 2022, 3, 822–828.
- Collins, J.E.; Vanagt, T.; Huys, I.; Vieira, H. Marine Bioresource Development—Stakeholder’s Challenges, Implementable Actions, and Business Models. Front. Mar. Sci. 2020, 7, 62.
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.M.; Calado, R. Trends in the Discovery of New Marine Natural Products from Invertebrates over the Last Two Decades—Where and What Are We Bioprospecting? PLoS ONE 2012, 7, e30580.
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a Source of New Marine Bioactive Compounds—An Overview of the Last Decade and Future Steps for Bioprospecting. Mar. Drugs 2011, 9, 1860–1886.
- Engku Noramalina Che Engku Chik, C.; Suryatie Kamaruzzan, A.; Ideris Abdul Rahim, A.; Lananan, F.; Endut, A.; Aslamyah, S.; Azman Kasan, N. Extraction and Characterization of Litopenaeus Vannamei’s Shell as Potential Sources of Chitosan Biopolymers. J. Renew. Mater. 2022, 11, 1181–1197.
- Cutajar, N.; Lia, F.; Deidun, A.; Galdies, J.; Arizza, V.; Mangion, M.Z. Turning Waste into a Resource: Isolation and Characterization of High-Quality Collagen and Oils from Atlantic Bluefin Tuna Discards. Appl. Sci. 2022, 12, 1542.
- Kumaran, S.; Perianaika Anahas, A.M.; Prasannabalaji, N.; Karthiga, M.; Bharathi, S.; Rajasekar, T.; Joseph, J.; Prasad, S.G.; Pandian, S.; Pugazhvendan, S.R.; et al. Chitin Derivatives of NAG and Chitosan Nanoparticles from Marine Disposal Yards and Their Use for Economically Feasible Fish Feed Development. Chemosphere 2021, 281, 130746.
- Selvakumar, G.; Kuttalam, I.; Mukundan, S.; Lonchin, S. Valorization of Toxic Discarded Fish Skin for Biomedical Application. J. Clean. Prod. 2021, 323, 129147.
- Ehrlich, H. Chitin and Collagen as Universal and Alternative Templates in Biomineralization. Int. Geol. Rev. 2010, 52, 661–699.
- Khrunyk, Y.; Lach, S.; Petrenko, I.; Ehrlich, H. Progress in Modern Marine Biomaterials Research. Mar. Drugs 2020, 18, 589.
- Dziedzic, I.; Voronkina, A.; Pajewska-Szmyt, M.; Kotula, M.; Kubiak, A.; Meissner, H.; Duminis, T.; Ehrlich, H. The Loss of Structural Integrity of 3D Chitin Scaffolds from Aplysina Aerophoba Marine Demosponge after Treatment with LiOH. Mar. Drugs 2023, 21, 334.
- Tsurkan, D.; Wysokowski, M.; Petrenko, I.; Voronkina, A.; Khrunyk, Y.; Fursov, A.; Ehrlich, H. Modern Scaffolding Strategies Based on Naturally Pre-Fabricated 3D Biomaterials of Poriferan Origin. Appl. Phys. A Mater. Sci. Process. 2020, 126, 382.
- Kritchenkov, A.S.; Egorov, A.R.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Yagafarov, N.Z.; Kurasova, M.N.; Dysin, A.P.; Kurliuk, A.V.; Shakola, T.V.; et al. Active Antibacterial Food Coatings Based on Blends of Succinyl Chitosan and Triazole Betaine Chitosan Derivatives. Food Packag. Shelf Life 2020, 25, 100534.
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers 2015, 7, 235–265.
- Ehrlich, H.; Wysokowski, M.; Jesionowski, T. The Philosophy of Extreme Biomimetics. Sustain. Mater. Technol. 2022, 32, e00447.
- Islam, N.; Hoque, M.; Taharat, S.F. Recent Advances in Extraction of Chitin and Chitosan. World J. Microbiol. Biotechnol. 2022, 39, 28.
- Sun, C.; Wang, Z.; Zheng, H.; Chen, L.; Li, F. Biodegradable and Re-Usable Sponge Materials Made from Chitin for Efficient Removal of Microplastics. J. Hazard. Mater. 2021, 420, 126599.
- Sagheer, F.A.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Marine Sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419.
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan Chemistry: Relevance to the Biomedical Sciences. In Polysaccharides I: Structure, Characterization and Use; Heinze, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 151–209. ISBN 9783540315834.
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393.
- Tarique, J.; Sapuan, S.M.; Aqil, N.F.; Farhan, A.; Faiz, J.I.; Shahrizan, S. A Comprehensive Review Based on Chitin and Chitosan Composites. In Composites from the Aquatic Environment; Sapuan, S.M., Ahmad, I., Eds.; Springer Nature: Singapore, 2023; pp. 15–66. ISBN 9789811953279.
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256.
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The Review of Versatile Application of Collagen: Versatile Application of Collagen. Polym. Adv. Technol. 2017, 28, 4–9.
- Ehrlich, H.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Jesionowski, T. Collagens of Poriferan Origin. Mar. Drugs 2018, 16, 79.
- Pozzolini, M.; Tassara, E.; Dodero, A.; Castellano, M.; Vicini, S.; Ferrando, S.; Aicardi, S.; Cavallo, D.; Bertolino, M.; Petrenko, I.; et al. Potential Biomedical Applications of Collagen Filaments Derived from the Marine Demosponges Ircinia Oros (Schmidt, 1864) and Sarcotragus Foetidus (Schmidt, 1862). Mar. Drugs 2021, 19, 563.
- Carvalho, A.M.; Marques, A.P.; Silva, T.H.; Reis, R.L. Evaluation of the Potential of Collagen from Codfish Skin as a Biomaterial for Biomedical Applications. Mar. Drugs 2018, 16, 495.
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39.
- Rigogliuso, S.; Campora, S.; Notarbartolo, M.; Ghersi, G. Recovery of Bioactive Compounds from Marine Organisms: Focus on the Future Perspectives for Pharmacological, Biomedical and Regenerative Medicine Applications of Marine Collagen. Molecules 2023, 28, 1152.
- Rajabimashhadi, Z.; Gallo, N.; Salvatore, L.; Lionetto, F. Collagen Derived from Fish Industry Waste: Progresses and Challenges. Polymers 2023, 15, 544.
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61.
- Abdullah, J.A.A.; Yemişken, E.; Guerrero, A.; Romero, A. Marine Collagen-Based Antibacterial Film Reinforced with Graphene and Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2022, 24, 648.
- Klüver, E.; Baltzer, M.; Langer, A.; Meyer, M. Additive Manufacturing with Thermoplastic Collagen. Polymers 2022, 14, 974.
- Harussani, M.M.; Sapuan, S.M.; Iyad, M.; Wong, H.K.A.; Farouk, Z.I.; Nazrin, A. Collagen Based Composites Derived from Marine Organisms: As a Solution for the Underutilization of Fish Biomass, Jellyfish and Sponges. In Composites from the Aquatic Environment; Springer Nature: Singapore, 2023; pp. 245–274. ISBN 9789811953262.
- Rahman, A.; Silva, T.H. Collagens from Marine Organisms towards Biomedical Applications. Mar. Drugs 2022, 20, 170.
- Rudovica, V.; Rotter, A.; Gaudêncio, S.P.; Novoveská, L.; Akgül, F.; Akslen-Hoel, L.K.; Burlakovs, J. Valorization of Marine Waste: Use of Industrial By-Products and Beach Wrack Towards the Production of High Added-Value Products. Front. Mar. Sci. 2021, 8, 723333.
- Markets andMarkets. Collagen Market by Product Type (Gelatin, Hydrolyzed Collagen, Native Collagen), Application, Source (Bovine, Porcine, Poultry, Marine, and Plant Sources), Form, Type, Extraction Process and Region—Global Forecast to 2030; Research and Markets: Dublin, Ireland, 2023.
- Lv, L.-C.; Huang, Q.-Y.; Ding, W.; Xiao, X.-H.; Zhang, H.-Y.; Xiong, L.-X. Fish Gelatin: The Novel Potential Applications. J. Funct. Foods 2019, 63, 103581.
- Ambayeram, V.; Rajulu, G.; Thirunavukkarasu, N.; Suryanarayanan, T.S. Endophytic Fungi of Marine Algae and Seagrasses: A Novel Source of Chitin Modifying Enzymes. Mycosphere 2015, 6, 345–355.
- Bradić, B.; Novak, U.; Likozar, B. Crustacean Shell Bio-Refining to Chitin by Natural Deep Eutectic Solvents. Green Process. Synth. 2019, 9, 13–25.
- Bisht, M.; Martins, M.; Dias, A.C.R.V.; Ventura, S.P.M.; Coutinho, J.A.P. Uncovering the Potential of Aqueous Solutions of Deep Eutectic Solvents on the Extraction and Purification of Collagen Type I from Atlantic Codfish (Gadus Morhua). Green Chem. 2021, 23, 8940–8948.
- Carrera, M.; Ezquerra-Brauer, J.M.; Aubourg, S.P. Characterization of the Jumbo Squid (Dosidicus Gigas) Skin By-Product by Shotgun Proteomics and Protein-Based Bioinformatics. Mar. Drugs 2019, 18, 31.
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; G Sotelo, C. Collagen Extraction Optimization from the Skin of the Small-Spotted Catshark (S. Canicula) by Response Surface Methodology. Mar. Drugs 2019, 17, 40.
- Benedetto, C.D.; Barbaglio, A.; Martinello, T.; Alongi, V.; Fassini, D.; Cullorà, E.; Patruno, M.; Bonasoro, F.; Barbosa, M.A.; Carnevali, M.D.C.; et al. Production, Characterization and Biocompatibility of Marine Collagen Matrices from an Alternative and Sustainable Source: The Sea Urchin Paracentrotus Lividus. Mar. Drugs 2014, 12, 4912–4933.
- Seixas, M.J.; Martins, E.; Reis, R.L.; Silva, T.H. Extraction and Characterization of Collagen from Elasmobranch Byproducts for Potential Biomaterial Use. Mar. Drugs 2020, 18, 617.
- Bardakova, K.N.; Akopova, T.A.; Kurkov, A.V.; Goncharuk, G.P.; Butnaru, D.V.; Burdukovskii, V.F.; Antoshin, A.A.; Farion, I.A.; Zharikova, T.M.; Shekhter, A.B.; et al. From Aggregates to Porous Three-Dimensional Scaffolds through a Mechanochemical Approach to Design Photosensitive Chitosan Derivatives. Mar. Drugs 2019, 17, 48.
- Batista, M.P.; Fernández, N.; Gaspar, F.B.; do Bronze, M.R.; Duarte, A.R.C. Extraction of Biocompatible Collagen from Blue Shark Skins through the Conventional Extraction Process Intensification Using Natural Deep Eutectic Solvents. Front. Chem. 2022, 10, 937036.
- Miron, A.; Sarbu, A.; Zaharia, A.; Sandu, T.; Iovu, H.; Fierascu, R.C.; Neagu, A.-L.; Chiriac, A.-L.; Iordache, T.-V. A Top-Down Procedure for Synthesizing Calcium Carbonate-Enriched Chitosan from Shrimp Shell Wastes. Gels 2022, 8, 742.
- Huang, C.-Y.; Kuo, C.-H.; Wu, C.-H.; Ku, M.-W.; Chen, P.-W. Extraction of Crude Chitosans from Squid (Illex Argentinus) Pen by a Compressional Puffing-Pretreatment Process and Evaluation of Their Antibacterial Activity. Food Chem. 2018, 254, 217–223.
- Uranga, J.; Etxabide, A.; Cabezudo, S.; de la Caba, K.; Guerrero, P. Valorization of Marine-Derived Biowaste to Develop Chitin/Fish Gelatin Products as Bioactive Carriers and Moisture Scavengers. Sci. Total Environ. 2020, 706, 135747.
- Acharya, P.P.; Kupendra, M.H.; Fasim, A.; More, S.S.; Murthy, V.K. A Comparative Assessment of Collagen Type 1 from Silver Carp (Fresh Water) and Milk Shark (Marine) Fish Waste. 3 Biotech 2022, 12, 82.
- Singh, A.; Benjakul, S.; Prodpran, T. Ultrasound-Assisted Extraction of Chitosan from Squid Pen: Molecular Characterization and Fat Binding Capacity. J. Food Sci. 2019, 84, 224–234.
- Amer, O.A.; Ali, S.S.; Azab, M.; El-Shouny, W.A.; Sun, J.; Mahmoud, Y.A.-G. Exploring New Marine Bacterial Species, Alcaligenes Faecalis Alca F2018 Valued for Bioconversion of Shrimp Chitin to Chitosan for Concomitant Biotechnological Applications. Int. J. Biol. Macromol. 2022, 196, 35–45.
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial Extraction of Chitin from Seafood Waste Using Sugars Derived from Fruit Waste-Stream. AMB Express 2020, 10, 17.
- Thomas, R.; Fukamizo, T.; Suginta, W. Bioeconomic Production of High-Quality Chitobiose from Chitin Food Wastes Using an in-House Chitinase from Vibrio Campbellii. Bioresour. Bioprocess. 2022, 9, 86.
- Chiarelli, P.G.; Pegg, R.B.; Dev Kumar, G.; Mis Solval, K. Exploring the Feasibility of Developing Novel Gelatin Powders from Salted, Dried Cannonball Jellyfish (Stomolophus Meleagris). Food Biosci. 2021, 44, 101397.
- Al-Ali, R.M.; Al-Hilifi, S.A.; Rashed, M.M.A. Fabrication, Characterization, and Anti-free Radical Performance of Edible Packaging-chitosan Film Synthesized from Shrimp Shell Incorporated with Ginger Essential Oil. J. Food Meas. Charact. 2021, 15, 2951–2962.
- El Harmoudi, H.; El Gaini, L.; Daoudi, E.; Rhazi, M.; Boughaleb, Y.; El Mhammedi, M.A.; Migalska-Zalas, A.; Bakasse, M. Removal of 2,4-D from Aqueous Solutions by Adsorption Processes Using Two Biopolymers: Chitin and Chitosan and Their Optical Properties. Opt. Mater. 2014, 36, 1471–1477.
- Akita, M.; Kono, T.; Lloyd, K.; Mitsui, T.; Morioka, K.; Adachi, K. Biochemical Study of Type I Collagen Purified from Skin of Warm Sea Teleost Mahi Mahi (Coryphaena Hippurus), with a Focus on Thermal and Physical Stability. J. Food Biochem. 2019, 43, e13013.
- Wisser, D.; Wisser, F.M.; Raschke, S.; Klein, N.; Leistner, M.; Grothe, J.; Brunner, E.; Kaskel, S. Biological Chitin-MOF Composites with Hierarchical Pore Systems for Air-Filtration Applications. Angew. Chem. Int. Ed. Engl. 2015, 54, 12588–12591.
- Sila, A.; Mlaik, N.; Sayari, N.; Balti, R.; Bougatef, A. Chitin and Chitosan Extracted from Shrimp Waste Using Fish Proteases Aided Process: Efficiency of Chitosan in the Treatment of Unhairing Effluents. J. Polym. Environ. 2014, 22, 78–87.
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Comparative Adsorption of Metal and Dye on Flake- and Bead-Types of Chitosans Prepared from Fishery Wastes. J. Hazard. Mater. 2000, 73, 63–75.
- Magnacca, G.; Guerretta, F.; Vizintin, A.; Benzi, P.; Valsania, M.C.; Nisticò, R. Preparation, Characterization and Environmental/Electrochemical Energy Storage Testing of Low-Cost Biochar from Natural Chitin Obtained via Pyrolysis at Mild Conditions. Appl. Surf. Sci. 2018, 427, 883–893.
- Mushi, N.E.; Kochumalayil, J.; Cervin, N.T.; Zhou, Q.; Berglund, L.A. Nanostructurally Controlled Hydrogel Based on Small-Diameter Native Chitin Nanofibers: Preparation, Structure, and Properties. ChemSusChem 2016, 9, 989–995.
- Sinha, S.; Tripathi, P.; Chand, S. A New Bifunctional Chitosanase Enzyme from Streptomyces sp. and Its Application in Production of Antioxidant Chitooligosaccharides. Appl. Biochem. Biotechnol. 2012, 167, 1029–1039.
- Eulálio, H.Y.C.; Vieira, M.; Fideles, T.B.; Tomás, H.; Silva, S.M.L.; Peniche, C.A.; Fook, M.V.L. Physicochemical Properties and Cell Viability of Shrimp Chitosan Films as Affected by Film Casting Solvents. I-Potential Use as Wound Dressing. Materials 2020, 13, 5005.
- Zhang, H.; Yun, S.; Song, L.; Zhang, Y.; Zhao, Y. The Preparation and Characterization of Chitin and Chitosan under Large-Scale Submerged Fermentation Level Using Shrimp by-Products as Substrate. Int. J. Biol. Macromol. 2017, 96, 334–339.
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Chitin Production from Crustacean Biomass: Sustainability Assessment of Chemical and Enzymatic Processes. J. Clean. Prod. 2018, 172, 4140–4151.
- Aboudamia, F.Z.; Kharroubi, M.; Neffa, M.; Aatab, F.; Hanoune, S.; Bouchdoug, M.; Jaouad, A. Potential of Discarded Sardine Scales (Sardina Pilchardus) as Chitosan Sources. J. Air Waste Manag. Assoc. 2020, 70, 1186–1197.
- Guerra, I.C.D.; de Oliveira, P.D.L.; Santos, M.M.F.; Lúcio, A.S.S.C.; Tavares, J.F.; Barbosa-Filho, J.M.; Madruga, M.S.; de Souza, E.L. The Effects of Composite Coatings Containing Chitosan and Mentha (Piperita L. or x Villosa Huds) Essential Oil on Postharvest Mold Occurrence and Quality of Table Grape Cv. Isabella. Innov. Food Sci. Emerg. Technol. 2016, 34, 112–121.
- Mittal, A.; Singh, A.; Aluko, R.E.; Benjakul, S. Pacific White Shrimp (Litopenaeus Vannamei) Shell Chitosan and the Conjugate with Epigallocatechin Gallate: Antioxidative and Antimicrobial Activities. J. Food Biochem. 2021, 45, e13569.
- Do Vale, D.A.; Vieira, C.B.; Vidal, M.F.; Claudino, R.L.; Andrade, F.K.; Sousa, J.R.; de Souza Filho, M.S.M.; da Silva, A.L.C.; de Souza, B.W.S. Chitosan-Based Edible Films Produced from Crab-Uçá (Ucides Cordatus) Waste: Physicochemical, Mechanical and Antimicrobial Properties. J. Polym. Environ. 2021, 29, 694–706.
- Nunes, C.; Maricato, É.; Cunha, Â.; Rocha, M.A.M.; Santos, S.; Ferreira, P.; Silva, M.A.; Rodrigues, A.; Amado, O.; Coimbra, J.; et al. Chitosan–Genipin Film, a Sustainable Methodology for Wine Preservation. Green Chem. 2016, 18, 5331–5341.
- Dehghani, M.H.; Dehghan, A.; Najafpoor, A. Removing Reactive Red 120 and 196 Using Chitosan/Zeolite Composite from Aqueous Solutions: Kinetics, Isotherms, and Process Optimization. J. Ind. Eng. Chem. 2017, 51, 185–195.
- Sun, W.Q.; Payne, G.F.; Moas, M.S.G.L.; Chu, J.H.; Wallace, K.K. Tyrosinase Reaction/Chitosan Adsorption for Removing Phenols from Wastewater. Biotechnol. Prog. 1992, 8, 179–186.
- Rizzi, V.; Gubitosa, J.; Fini, P.; Romita, R.; Nuzzo, S.; Cosma, P. Chitosan Biopolymer from Crab Shell as Recyclable Film to Remove/Recover in Batch Ketoprofen from Water: Understanding the Factors Affecting the Adsorption Process. Materials 2019, 12, 3810.
- Jaiswal, M.; Chauhan, D.; Sankararamakrishnan, N. Copper Chitosan Nanocomposite: Synthesis, Characterization, and Application in Removal of Organophosphorous Pesticide from Agricultural Runoff. Environ. Sci. Pollut. Res. Int. 2012, 19, 2055–2062.
- Sun, T.-C.; Yan, B.-Y.; Ning, X.-C.; Tang, Z.-Y.; Hui, C.; Hu, M.-Z.; Ramakrishna, S.; Long, Y.-Z.; Zhang, J. A Nanofiber Hydrogel Derived Entirely from Ocean Biomass for Wound Healing. Nanoscale Adv. 2022, 5, 160–170.
- Babeanu, N.; Radu, N.; Enascuta, C.-E.; Alexandrescu, E.; Ganciarov, M.; Mohammed, M.S.O.; Suica-Bunghez, I.R.; Senin, R.; Ursu, M.; Bostan, M. Obtaining and Characterizing Composite Biomaterials of Animal Resources with Potential Applications in Regenerative Medicine. Polymers 2022, 14, 3544.
- El-Beltagi, H.S.; El-Mahdy, O.M.; Mohamed, H.I.; El-Ansary, A.E. Antioxidants, Antimicrobial, and Anticancer Activities of Purified Chitinase of Talaromyces Funiculosus Strain CBS 129594 Biosynthesized Using Crustacean Bio-Wastes. Agronomy 2022, 12, 2818.
- Rodriguez-Veiga, I.; Acosta, N.; Aranaz, I.; Dobrzycka-Krahel, A. Exploring Saduria Entomon (Crustacea Isopoda) as a New Source for Chitin and Chitosan Isolation. Int. J. Mol. Sci. 2022, 23, 16125.
- Abdullah, N.A.S.; Mohamad, Z. The Effect of Dynamic Vulcanization on the Morphological and Mechanical Properties of the Toughened Poly (Lactic Acid)/Epoxidized Natural Rubber. Malays. J. Fundam. Appl. Sci. 2018, 14, 348–352.
- Balitaan, J.N.I.; Yeh, J.-M.; Santiago, K.S. Marine Waste to a Functional Biomaterial: Green Facile Synthesis of Modified-β-Chitin from Uroteuthis Duvauceli Pens (Gladius). Int. J. Biol. Macromol. 2020, 154, 1565–1575.
- Gallo, N.; Natali, M.L.; Quarta, A.; Gaballo, A.; Terzi, A.; Sibillano, T.; Giannini, C.; De Benedetto, G.E.; Lunetti, P.; Capobianco, L.; et al. Aquaponics-Derived Tilapia Skin Collagen for Biomaterials Development. Polymers 2022, 14, 1865.
- Ahmed, M.; Anand, A.; Verma, A.K.; Patel, R. In-Vitro Self-Assembly and Antioxidant Properties of Collagen Type I from Lutjanus Erythropterus, and Pampus Argenteus Skin. Biocatal. Agric. Biotechnol. 2022, 43, 102412.
- Addad, S.; Exposito, J.-Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, Characterization and Biological Evaluation of Jellyfish Collagen for Use in Biomedical Applications. Mar. Drugs 2011, 9, 967–983.
- Tziveleka, L.-A.; Kikionis, S.; Karkatzoulis, L.; Bethanis, K.; Roussis, V.; Ioannou, E. Valorization of Fish Waste: Isolation and Characterization of Acid- and Pepsin-Soluble Collagen from the Scales of Mediterranean Fish and Fabrication of Collagen-Based Nanofibrous Scaffolds. Mar. Drugs 2022, 20, 664.
More
Information
Subjects:
Marine & Freshwater Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
886
Revisions:
2 times
(View History)
Update Date:
06 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




