Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yuki Daigo | -- | 2543 | 2023-12-04 09:50:55 | | | |
| 2 | Fanny Huang | -6 word(s) | 2537 | 2023-12-06 03:22:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Daigo, Y.; Daigo, E.; Fukuoka, H.; Fukuoka, N.; Idogaki, J.; Taniguchi, Y.; Tsutsumi, T.; Ishikawa, M.; Takahashi, K. Treatment of the Lips Using a CO2 Laser. Encyclopedia. Available online: https://encyclopedia.pub/entry/52314 (accessed on 07 February 2026).
Daigo Y, Daigo E, Fukuoka H, Fukuoka N, Idogaki J, Taniguchi Y, et al. Treatment of the Lips Using a CO2 Laser. Encyclopedia. Available at: https://encyclopedia.pub/entry/52314. Accessed February 07, 2026.
Daigo, Yuki, Erina Daigo, Hiroshi Fukuoka, Nobuko Fukuoka, Jun Idogaki, Yusuke Taniguchi, Takashi Tsutsumi, Masatsugu Ishikawa, Kazuya Takahashi. "Treatment of the Lips Using a CO2 Laser" Encyclopedia, https://encyclopedia.pub/entry/52314 (accessed February 07, 2026).
Daigo, Y., Daigo, E., Fukuoka, H., Fukuoka, N., Idogaki, J., Taniguchi, Y., Tsutsumi, T., Ishikawa, M., & Takahashi, K. (2023, December 04). Treatment of the Lips Using a CO2 Laser. In Encyclopedia. https://encyclopedia.pub/entry/52314
Daigo, Yuki, et al. "Treatment of the Lips Using a CO2 Laser." Encyclopedia. Web. 04 December, 2023.
Copy Citation
A number of studies have recently demonstrated the effectiveness of CO2 laser irradiation for the repair and regeneration of scar tissue from injuries or surgical wounds. However, such studies of the oral mucosa are highly limited. Previous studies using CO2 laser irradiation have indicated that two factors contribute to esthetic healing, namely, artificial scabs, which are a coagulated and carbonized blood layer formed on the wound surface, and photobiomodulation therapy (PBMT) for suppressing wound scarring and promoting wound healing.
CO2 laser
scar
open lip
1. Introduction
During the healing of injuries and surgical wounds with small parenchymal defects in oral soft tissues, scarring (e.g., formation of hypertrophic scars, contracture and epithelial concavity) sometimes occurs, affecting esthetic and functional outcomes. Scarring and associated problems can also occur due to suturing performed to achieve early wound closure and prevent wound infections [1][2] and due to the use of a wound dressing [3][4].
Recently, the effectiveness of CO2 lasers (a fractional CO2 laser, in particular) for the treatment of epithelial scars from trauma, surgical wounds and acne was reported in clinical studies using biopsy samples [5][6][7][8][9], in studies using a scar scale or a visual analog scale [10][11][12][13][14][15][16] and in basic studies using experimental animals [17][18][19][20][21][22][23][24].
2. Characteristics of CO2 Laser
Dental CO2 lasers emit long-wavelength light (10.6 mm) that is strongly absorbed by water. Therefore, upon irradiation of the skin, the thermal energy of a CO2 laser is mostly absorbed before reaching a depth of 0.05 mm beneath the skin surface, causing no impact on the deep tissue [25][26][27]. Similarly, in the irradiation of blood, coagulation and carbonization occur only at the surface, and blood under the surface layer is unaffected.
However, excessive CO2 laser irradiation due to inappropriate conditions (e.g., output, irradiation mode, irradiation time, irradiation distance) and inappropriate maneuvers can cause irreversible changes and delayed healing.
3. Secondary Healing-like Effect Using a CO2 Laser
The two factors of artificial scabs and PBMT are involved in achieving secondary healing using a CO2 laser. These factors are explained below.
3.1. Presence of Artificial Scabs
Artificial scabs, formed by coagulation and carbonization of blood at the wound surface by HILT, are necessary for the repair and regeneration of parenchymal defects associated with injuries and surgical wounds to obtain the original tissue form [28][29][30][31][32]. These artificial scabs play a role similar to that of wound dressings in dressing therapy (Figure 1b). They have a “space-making effect” to retain effusion and blood required for repair and regeneration of tissue to its original form, preserve the moist condition for moist wound healing and protect immature epithelial and granulation tissues from contamination and infection, thereby facilitating early wound healing. Problems associated with wound dressings, are absent in the case of artificial scabs: problems (1) and (2) are unlikely because artificial scabs are breathable; problem (3) is unlikely because they fall off naturally rather than requiring forced removal; and problem (4) is unlikely because laser soldering of artificial scabs formed over the mobile tissue to the surrounding tissues reduces the risk of peeling [33][34].
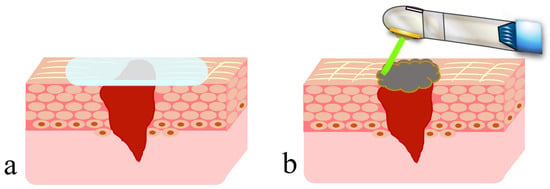
Figure 1. Dressing therapy with wound dressing and wound covering with an artificial scab formed using a CO2 laser: (a) wound dressing; (b) artificial scab.
3.2. Preventive Effect of PBMT on Scarring
PBMT is expected to promote wound healing and prevent scar formation. Those who are interested in the promotion of healing by PBMT are encouraged to refer to studies [28][29][30]. PBMT inhibits differentiation of fibroblasts to myofibroblasts in granulation tissue and inhibits or improves scarring.
Recently, the effectiveness of PBMT using a CO2 laser for inhibiting or improving scar formation associated with trauma, burns, surgical wounds and acne in skin tissue was demonstrated in clinical studies using a biopsy or a scar scale. Pathohistological and biochemical examination of wound biopsy samples showed inhibition of collagen fiber production [5][6][9], normalization of collagen fiber orientation [5], normal regeneration of and increases in dermal collagen with elastic fibers [8][10], thinning in the stratum corneum [5][9] and downregulation of expression of TGF-b1 involved in differentiation of fibroblasts into myofibroblasts [5][7][9]. With respect to appearance, studies of scar healing, such as those using the Vancouver Scar Scale or ultrasound measurement, have demonstrated improvements in the hardness [10][14], thickness [11][16] and flexibility and elasticity [10][14] of scars. In studies of motor function at scar sites [12], studies using the Patient Scar Assessment Questionnaire showed improvements in appearance and scar awareness [10][11][12][13][14][15][16].
Basic studies using experimental animals with artificially formed scar tissue in skin tissue have shown that scar formation can be alleviated by PBMT through downregulation of TGF-b1 expression [17][20][21], which decreases expression of the myofibroblast marker α-smooth muscle actin (α-SMA) [21][22], decreases the amount and density of excessive collagen fibers produced in granulation tissue [17][18][19][20][21], decreases the disruption of fiber orientation [19][20], decreases the scar elevation index [21], decreases the micro-vessel density [18] and thins the fiber layer [20]. Also, PBMT induces downregulation of fibroblast expression in scar tissue [18][19][21], where basic fibroblast growth factor (bFGF) plays a role. During the maturation phase of wound healing, a scar forms when many fibroblasts are present in the tissue. Secretion of bFGF induces apoptosis of fibroblasts, thereby inhibiting and reducing scar tissue formation due to the production of excessive collagen fibers. On the other hand, during the inflammatory and proliferative phases, bFGF promotes cell division to increase fibroblasts, thereby promoting repair and regeneration of the wound. During these phases, PBMT stimulates bFGF secretion to promote repair and regeneration of normal tissue without scarring [23][24].
The relationship between PBMT and TGF-b1 has been studied with respect to suppression of scarring and promotion of wound healing. Laser beams affect mitochondrial cytochrome-C oxidase, thereby influencing the production of adenosine triphosphate (ATP), which is a major source of energy for cell functions. These responses include the generation of reactive oxygen species (ROS) that activate nuclear factor-κB, which plays a role in the signaling cascade, including wound shrinkage, fibroblast differentiation and collagen production. ROS induces extracellular activation of TGF-b1. Also, downregulation of TGF-b1 signaling enhances the formation of keloids and hypertrophic scars. However, PBMT increases ATP production, but the level of ROS remains low. As a result, the downregulation of TGF-b1 by PBMT affects the reduction and inhibition of profibrotic gene synthesis and collagen synthesis. These are likely to indicate the suppression of wound tissue scarring [35][36]. On the other hand, TGF-b1 is a potent regulator of inflammatory responses and is usually upregulated in the early phase of wound healing. However, when comparing treatment with and without PBMT, researchers found no significant differences in TGF-b1 expression in the inflammatory phase but a significant decrease by PBMT in the proliferative phase of wound healing, indicating a suppressive effect on wound tissue scarring [29][31].
After PBMT, TGF-b2, like TGF-b1, is also involved in recruiting fibroblasts and immune cells from the circulation and wound edges to the wounded area, thereby promoting granulation tissue formation and collagen synthesis [37]. On the other hand, a different study showed the appearance of apoptotic epithelial cells and fibroblasts after PBMT [38]. This phenomenon is likely to show suppression of tissue scarring through a reduction in excessive collagen production associated with wound healing. In contrast to TGF-b1 and TGF-b2, TGF-b3 possibly reduces scarring in adults and promotes scarless healing in fetuses, but no study has provided evidence on TGF-b3 in relation to PBMT. Basic studies on PBMT using a CO2 laser and TGF-b are limited, and future studies are awaited.
Taken together, both clinical and basic studies have demonstrated that PBMT is effective in suppressing scar tissue formation. At present, although suppression of scar formation has been verified, complete healing is difficult solely with PBMT using a CO2 laser. To address this, combination with a laser of a different wavelength or an agent has been studied to improve the reliability of treatment effectiveness [7][9][17][18][19][20][21].
As described above, studies of scars in the oral mucosa, are limited compared with those of scars in skin tissue. This can be explained by the rarity of scar formation in the oral mucosa due to differences in turnover (a few days to 2 weeks for the oral mucosa vs. approximately 4 weeks for skin tissue). However, depending on the treatment procedures used, mucosal concavity and recession occur, influencing the esthetic outcomes and motor functions of the tongue, lips and cheeks. Also, it is important to understand that the lips are complex tissues with a transition between oral mucosa and skin tissue.
Next, secondary healing achieved in clinical cases by treatment procedures using a CO2 laser are described (Figure 2).
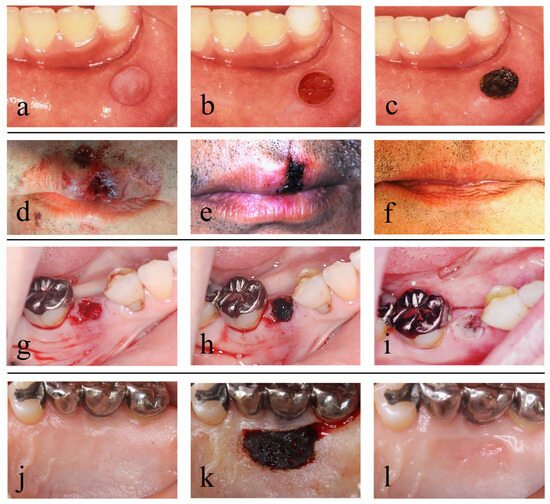
Figure 2. Cases and surgical methods with artificial scabs formed using a CO2 laser: (a–c) removal of a mucocele; (d–f) treatment of open lip vermillion wounds; (g–i) treatment after tooth extraction; (j–l) treatment of the donor site for a free gingival graft; (a) preoperative; (b) after removal of the mucocele; (c) an artificial scab formed on the surface of the resection wound; (d) immediately after open lip vermillion wounds; (e) artificial scab formed in open lip vermillion wound; (f) 6 months after open lip vermillion wound; (g) the extraction socket fully filled with blood immediately after tooth extraction; (h) an artificial scab formed on the surface of blood filled in the extraction socket; (i) day 2 after tooth extraction; (j) preoperative; (k) an artificial scab formed after the donor site of a gingival flap filled with blood clots; (l) 1 month after surgery. Photos by (d–i) co-author Dr. H. Fukuoka and (j–k) Dr. Funakoshi. Consent was obtained from the patients in all cases. Reproduced from [32] under Creative Commons CC BY-NC 4.0.
4. Treatment of the Lips Using a CO2 Laser
Achieving favorable esthetic outcomes is crucial in the facial area, including the lips, so careful postoperative treatment is important.
4.1. Mucocele of the Lip
A mucocele of the lip is thought to be caused by impaired outflow of mucus from a minor salivary gland due to damage to the opening of the minor salivary gland duct present under the labial mucosa.
In conventional mucocele removal, infiltration anesthesia is applied to the area around the mucocele, and an incision is made on the normal mucosa without damaging the mucocele using a scalpel. Then, the lesion is fully enucleated from the surrounding tissue using a surgical scalpel, mucosal elevator or mosquito forceps, and lastly, suturing is performed (Figure 3a–e) [39].
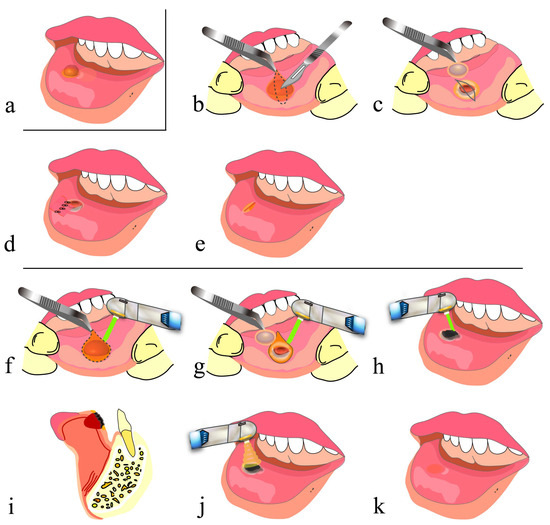
Figure 3. Removal of mucocele using a CO2 laser: (a) before removal of mucocele; (b–e) conventional surgical method; (f–k) surgical method using CO2 laser; (b) designing a spindle incision line over the mucocele along a lip wrinkle and making a submucosal incision; (c) removing a mucocele without damaging it; (d) hemostatic suturing; (e) high likelihood of formation of a linear scar and a mucosal concavity along the sutured wound edges; (f) making an incision in the shape of the mucocele using a CO2 laser; (g) removing a mucocele as in (c); (h) letting blood accumulate in the shape of the resection wound, and forming an artificial scab on the surface; (i) sagittal cross section of (h); (j) performing PBMT; (k) high likelihood of healing without scar formation.
On the other hand, in mucocele removal using a CO2 laser, tissues are cut and vaporized by a CO2 laser instead of being cut by a scalpel, and basically, no suturing is required. The most important point of this method is to retain blood in the space generated after mucocele removal and along the lip morphology and to form an artificial scab on the surface. Then, artificial scabs are strongly soldered to the surrounding tissues to avoid detachment [33][34], and PBMT is subsequently applied several times before the end of the procedure (Figure 2a–c and Figure 3a,f–k). This method frees surgeons from suturing and also frees patients from pain associated with suturing and uncomfortable symptoms such as tension and contracture of the lip. The surgical procedure for hemangioma resection is basically the same as that described above [40].
Recently, clinical studies have shown that this treatment procedure can reduce bleeding and pain, requires a short time and is associated with almost no or no recurrence [41][42]. However, findings regarding esthetic and motor function outcomes associated with scar formation in treated tissue have not yet been reported in detail.
Clinical studies using lasers of other wavelengths have also been reported [43][44], but no studies using biopsy have yet been reported. Also, there are specific problems associated with the use of lasers. For example, when a laser tip is in direct contact with the skin for vaporization and cutting in treatment using a laser that penetrates tissue (e.g., a diode laser), bleeding during the treatment is reduced, but heating of the surrounding tissue induces protein coagulation, which is associated with a risk of delayed wound healing and some scar formation [45][46]. Compared with CO2 lasers, when Er.YAG (erbium-yttrium-aluminum-garnet) lasers, which are absorbed at the surface, are used, more bleeding is expected, albeit without the effect of heating the surrounding tissue during treatment [27][45][47].
4.2. Open Lip Vermillion Wounds
The lips are the part of the face most prone to injury. Treatment of open lip vermillion wounds includes thorough debridement of contaminated tissue at the injured site and then advanced suturing (e.g., V-Y advancement flap [1][2]) that achieves esthetic and functional reconstruction of the lips. Although debridement is necessary, it must be kept to a minimum when treating tissues (e.g., the face) where favorable esthetic outcomes are particularly important. It is highly likely that forceful reefing results in scar formation along the wound edges that are closed and consequent interruption of the vermillion border (Figure 4a–e). Similar findings were shown in suturing in the surgery of the cleft lip [48][49]. Also, because the lips are highly mobile, wound dehiscence and the formation of dead space in the deep part beneath the wound may occur.
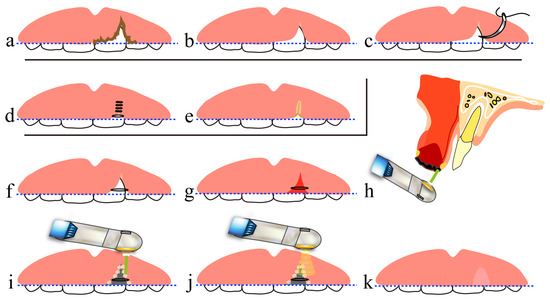
Figure 4. Treatment of open lip vermillion wounds using a CO2 laser: (a–c) procedures common to both hemostatic suturing and treatment with a CO2 laser; (d,e) treatment with hemostatic suturing only; (f–k) treatment using CO2 laser; (a) immediately after injury; (b) after debridement; (c) before hemostatic suturing; (d) suturing with prioritization of wound closure but without consideration of esthetic outcomes of the lip; (e) an interrupted inferior vermillion border of the upper lip, with a high possibility of healing with a linear scar; (f) minimum suturing to make an uninterrupted inferior vermillion border of the upper lip; (g) letting blood accumulate in the shape of a parenchymal defect in the tissue; (h) sagittal cross section of (g); (i) forming an artificial scab on the blood at the wound surface; (j) performing PBMT; (k) high likelihood of healing without mucosal scars. A dotted line indicates the inferior vermillion border of the lip.
On the other hand, in treatment using a CO2 laser, debridement and artificial scab formation are accomplished. In this procedure, after minimum vaporization of contaminated tissue by the laser, blood is allowed to accumulate in the dead space formed at the site of the tissue defect and along the lip morphology, and artificial scabs are formed on the blood surface and strongly soldered to the surrounding tissues [33][34]. Then, PBMT is performed several times (Figure 2d–f and Figure 4a–c,f–k). It should be noted that the formation of artificial scabs without careful consideration results in irregular closure of the lip wound edges, potentially causing concavity, unevenness and hypertrophic scar formation. Thus, it is important to align and close the lip wound edges by light suturing and then to form artificial scabs [32].
References
- Bayramicli, M.; Numanoğlu, A.; Tezel, E. The mental V-Y island advancement flap in functional lower lip reconstruction. Plast. Reconstr. Surg. 1997, 100, 1682–1690.
- Kim, J.H.; Ahn, C.H.; Kim, S.; Lee, W.S.; Oh, S.H. Effective method for reconstruction of remaining lower lip vermilion defect after a mental V-Y advancement flap. Arch. Craniofac. Surg. 2019, 20, 76–83.
- Dalisson, B.; Barralet, J. Bioinorganics and wound healing. Adv. Healthc. Mater. 2019, 8, e1900764.
- Nuutila, K.; Eriksson, E. Moist wound healing with commonly available dressings. Adv. Wound Care 2021, 10, 685–698.
- Makboul, M.; Makboul, R.; Abdelhafez, A.H.K.; Hassan, S.S.; Youssif, S.M. Evaluation of the effect of fractional CO2 laser on histopathological picture and TGF-β1 expression in hypertrophic scar. J. Cosmet. Dermatol. 2014, 13, 169–179.
- Qu, L.; Liu, A.; Zhou, L.; He, C.; Grossman, P.H.; Moy, R.L.; Mi, Q.S.; Ozog, D. Clinical and molecular effects on mature burn scars after treatment with a fractional CO2 laser. Lasers Surg. Med. 2012, 44, 517–524.
- Sabry, H.H.; Rahman, S.H.A.; Hussein, M.S.; Sanad, R.R.; Abd El Azez, T.A. The efficacy of combining fractional carbon dioxide laser with verapamil hydrochloride or 5-fluorouracil in the treatment of hypertrophic scars and keloids: A clinical and immunohistochemical study. Dermatol. Surg. 2019, 45, 536–546.
- Karmisholt, K.E.; Taudorf, E.H.; Wulff, C.B.; Wenande, E.; Philipsen, P.A.; Haedersdal, M. Fractional CO2 laser treatment of caesarean section scars-A randomized controlled split-scar trial with long term follow-up assessment. Lasers Surg. Med. 2017, 49, 189–197.
- Tawfic, S.O.; El-Tawdy, A.; Shalaby, S.; Foad, A.; Shaker, O.; Sayed, S.S.; Metwally, D. Evaluation of fractional CO2 versus long pulsed Nd:YAG lasers in treatment of hypertrophic scars and keloids: A randomized clinical trial. Lasers Surg. Med. 2020, 52, 959–965.
- Peng, W.; Zhang, X.; Kong, X.; Shi, K. The efficacy and safety of fractional CO2 laser therapy in the treatment of burn scars: A meta-analysis. Burns 2021, 47, 1469–1477.
- Issler-Fisher, A.C.; Fisher, O.M.; Haertsch, P.A.; Li, Z.; Maitz, P.K.M. Effectiveness and safety of ablative fractional CO2 laser for the treatment of burn scars: A case-control study. Burns 2021, 47, 785–795.
- Jahanbin, A.; Eslami, N.; Layegh, P.; Saeidi, M.; Kazemi, M.; Shahabi, M.; Raisolsadat, S.M.A. Fractional CO2 laser treatment for post-surgical lip scars in cleft lip and palate patients. Lasers Med. Sci. 2019, 34, 1699–1703.
- Zhang, N.; Yu, X.; Zhao, J.; Yu, J.; Shi, K.; Liu, T. Fractional CO2 laser therapy for cesarean scar under the guidance of multiple evaluation methods: A retrospective study. J. Cosmet. Dermatol. 2021, 20, 2119–2124.
- Hedelund, L.; Haak, C.S.; Togsverd-Bo, K.; Bogh, M.K.; Bjerring, P.; Hædersdal, M. Fractional CO2 laser resurfacing for atrophic acne scars: A randomized controlled trial with blinded response evaluation. Lasers Surg. Med. 2012, 44, 447–452.
- Cox, C.; Bettiol, P.; Le, A.; MacKay, B.J.; Griswold, J.; McKee, D. CO2 laser resurfacing for burn and traumatic scars of the hand and upper extremity. Scars Burn. Heal. 2022, 8, 20595131211047694.
- Bohan, P.M.K.; Cooper, L.E.; Lu, K.N.; Raper, D.M.; Batchinsky, M.; Carlsson, A.H.; Cancio, L.C.; Chan, R.K. Fractionated ablative carbon dioxide laser therapy decreases ultrasound thickness of hypertrophic burn scar: A prospective process improvement initiative. Ann. Plast. Surg. 2021, 86, 273–278.
- Zhang, J.; Zhou, S.; Xia, Z.; Peng, Z.; Cheng, X.; Yang, X.; Luo, W.; Yang, R. 595-nm pulsed dye laser combined with fractional CO2 laser reduces hypertrophic scar through down-regulating TGFβ1 and PCNA. Lasers Med. Sci. 2021, 36, 1625–1632.
- Chen, H.Y.; Lei, Y.; OuYang, H.W.; Gold, M.H.; Tan, J. Experimental comparative study of the effect of fractional CO2 laser combined with pulsed dye laser versus each laser alone on the treatment of hypertrophic scar of rabbit ears. J. Cosmet. Dermatol. 2022, 21, 979–990.
- Zhang, J.; Xia, Z.; Zhou, S.; Luo, W.; Peng, Z.; Yang, R. Effect of artesunate combined with fractional CO2 laser on the hypertrophic scar in a rabbit model. Lasers Surg. Med. 2021; online ahead of print.
- Huang, J.; Chen, J.; Wo, Y.; Wang, X.; Zhang, Y.; Chen, X.; Zhang, Z.; Biskup, E. CO2 fractional laser combined with 5-fluorouracil ethosomal gel treatment of hypertrophic scar macro-, microscopic, and molecular mechanism of action in a rabbit animal model. Rejuvenation Res. 2021, 24, 131–138.
- Xiong, J.; Li, X.; Xu, G.; Wang, Y.; Wen, H. Effectiveness of fractional carbon dioxide laser combined with botulinum toxin type A in a rabbit ear model with the underlying mechanism. J. Cosmet. Dermatol. 2023; online ahead of print.
- de Freitas, A.C.; Pinheiro, A.L.B.; Gerardt de Oliveira, M.; Pedreira Ramalho, L.M. Assessment of the behavior of myofibroblasts on scalpel and CO2 laser wounds: An immunohistochemical study in rats. J. Clin. Laser Med. Surg. 2002, 20, 221–225.
- Nowak, K.C.; McCormack, M.; Koch, R.J. The effect of superpulsed carbon dioxide laser energy on keloid and normal dermal fibroblast secretion of growth factors: A serum-free study. Plast. Reconstr. Surg. 2000, 105, 2039–2048.
- Cheng, E.T.; Nowak, K.C.; Koch, R.J. Effect of blended carbon dioxide and erbium: YAG laser energy on preauricular and ear lobule keloid fibroblast secretion of growth factors: A serum-free study. Arch. Facial Plast. Surg. 2001, 3, 252–257.
- Tuncer, I.; Özçakır-Tomruk, C.; Şencift, K.; Çöloğlu, S. Comparison of conventional surgery and CO2 laser on intraoral soft tissue pathologies and evaluation of the collateral thermal damage. Photomed. Laser Surg. 2010, 28, 75–79.
- Bornstein, M.M.; Winzap-Kälin, C.; Cochran, D.L.; Buser, D. The CO2 laser for excisional biopsies of oral lesions: A case series study. Int. J. Periodontics Restor. Dent. 2005, 25, 221–229.
- Suter, V.G.A.; Altermatt, H.J.; Bornstein, M.M. A randomized controlled clinical and histopathological trial comparing excisional biopsies of oral fibrous hyperplasias using CO2 and Er:YAG laser. Lasers Med. Sci. 2017, 32, 573–581.
- Fukuoka, H.; Daigo, Y.; Enoki, N.; Taniguchi, K.; Sato, H. Influence of carbon dioxide laser irradiation on the healing process of extraction sockets. Acta Odontol. Scand. 2011, 69, 33–40.
- Daigo, Y.; Daigo, E.; Hasegawa, A.; Fukuoka, H.; Ishikawa, M.; Takahashi, K. Utility of high-intensity laser therapy combined with photobiomodulation therapy for socket preservation after tooth extraction. Photobiomodul. Photomed. Laser Surg. 2020, 38, 75–83.
- Daigo, Y.; Daigo, E.; Fukuoka, H.; Fukuoka, N.; Ishikawa, M.; Takahashi, K. Wound healing and cell dynamics including mesenchymal and dental pulp stem cells induced by photobiomodulation therapy: An example of socket-preserving effects after tooth extraction in rats and a literature review. Int. J. Mol. Sci. 2020, 21, 6850.
- Taniguchi, Y.; Matsuzaki, E.; Daigo, Y.; Tsutsumi, T.; Fukuoka, H.; Kakura, K.; Egashira, K.; Takahashi, K.; Kido, H. Space-making effect for new bone formation by suppressing scar contraction of mucosal epithelium of rat tooth extraction wound using diode laser and CO2 laser treatment. J. Dent. Sci. 2022, 17, 1001–1008.
- Fukuoka, H.; Fukuoka, N.; Daigo, Y.; Daigo, E.; Ishikawa, M.; Kibe, T. Healing of open upper lip vermillion wounds irradiated with CO2 laser immediately after injury. Photobiomodul. Photomed. Laser Surg. 2021, 39, 612–616.
- Levanon, D.; Katzir, A.; Ravid, A. A scanning electron microscopy study of CO2 laser-albumin soldering in the rabbit model. Photomed. Laser Surg. 2004, 22, 461–469.
- McNally, K.M.; Sorg, B.S.; Welch, A.J.; Dawes, J.M.; Owen, E.R. Photothermal effects of laser tissue soldering. Phys. Med. Biol. 1999, 44, 983–1002.
- Han, B.; Fan, J.; Liu, L.; Tian, J.; Gan, C.; Yang, Z.; Jiao, H.; Zhang, T.; Liu, Z.; Zhang, H. Adipose-derived mesenchymal stem cells treatments for fibroblasts of fibrotic scar via downregulating TGF-β1 and Notch-1 expression enhanced by photobiomodulation therapy. Lasers Med. Sci. 2019, 1, 1–10.
- Santiago, R.; Gomes, S.; Ozsarfati, J.; Zitney, M. Photobiomodulation for modulation of neuropathic pain and improvement of scar tissue. Scars Burn Heal. 2022, 8, 20595131221134052.
- Rocha Júnior, A.M.; Vieira, B.J.; de Andrade, L.C.; Aarestrup, F.M. Low-level laser therapy increases transforming growth factor-beta2 expression and induces apoptosis of epithelial cells during the tissue repair process. Photomed. Laser Surg. 2009, 27, 303–307.
- Keshri, G.K.; Gupta, A.; Yadav, A.; Sharma, S.K.; Singh, S.B. Photobiomodulation with pulsed and continuous wave near-infrared laser (810 nm, Al-Ga-As) augments dermal wound healing in immunosuppressed rats. PLoS ONE 2016, 11, e0166705.
- Ying, B. Adjacent flaps for lower lip reconstruction after mucocele resection. J. Craniofac. Surg. 2012, 23, 556–557.
- Nammour, S.; El Mobadder, M.; Namour, M.; Namour, A.; Arnabat-Dominguez, J.; Grzech-Leśniak, K.; Vanheusden, A.; Vescovi, P. Aesthetic treatment outcomes of capillary hemangioma, venous lake, and venous malformation of the lip using different surgical procedures and laser wavelengths (Nd:YAG, Er,Cr:YSGG, CO2, and diode 980 nm). Int. J. Environ. Res. Public Health 2020, 17, 8665.
- Huang, I.Y.; Chen, C.M.; Kao, Y.H.; Worthington, P. Treatment of mucocele of the lower lip with carbon dioxide laser. J. Oral Maxillofac. Surg. 2007, 65, 855–858.
- Yagüe-García, J.; España-Tost, A.J.; Berini-Aytés, L.; Gay-Escoda, C. Treatment of oral mucocele-scalpel versus CO2 laser. Med. Oral Patol. Oral Cir. Bucal. 2009, 14, e469–e474.
- Ramkumar, S.; Ramkumar, L.; Malathi, N.; Suganya, R. Excision of mucocele using diode laser in lower lip. Case Rep. Dent. 2016, 2016, 1746316.
- Boj, J.R.; Poirier, C.; Espasa, E.; Hernandez, M.; Espanya, A. Lower lip mucocele treated with an erbium laser. Pediatr. Dent. 2009, 31, 249–252.
- Monteiro, K.; Delgado, M.L.; Garcês, F.; Machado, M.; Ferreira, F.; Martins, M.; Salazar, F.; Pacheco, J.J. A histological evaluation of the surgical margins from human oral fibrous-epithelial lesions excised with CO2 laser, diode laser, Er:YAG laser, Nd:YAG laser, electrosurgical scalpel and cold scalpel. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e271–e280.
- Li, H.; Liu, Y.; Li, X.; Du, J.; Guo, L.; Liu, Y. A histological evaluation of the mice oral mucosal tissue wounds excised with diode laser, Er:YAG laser, and cold scalpel. Lasers Med. Sci. 2022, 37, 2707–2715.
- Suter, V.G.A.; Altermatt, H.J.; Bornstein, M.M. A randomized controlled trial comparing surgical excisional biopsies using CO2 laser, Er:YAG laser and scalpel. Int. J. Oral Maxillofac. Surg. 2020, 49, 99–106.
- Bartkowska, P.; Komisarek, O. Scar management in patients after cleft lip repair-Systematic review Cleft lip scar management. J. Cosmet. Dermatol. 2020, 19, 1866–1876.
- Frans, F.A.; van Zuijlen, P.P.M.; Griot, J.P.W.D.; van Der Horst, C.M.A.M. Assessment of scar quality after cleft lip closure. Cleft Palate Craniofac. J. 2012, 49, 171–176.
More
Information
Subjects:
Surgery
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
2 times
(View History)
Update Date:
06 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




