Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dalila Di Francesco | -- | 2233 | 2023-12-04 10:23:07 | | | |
| 2 | Lindsay Dong | Meta information modification | 2233 | 2023-12-06 01:34:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Di Francesco, D.; Pigliafreddo, A.; Casarella, S.; Di Nunno, L.; Mantovani, D.; Boccafoschi, F. Biological Materials for Tissue-Engineered Vascular Grafts. Encyclopedia. Available online: https://encyclopedia.pub/entry/52320 (accessed on 07 March 2026).
Di Francesco D, Pigliafreddo A, Casarella S, Di Nunno L, Mantovani D, Boccafoschi F. Biological Materials for Tissue-Engineered Vascular Grafts. Encyclopedia. Available at: https://encyclopedia.pub/entry/52320. Accessed March 07, 2026.
Di Francesco, Dalila, Alexa Pigliafreddo, Simona Casarella, Luca Di Nunno, Diego Mantovani, Francesca Boccafoschi. "Biological Materials for Tissue-Engineered Vascular Grafts" Encyclopedia, https://encyclopedia.pub/entry/52320 (accessed March 07, 2026).
Di Francesco, D., Pigliafreddo, A., Casarella, S., Di Nunno, L., Mantovani, D., & Boccafoschi, F. (2023, December 04). Biological Materials for Tissue-Engineered Vascular Grafts. In Encyclopedia. https://encyclopedia.pub/entry/52320
Di Francesco, Dalila, et al. "Biological Materials for Tissue-Engineered Vascular Grafts." Encyclopedia. Web. 04 December, 2023.
Copy Citation
The clinical demand for tissue-engineered vascular grafts is still rising, and there are many challenges that need to be overcome to obtain functional grafts with appropriate biological and mechanical properties. The many advances made in cell culture, biomaterials, manufacturing techniques, and tissue engineering methods have led to various promising solutions for vascular graft production, and materials from natural sources have recently gained more attention for vascular tissue engineering, as new strategies have been developed to solve the disadvantages related to their use.
vascular tissue engineering
natural biomaterials
tissue-engineered vascular grafts
1. Introduction
Cardiovascular diseases (CVDs) are a group of pathologies that affect the cardiac and vascular systems; they include coronary heart disease, cerebrovascular pathologies, rheumatic heart disease, and other conditions. CVDs are the main cause of death in the world, with 17.9 million deaths per year, according to the World Health Organization (WHO). Almost 85% of CVD-related deaths are caused by heart attack and strokes. [1]. The main cause of these pathologies is atherosclerosis, a progressive condition characterized by the formation of atherosclerotic plaques that develop in the intima layer of blood vessels and lead to the partial or total obstruction of vessels [2]. The most common treatment is pharmaceutical therapy, coupled with a healthy lifestyle and balanced diet; however, in the case of occlusive CVDs, the ultimate treatment options are surgical, represented by vascular stents, substitution surgery, or vascular bypass. The latter solutions aim at replacing the damaged vessel or at redirecting blood flow around it through the use of vascular grafts [3]. For these purposes, the main source of vascular grafts are autologous blood vessels, which are naturally biocompatible, non-thrombogenic, and have the necessary mechanical properties to suit vascular application [4]. However, this treatment option is still hampered by different problems, the main one being implant failure [5]. Media layer hyperplasia or vein graft disease can also arise after implantation, which lead to the occlusion of the lumen and implanted graft [6][7][8]. Moreover, the use of autologous blood vessels is not always an option, due to either the multiple surgical procedures required, the patients’ age and health conditions, or the mismatch of blood vessel dimensions [9]. As an alternative, commercial vascular grafts can be used. Most of these are usually made from synthetic materials such as polyethylene terephthalate (PET) and polytetrafluoroethylene (e-PTFE); however, some biological commercial products can also be found. LeMaitre Vascular produces natural commercial grafts such as Artegraft®, a xenograft from bovine collagen, ProCol®, a bioprothesis from the bovine mesenteric vein, or Omniflow II®, which is a biosynthetic graft. Bioprotec S.A.S.U. produces human-derived vascular grafts from the saphenous vein which range from small- to medium-caliber applications. Even though there are commercial alternatives to autologous bypass, they still present some limitations: their mechanical properties are not always appropriate and they may cause aneurysms and/or thrombosis, allergic reactions, and, generally, have a high implant failure rate, and low patency rates for small-diameter grafts [10][11][12][13][14][15][16][17].
The success of the existing approaches is still constrained by the aforementioned problems; thus, valid alternatives to obtain functional vascular conduits are needed. One approach that represents a potential solution is vascular tissue engineering (VTE), a branch of regenerative medicine that aims at producing innovative solutions to substitute damaged blood vessels. VTE relies on the production of tissue-engineered vascular grafts (TEVGs), which are made in vitro by combining cells, biomaterials, fabrication techniques, and tissue maturation methods [18][19]. Appropriate mechanical and biological properties for TEVGs’ success are difficult to achieve, and grafts’ failure greatly depends on the choice of cells, biomaterials, the technique used to manufacture the graft, and how these parts interact with each other, however, innovations in the field have allowed to bring advancements in vascular graft production, especially using natural biomaterials [20][21].
2. Vascular Tissue Engineering Requirements and Fundamentals
TEVGs should have specific requirements that allow them to closely mimic physiological conditions [22]. The structure of native blood vessels, as presented in Figure 1, allows to achieve the necessary biological and mechanical properties for correct blood vessel function, therefore, to prepare TEVGs, certain requirments should be met, such as noncytotoxicity, nonthrombogenicity, hemocompatibility, cell repopulation potential, ability to bear blood pressure, compliace and patency maintainance [23][24][25][26].
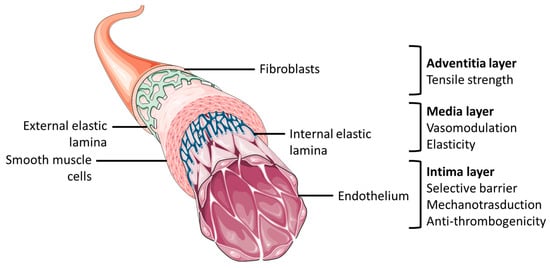
Figure 1. General anatomical structure of blood vessel and layers’ functional characteristics. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
In order to achieve these complex properties, appropriate cell sources, like vascular cells either stem cells or primary cell lines, optimal biomaterial, such as synthetic, natural or hybrid biomaterials, fabrication techniques, likes electrospinning, 3D-bioprinting, or cell sheet engineering, and maturation techniques, such as static or dynamic cell maturation, are important elements to consider; and, as a result, these constitute the fundamentals of VTE (Figure 2).
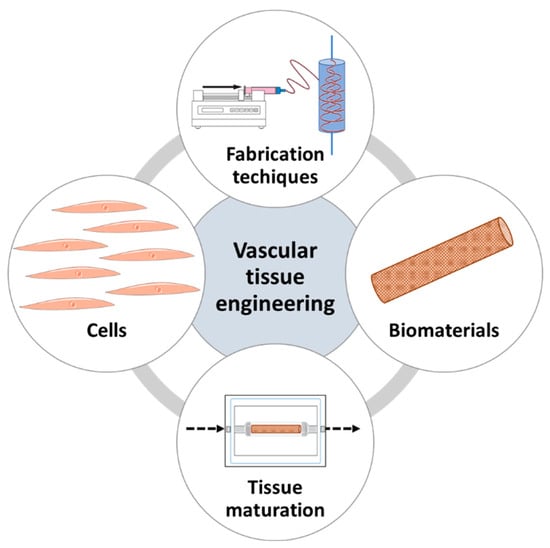
Figure 2. The fundamentals of vascular tissue engineering.
3. Natural Biomaterials for Vascular Tissue Engineering
The success of a TEVG is highly dependent on the choice of the biomaterial used, which should have some key features to appropriately fulfil its use. As of late, bioactivity has been regarded as one of the most important biomaterial properties for tissue engineering applications [27], and this property is mainly found in naturally derived biomaterials, which are able to provide necessary biological cues to interact and guide cell functions. These biomaterials display biocompatibility, bioactivity, and biodegradation, and are mostly nonimmunogenic; however, they are difficult to fine-tune, show batch-to-batch variability, and commonly display weak mechanical properties [28][29][30]. For VTE applications, the appropriate interaction between the material and the blood interface is a necessary feature for a TEVG’s success, together with the endothelialization process and the control of SMCs’ proliferation and functionality. Thus, the domains found in naturally derived materials are suitable for this, as they allow for cell recognition and adhesion [3].
3.1. Collagen
Collagen is the most abundant protein in the body, representing a third of total proteins and the main component of ECM [31][32]. The use of collagen in tissue engineering is common due to its many advantages such as biocompatibility, cell adhesion sites, biodegradability, nonimmunogenicity, availability, and hydrophilicity. Collagen is the most commonly used natural material for VTE, as it is the main ECM component of the vascular wall, responsible for load carrying and pressure resistance [33]. Its use for vascular graft fabrication dates back to 1986 [34], and, with time, more complex TEVGs were fabricated with collagen as the core biomaterial [33][35][36]. Recent research works have shown the different advances made in the use of collagen for TEVGs production, achieving collagen constructs with better mechanical properties [37][38][39]. The use of collagen still presents limitations, however, due to the excellent biological, potential it is still one of the most interesting natural materials for TEVG production.
3.2 Gelatin
Gelatin is a material derived from the denaturation of collagen’s triple helix. Thanks to its biocompatibility, biodegradability, low cytotoxicity, immunogenicity, and, finally, low cost, gelatin has been widely used as a biomaterial for tissue engineering. A disadvantage of gelatin is related to the need for chain reticulation in order to maintain its stability, thus it is mainly used in the functionalized form: gelatin methacryloyl (GelMA) and is often reticulated with other materials [40][41]. Still, different works have shown the potential of gelatin biomaterials for VTE [42][43][44], and its use in combination with other materials for VTE is promising to enhance biological properties and endothelialization.
3.3. Fibrin
Fibrin is the active and insoluble form of fibrinogen, a protein involved in the coagulation cascade and wound healing. Because of its adhesive properties, it is widely used as a sealant in biomedical applications [45]. It is an interesting material for scaffold design, both in gel and fiber form and it can be isolated from a patient’s plasma, representing an interesting option in terms of personalized tailored biomaterials, limiting immunological reaction risks [46]. Fibrin fibers can closely replicate the structure of ECM and guide cell functions and remodeling [47] so, it is frequently found used in combination with other materials for VTE purposes [48][49][50]. Fibrin undoubtedly demonstrates many advantages for TEVGs’ production, especially because of the possibility of obtaining it from patients’ blood. However, the studies performed still use it in combination with other materials in order to achieve the necessary mechanical properties.
3.4. Elastin
Elastin is a protein of the connective tissue responsible for tissue elasticity. The tropoelastin fibers provide recoil to tissues that undergo stretching forces and in the vascular tissue, elastin plays both a mechanical and biosensing role, allowing for elastic expansion and contraction. Moreover, elastin plays a role in the inhibition of SMCs’ hyperproliferation and has antithrombotic properties [51]. Collagen and elastin are also often used in combination for VTE, as they are both major components of blood vessels and elastin is used to provide elasticity to the scaffold. These types of scaffolds present higher porosity and structural features that promote their use for small-caliber TEVGs, while stimulating endothelialization and preventing SMCs’ hyperplasia [40]. Aside from collagen and elastin composites, elastin has been widely reported to increase the elastic properties of TEVGs in combination with other materials [52]. Even though the use of elastin retains the limitation of its insolubility, its use has gained interest in the field of VTE, demonstrating the ability to improve mechanical and biological properties and decreasing platelet adhesion.
3.5. Silk
Silk is a versatile biopolymer mainly produced by insects. This natural material is extremely resistant to traction, aside from being very biocompatible, and has been broadly used for surgical sutures. In recent decades, silk has also gathered interest for the production of bioscaffolds in VTE, because it demonstrates interesting advantages such as controllable biodegradation, low immunogenicity, extraordinary mechanical strength, and wide scaffolding applications, as it can be used in the form of films, hydrogels, nanofibers, and nanoparticles. Moreover, it is an easily accessible material, both eco-sustainable and low cost [53][54]. Silk can be used alone or in combination with other materials for TEVGs [55][56][57], demonstrating its suitability for this application.
3.6. Chitosan
Chitosan is a linear polysaccharide derived from chitin’s partial deacetylation, found in the exoskeleton of arthropods. Structurally, chitosan is very similar to glycosaminoglycans contained in the ECM, and as a biomaterial it presents several advantages: it is easily sterilisable, low cost, bioactive, and highly hydrophilic. Its degradability can be controlled, and, notably, it has antibacterial and antifungal properties. Thus, it was also explored as a scaffold for TEVGs production. However, its mechanical properties are far from resembling those of native blood vessels; to overcome this limitation, chitosan is often reticulated with other polymers [40][58][59][60]. Chitosan shows unique antibacterial and antithrombogenic properties; therefore, it is used in VTE to improve these aspects when they are lacking in other materials. However, it remains difficult to find uses for chitosan as a sole material for TEVG production. With further innovations in manufacturing and modifications, technologies may bring research closer to improving its use for VTE.
3.7. Decellularized Extracellular Matrix
Decellularized vessels were explored as a means to obtain a tubular scaffold directly from the vessel, and this strategy has led to the development of several vascular grafts, which have indeed reached clinical trials. However, decellularized extracellular matrix (dECM) derived from other tissue sources can also be used for scaffold production. In particular, after decellularization, the matrix can be further processed in order to be solubilized, then used as hydrogels, bioinks, or electrospinning solution. dECM is one of the most bioactive materials that can be found, as it is the one that most closely resembles the native ECM environment. In VTE, it has been widely used, both alone and in combination with other materials, to enhance their mechanical properties and provide them with superior bioactivity [61][62][63][64][65][65].
4. Conclusions
In a context where CDVs are the main cause of death in the world and vessel substitution or bypass are often required, VTE has been shown to be a promising alternative to autologous vascular grafts. Although it is a challenge to replicate the necessary biological and mechanical properties, great progress has been made in the production of TEVGs. New fabrication techniques, insights into biomaterial design, and innovative tissue maturation strategies have led to improved results. Natural materials have received more attention due to their innate bioactivity, and, thanks to the progress made in the past decades, some of the problems tied to their use have been overcome. In particular, the advances in fabrication techniques have allowed better manipulation and tailorability of natural materials, which were significant challenges in the use of biological materials; at the same time, many research works reported herein also demonstrated results in obtaining natural-based TEVGs with appropriate mechanical properties for blood vessel replacement. Astonishingly, many research works even reported the production of small-caliber TEVGs made with natural materials, an achievement that has challenged researchers for many years. These innovations have also led to many works obtaining promising results with in vivo experimentation using natural-based TEVGs. Biomaterials like collagen and elastin remain the top choices when it concerns biomaterials for VTE; some collagen products have reached the market, such as Artegraft® or ProCol®, while new manufacturing techniques, such as electrospinning and 3D printing, are taking over and being used more frequently to obtain highly controlled graft ultrastructures. However, challenges with the use of all-natural TEVGs still remain, such as the ability to obtain functional and effective vascular grafts (encompassing both biological and mechanical properties, made of a single and natural component) and if—and when—most of these will be able to achieve commercialization. However, considering the positive advances reported herein, TEVGs from natural material scaffolds show potential for being translated from research to clinical practice in the near future, while other natural TEVGs, such as cell-derived TEVGs or hybrid TEVGs, have already been utilized in human trials [66][67][68].
References
- Kim, H.C. Epidemiology of Cardiovascular Disease and Its Risk Factors in Korea. Glob. Health Med. 2021, 3, 134–141.
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS): Document Covering Atherosclerotic Disease of Extracranial Carotid and Vertebral, Mesenteric, Renal, Upper and Lower Extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816.
- Moore, M.J.; Tan, R.P.; Yang, N.; Rnjak-Kovacina, J.; Wise, S.G. Bioengineering Artificial Blood Vessels from Natural Materials. Trends Biotechnol. 2022, 40, 693–707.
- Farkouh, M.E.; Domanski, M.; Sleeper, L.A.; Siami, F.S.; Dangas, G.; Mack, M.; Yang, M.; Cohen, D.J.; Rosenberg, Y.; Solomon, S.D.; et al. Strategies for Multivessel Revascularization in Patients with Diabetes. N. Engl. J. Med. 2012, 367, 2375–2384.
- Hall, A.B.; Brilakis, E.S. Saphenous Vein Graft Failure: Seeing the Bigger Picture. J. Thorac. Dis. 2019, 11, S1441–S1444.
- de Vries, M.R.; Simons, K.H.; Jukema, J.W.; Braun, J.; Quax, P.H.A. Vein Graft Failure: From Pathophysiology to Clinical Outcomes. Nat. Rev. Cardiol. 2016, 13, 451–470.
- Motwani, J.G.; Topol, E.J. Aortocoronary Saphenous Vein Graft Disease: Pathogenesis, Predisposition, and Prevention. Circulation 1998, 97, 916–931.
- Harskamp, R.E.; Lopes, R.D.; Baisden, C.E.; de Winter, R.J.; Alexander, J.H. Saphenous Vein Graft Failure After Coronary Artery Bypass Surgery: Pathophysiology, Management, and Future Directions. Ann. Surg. 2013, 257, 824–833.
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft—Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100.
- Stöwe, I.; Pissarek, J.; Moosmann, P.; Pröhl, A.; Pantermehl, S.; Bielenstein, J.; Radenkovic, M.; Jung, O.; Najman, S.; Alkildani, S.; et al. Ex Vivo and In Vivo Analysis of a Novel Porcine Aortic Patch for Vascular Reconstruction. Int. J. Mol. Sci. 2021, 22, 7623.
- van de Laar, B.C.; van Heusden, H.C.; Pasker-de Jong, P.C.; van Weel, V. Omniflow II Biosynthetic Grafts versus Expanded Polytetrafluoroethylene Grafts for Infrainguinal Bypass Surgery. A Single-Center Retrospective Analysis. Vascular 2022, 30, 749–758.
- Lin, C.-H.; Hsia, K.; Ma, H.; Lee, H.; Lu, J.-H. In Vivo Performance of Decellularized Vascular Grafts: A Review Article. Int. J. Mol. Sci. 2018, 19, 2101.
- Stieglmeier, F.; Grab, M.; König, F.; Büch, J.; Hagl, C.; Thierfelder, N. Mapping of Bovine Pericardium to Enable a Standardized Acquirement of Material for Medical Implants. J. Mech. Behav. Biomed. Mater. 2021, 118, 104432.
- Obiweluozor, F.O.; Emechebe, G.A.; Kim, D.-W.; Cho, H.-J.; Park, C.H.; Kim, C.S.; Jeong, I.S. Considerations in the Development of Small-Diameter Vascular Graft as an Alternative for Bypass and Reconstructive Surgeries: A Review. Cardiovasc. Eng. Technol. 2020, 11, 495–521.
- Li, M.-X.; Wei, Q.-Q.; Mo, H.-L.; Ren, Y.; Zhang, W.; Lu, H.-J.; Joung, Y.K. Challenges and Advances in Materials and Fabrication Technologies of Small-Diameter Vascular Grafts. Biomater. Res. 2023, 27, 58.
- Rodríguez-Soto, M.A.; Polanía-Sandoval, C.A.; Aragón-Rivera, A.M.; Buitrago, D.; Ayala-Velásquez, M.; Velandia-Sánchez, A.; Peralta Peluffo, G.; Cruz, J.C.; Muñoz Camargo, C.; Camacho-Mackenzie, J.; et al. Small Diameter Cell-Free Tissue-Engineered Vascular Grafts: Biomaterials and Manufacture Techniques to Reach Suitable Mechanical Properties. Polymers 2022, 14, 3440.
- Skovrind, I.; Harvald, E.B.; Juul Belling, H.; Jørgensen, C.D.; Lindholt, J.S.; Andersen, D.C. Concise Review: Patency of Small-Diameter Tissue-Engineered Vascular Grafts: A Meta-Analysis of Preclinical Trials. Stem Cells Transl. Med. 2019, 8, 671–680.
- Sin-Guang Chen; Felix Ugwu; Wan-Chun Li; Noel M. Caplice; Eugen Bogdan Petcu; Shea Ping Yip; Chien-Ling Huang; Vascular Tissue Engineering: Advanced Techniques and Gene Editing in Stem Cells for Graft Generation. Tissue Eng. Part B: Rev. 2021, 27, 14-28.
- Prerak Gupta; Biman B. Mandal; Silk biomaterials for vascular tissue engineering applications. Acta Biomater. 2021, 134, 79-106.
- Ryuma Iwaki; Toshihiro Shoji; Yuichi Matsuzaki; Anudari Ulziibayar; Toshiharu Shinoka; Current status of developing tissue engineering vascular technologies. Expert Opin. Biol. Ther. 2021, 22, 433-440.
- Maria A. Rodriguez-Soto; Natalia Suarez Vargas; Alejandra Riveros; Carolina Muñoz Camargo; Juan C. Cruz; Nestor Sandoval; Juan C. Briceño; Failure Analysis of TEVG’s I: Overcoming the Initial Stages of Blood Material Interaction and Stabilization of the Immune Response. Cells 2021, 10, 3140.
- Wei, Y.; Wang, F.; Guo, Z.; Zhao, Q. Tissue-Engineered Vascular Grafts and Regeneration Mechanisms. J. Mol. Cell. Cardiol. 2022, 165, 40–53.
- Samand Pashneh-Tala; Sheila MacNeil; Frederik Claeyssens; Rachel E. Gurlin; Jaime A. Giraldo; Esther Latres; Alicia Fernández-Colino; Stefan Jockenhoevel; Renee Duijvelshoff; Andrea di Luca; et al.Eline E. van HaaftenSylvia DekkerSerge H.M. SöntjensHenk M. JanssenAnthal I.P.M. SmitsPatricia Y.W. DankersCarlijn V.C. BoutenJaime A GiraldoSin-Guang ChenFelix UgwuWan-Chun LiNoel M. CapliceEugen PetcuShea Ping YipChien-Ling HuangXiaoqiang CongShang-Min ZhangLuke BattyJiesi LuoMaziyar KeshavarzianClark A. MeyerHeather N. HayengaWouter Jan GeelhoedReshma A. LalaiJoep H. SinnigePatrick J. JongeleenCornelis StormJoris I. RotmansZiba RoveimiabFrancis LinJudy E. AndersonRui Han LiuChin Siang OngTakuma FukunishiKingsfield OngNarutoshi HibinoYukiharu SugimuraAnna Kathrin SchmidtArtur LichtenbergAlexander AssmannPayam AkhyariNitsan DahanUdi SarigTomer BronshteinLimor BaruchTony KarramAaron HoffmanMarcelle Machluf The Tissue-Engineered Vascular Graft—Past, Present, and Future. Tissue Eng. Part B: Rev. 2016, 22, 68-100.
- David Durán-Rey; Verónica Crisóstomo; Juan A. Sánchez-Margallo; Francisco M. Sánchez-Margallo; Systematic Review of Tissue-Engineered Vascular Grafts. Front. Bioeng. Biotechnol. 2021, 9, 771400.
- Prerak Gupta; Biman B. Mandal; Tissue‐Engineered Vascular Grafts: Emerging Trends and Technologies. Adv. Funct. Mater. 2021, 31, 2100027.
- J Chlupáč; E Filová; L Bačáková; Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol. Res. 2009, 58 Suppl 2, S119-S140.
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive Potential of Natural Biomaterials: Identification, Retention and Assessment of Biological Properties. Sig Transduct. Target. Ther. 2021, 6, 122.
- Brovold, M.; Almeida, J.I.; Pla-Palacín, I.; Sainz-Arnal, P.; Sánchez-Romero, N.; Rivas, J.J.; Almeida, H.; Dachary, P.R.; Serrano-Aulló, T.; Soker, S.; et al. Naturally-Derived Biomaterials for Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1077, 421–449.
- Ullah, S.; Chen, X. Fabrication, Applications and Challenges of Natural Biomaterials in Tissue Engineering. Appl. Mater. Today 2020, 20, 100656.
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115.
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958.
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307.
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166.
- Weinberg, C.B.; Bell, E. A Blood Vessel Model Constructed from Collagen and Cultured Vascular Cells. Science 1986, 231, 397–400.
- He, W.; Ma, Z.; Yong, T.; Teo, W.E.; Ramakrishna, S. Fabrication of Collagen-Coated Biodegradable Polymer Nanofiber Mesh and Its Potential for Endothelial Cells Growth. Biomaterials 2005, 26, 7606–7615.
- Lo, S.; Fauzi, M.B. Current Update of Collagen Nanomaterials—Fabrication, Characterisation and Its Applications: A Review. Pharmaceutics 2021, 13, 316.
- Dimitria B. Camasão; Ling Li; Bernard Drouin; Cori Lau; Dieter P. Reinhardt; Diego Mantovani; Physiologically relevant platform for an advanced in vitro model of the vascular wall: focus on in situ fabrication and mechanical maturation. Vitr. Model. 2022, 1, 179-195.
- Èlia Bosch-Rué; Leire Díez-Tercero; Luis M. Delgado; Román A. Pérez; Biofabrication of Collagen Tissue-Engineered Blood Vessels with Direct Co-Axial Extrusion. Int. J. Mol. Sci. 2022, 23, 5618.
- Alexander W. Justin; Federico Cammarata; Andrew A Guy; Silas R Estevez; Sebastian Burgess; Hongorzul Davaapil; Agavi Stavropoulou-Tatla; John Ong; Aishwarya G. Jacob; Kourosh Saeb-Parsy; Sanjay Sinha; Athina E. Markaki. Densified Collagen Tubular Grafts for Human Tissue Replacement and Disease Modelling Applications; Cold Spring Harbor Laboratory: Woodbury, NY, United States, 2021; pp. 213245.
- Thottappillil, N.; Nair, P.D. Scaffolds in Vascular Regeneration: Current Status. Vasc. Health Risk Manag. 2015, 11, 79–91.
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177.
- Peng, K.; Liu, X.; Zhao, H.; Lu, H.; Lv, F.; Liu, L.; Huang, Y.; Wang, S.; Gu, Q. 3D Bioprinting of Reinforced Vessels by Dual-Cross-Linked Biocompatible Hydrogels. ACS Appl. Bio Mater. 2021, 4, 4549–4556.
- Joy, J.; Pereira, J.; Aid-Launais, R.; Pavon-Djavid, G.; Ray, A.R.; Letourneur, D.; Meddahi-Pellé, A.; Gupta, B. Gelatin—Oxidized Carboxymethyl Cellulose Blend Based Tubular Electrospun Scaffold for Vascular Tissue Engineering. Int. J. Biol. Macromol. 2018, 107, 1922–1935.
- Xingmao Li; Lin Huang; Long Li; Ya Tang; Qibin Liu; Haibo Xie; Jialiang Tian; Shaobing Zhou; Geng Tang; Biomimetic dual-oriented/bilayered electrospun scaffold for vascular tissue engineering. J. Biomater. Sci. Polym. Ed. 2019, 31, 439-455.
- Jackson, M.R. Fibrin Sealants in Surgical Practice: An Overview. Am. J. Surg. 2001, 182, S1–S7.
- Catto, V.; Farè, S.; Freddi, G.; Tanzi, M. Vascular Tissue Engineering: Recent Advances in Small Diameter Blood Vessel Regeneration. ISRN Vasc. Med. 2014, 2014, 27.
- Liu, R.H.; Ong, C.S.; Fukunishi, T.; Ong, K.; Hibino, N. Review of Vascular Graft Studies in Large Animal Models. Tissue Eng. Part B Rev. 2018, 24, 133–143.
- Elliott, M.B.; Matsushita, H.; Shen, J.; Yi, J.; Inoue, T.; Brady, T.; Santhanam, L.; Mao, H.-Q.; Hibino, N.; Gerecht, S. Off-the-Shelf, Heparinized Small Diameter Vascular Graft Limits Acute Thrombogenicity in a Porcine Model. Acta Biomater. 2022, 151, 134–147.
- Lei Yang; Xiafei Li; Yiting Wu; Pengchong Du; Lulu Sun; Zhenyang Yu; Shuang Song; Jianshen Yin; Xianfen Ma; Changqin Jing; et al.Junqiang ZhaoHongli ChenYuzhen DongQiqing ZhangLiang Zhao Preparation of PU/Fibrin Vascular Scaffold with Good Biomechanical Properties and Evaluation of Its Performance in vitro and in vivo. Int. J. Nanomed. 2020, ume 15, 8697-8715.
- Liang Zhao; Xiafei Li; Lei Yang; Lulu Sun; Songfeng Mu; Haibin Zong; Qiong Li; Fengyao Wang; Shuang Song; Chengqiang Yang; et al.Changhong ZhaoHongli ChenRui ZhangShicheng WangYuzhen DongQiqing Zhang Evaluation of remodeling and regeneration of electrospun PCL/fibrin vascular grafts in vivo. Mater. Sci. Eng. C 2020, 118, 111441-111441.
- Wang, Z.; Liu, L.; Mithieux, S.M.; Weiss, A.S. Fabricating Organized Elastin in Vascular Grafts. Trends Biotechnol. 2021, 39, 505–518.
- Tanaka, T.; Abe, Y.; Cheng, C.-J.; Tanaka, R.; Naito, A.; Asakura, T. Development of Small-Diameter Elastin-Silk Fibroin Vascular Grafts. Front. Bioeng. Biotechnol. 2021, 8, 622220.
- Alberto Settembrini; Gianluca Buongiovanni; Piergiorgio Settembrini; Antonio Alessandrino; Giuliano Freddi; Giulia Vettor; Eugenio Martelli; In-vivo evaluation of silk fibroin small-diameter vascular grafts: state of art of preclinical studies and animal models. Front. Surg. 2023, 10, 1090565.
- Guru Janani; Manishekhar Kumar; Dimple Chouhan; Joseph Christakiran Moses; Ankit Gangrade; Sohenii Bhattacharjee; Biman B. Mandal; Insight into Silk-Based Biomaterials: From Physicochemical Attributes to Recent Biomedical Applications. ACS Appl. Bio Mater. 2019, 2, 5460-5491.
- Rodriguez, M.; Kluge, J.A.; Smoot, D.; Kluge, M.A.; Schmidt, D.F.; Paetsch, C.R.; Kim, P.S.; Kaplan, D.L. Fabricating Mechanically Improved Silk-Based Vascular Grafts by Solution Control of the Gel-Spinning Process. Biomaterials 2020, 230, 119567.
- Rizzi, S.; Mantero, S.; Boschetti, F.; Pesce, M. Luminal Endothelialization of Small Caliber Silk Tubular Graft for Vascular Constructs Engineering. Front. Cardiovasc. Med. 2022, 9, 1013183.
- Xinyu Shi; Xiaoyu Wang; Wei Shen; Wanfu Yue; Biocompatibility of silk methacrylate/gelatin-methacryloyl composite hydrogel and its feasibility as a vascular tissue engineering scaffold. Biochem. Biophys. Res. Commun. 2023, 650, 62-72.
- Szulc, M.; Lewandowska, K. Biomaterials Based on Chitosan and Its Derivatives and Their Potential in Tissue Engineering and Other Biomedical Applications—A Review. Molecules 2022, 28, 247.
- Wang, Q.; Wang, X.; Feng, Y. Chitosan Hydrogel as Tissue Engineering Scaffolds for Vascular Regeneration Applications. Gels 2023, 9, 373.
- Iffa A. Fiqrianti; Prihartini Widiyanti; Muhammad A. Manaf; Claudia Y. Savira; Nadia R. Cahyani; Fitria R. Bella; Poly-L-lactic Acid (PLLA)-Chitosan-Collagen Electrospun Tube for Vascular Graft Application. J. Funct. Biomater. 2018, 9, 32.
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized Extracellular Matrix: New Promising and Challenging Biomaterials for Regenerative Medicine. Biomaterials 2022, 289, 121786.
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.-W. Decellularized Extracellular Matrix-Based Bioinks for Engineering Tissue- and Organ-Specific Microenvironments. Chem. Rev. 2020, 120, 10608–10661.
- Liao, J.; Xu, B.; Zhang, R.; Fan, Y.; Xie, H.; Li, X. Applications of Decellularized Materials in Tissue Engineering: Advantages, Drawbacks and Current Improvements, and Future Perspectives. J. Mater. Chem. B 2020, 8, 10023–10049.
- Gao, G.; Lee, J.H.; Jang, J.; Lee, D.H.; Kong, J.-S.; Kim, B.S.; Choi, Y.-J.; Jang, W.B.; Hong, Y.J.; Kwon, S.-M.; et al. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease. Adv. Funct. Mater. 2017, 27, 1700798.
- Jie Shi; Yanjiao Teng; Duo Li; Ju He; Adam C. Midgley; Xiaoqin Guo; Xiudan Wang; Xinran Yang; Shufang Wang; Yakai Feng; et al.Qi LvShike Hou Biomimetic tri-layered small-diameter vascular grafts with decellularized extracellular matrix promoting vascular regeneration and inhibiting thrombosis with the salidroside. Mater. Today Bio 2023, 21, 100709.
- Gutowski, P.; Gage, S.M.; Guziewicz, M.; Ilzecki, M.; Kazimierczak, A.; Kirkton, R.D.; Niklason, L.E.; Pilgrim, A.; Prichard, H.L.; Przywara, S.; et al. Arterial Reconstruction with Human Bioengineered Acellular Blood Vessels in Patients with Peripheral Arterial Disease. J. Vasc. Surg. 2020, 72, 1247–1258.
- Lawson, J.H.; Glickman, M.H.; Ilzecki, M.; Jakimowicz, T.; Jaroszynski, A.; Peden, E.K.; Pilgrim, A.J.; Prichard, H.L.; Guziewicz, M.; Przywara, S.; et al. Bioengineered Human Acellular Vessels for Dialysis Access in Patients with End-Stage Renal Disease: Two Phase 2 Single-Arm Trials. Lancet 2016, 387, 2026–2034.
- Drews, J.D.; Pepper, V.K.; Best, C.A.; Szafron, J.M.; Cheatham, J.P.; Yates, A.R.; Hor, K.N.; Zbinden, J.C.; Chang, Y.-C.; Mirhaidari, G.J.M.; et al. Spontaneous Reversal of Stenosis in Tissue-Engineered Vascular Grafts. Sci. Transl. Med. 2020, 12, eaax6919.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
616
Revisions:
2 times
(View History)
Update Date:
06 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





