You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | SIMONA CLAUDIA CAMBREA | -- | 1360 | 2023-12-01 10:36:45 | | | |
| 2 | Camila Xu | Meta information modification | 1360 | 2023-12-04 01:05:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cambrea, S.C.; Badiu, D.; Ionescu, C.; Penciu, R.; Pazara, L.; Mihai, C.M.; Cambrea, M.A.; Mihai, L. Boutonneuse Fever in Southeastern Romania. Encyclopedia. Available online: https://encyclopedia.pub/entry/52261 (accessed on 23 December 2025).
Cambrea SC, Badiu D, Ionescu C, Penciu R, Pazara L, Mihai CM, et al. Boutonneuse Fever in Southeastern Romania. Encyclopedia. Available at: https://encyclopedia.pub/entry/52261. Accessed December 23, 2025.
Cambrea, Simona Claudia, Diana Badiu, Constantin Ionescu, Roxana Penciu, Loredana Pazara, Cristina Maria Mihai, Mara Andreea Cambrea, Larisia Mihai. "Boutonneuse Fever in Southeastern Romania" Encyclopedia, https://encyclopedia.pub/entry/52261 (accessed December 23, 2025).
Cambrea, S.C., Badiu, D., Ionescu, C., Penciu, R., Pazara, L., Mihai, C.M., Cambrea, M.A., & Mihai, L. (2023, December 01). Boutonneuse Fever in Southeastern Romania. In Encyclopedia. https://encyclopedia.pub/entry/52261
Cambrea, Simona Claudia, et al. "Boutonneuse Fever in Southeastern Romania." Encyclopedia. Web. 01 December, 2023.
Copy Citation
Boutonneuse fever (BF) is an eruptive disease and is classified as a spotted fever, which is endemic in the Mediterranean basin (i.e., Marseille fever or Mediterranean spotted fever) and the Black Sea, caused by Rickettsia conorii, with dog ticks being a vector (i.e., Rhipicephalus sanguineus).
boutonneuse fever
Dobruja region
Rickettsia conorii
southeastern Romania
1. Introduction
Boutonneuse, French for spotty fever (BF) or Mediterranean spotted fever (MSF), is a tick-borne disease caused by Rickettsia conorii and transmitted to humans by the brown dog tick, Rhipicephalus sanguineus. BF is traditionally considered to be endemic to the regions bordering the Mediterranean basin, including southern Europe and northern Africa. Among the European countries with relatively high R. conorii infection rates are Portugal, Spain, France and Italy [1].
The tick R. sanguineus is also the main reservoir of a pathogen from R. conorii due to rickettsial permanent transstadial perpetuation which ensures the permanent survival of bacteria [2]. The most important natural reservoirs for R. conorii in Romania are dogs, but other mammalian species may also be involved like sheep, cattle and, very rarely, cats [3]. It is well known that in tropical and subtropical areas, R. sanguineus is found throughout the year [4]. In temperate regions like Romania, this tick is found during late spring and early autumn [5].
In a study performed by Sandor and contributors, they found that in wetlands of the Danube Delta, Rickettsia rossicus had a dominant occurrence in dogs from this area [6].
Although the disease occurs most frequently in Constanta and Tulcea counties, which includes in its northeast corner the large and thinly populated estuary of the Danube, recently, other counties have started to report cases of BF: Prahova, Dîmbovita, Calarași and Buzău [7]. Throughout Central Europe, including Romania, isolated cases of BF have been reported [8]. In the western counties of Romania, since this condition is not endemic, the disease is more difficult to be recognized and diagnosed; but, with the complete anamnesis of patients including recent holidays to these endemic areas, the disease should be taken into consideration.
2. History and Epidemiology
The disease was first described in Tunis in 1920 by Connor and Bruch, and its name is related to a papular skin rash noticed during disease discovery [9]. Carducci in 1920 in Italy and Olmer in Marseilles in 1925 described a Mediterranean feverish disease that in 1932 received the name of Mediterranean BF. Meanwhile, in 1925, Pieri described the tache noir, or black spot or inoculation eschar, as characteristic of the disease. In 1930, Durand and Conseil showed the role of the dog tick R. sanguineus in disease transmission. In 1932, Brumpt discovered the causal agent, a rickettsia that he named in honor of Connor: R. conorii [10].
In Romania, the first reported outbreak of BF occurred during the summer of 1931 in Constanta county, involving 34 individuals. Suspicion of a new disease different to typhus had been diagnosed in Romania since 1910 (i.e., 11 persons in 1910 and 4 persons between 1932 and 1934) [11][12].
Its appearance in a non-Mediterranean country was most probably because of intense commerce with sheep between two harbors, Marseille in France and Constanta in Romania. At that moment, it was considered that sheep were the main reservoir of R. conorii [3][13]. In 1931, it was considered that the number of cases in Constanta was higher because there were more inapparent or oligosymptomatic cases [3][13]. After World War II, the disease spread in the Bucharest area, between 1948 and 1951, with 89 cases detected [12]. The isolation of rickettsia from the blood of one of the patients and the presence of dogs parasitized by R. sanguineus seems to appear between 1931 and 1948 [11][12].
The overall incidence of BF varied between 0.3 and 4.2 per 100,000 inhabitants in the period 2000–2016 in southeastern Romania. The highest incidence was observed in two counties in the Dobruja region: Tulcea county with an incidence of 15.76 per 100,000 inhabitants and Constanta county with an incidence of 5.73 per 100,000 inhabitants. In 2016, the highest number of cases was registered, especially in May and July [7].
BF still represents a public health problem in Constanta and Tulcea counties, as it can be seen in Figure 1 [7].
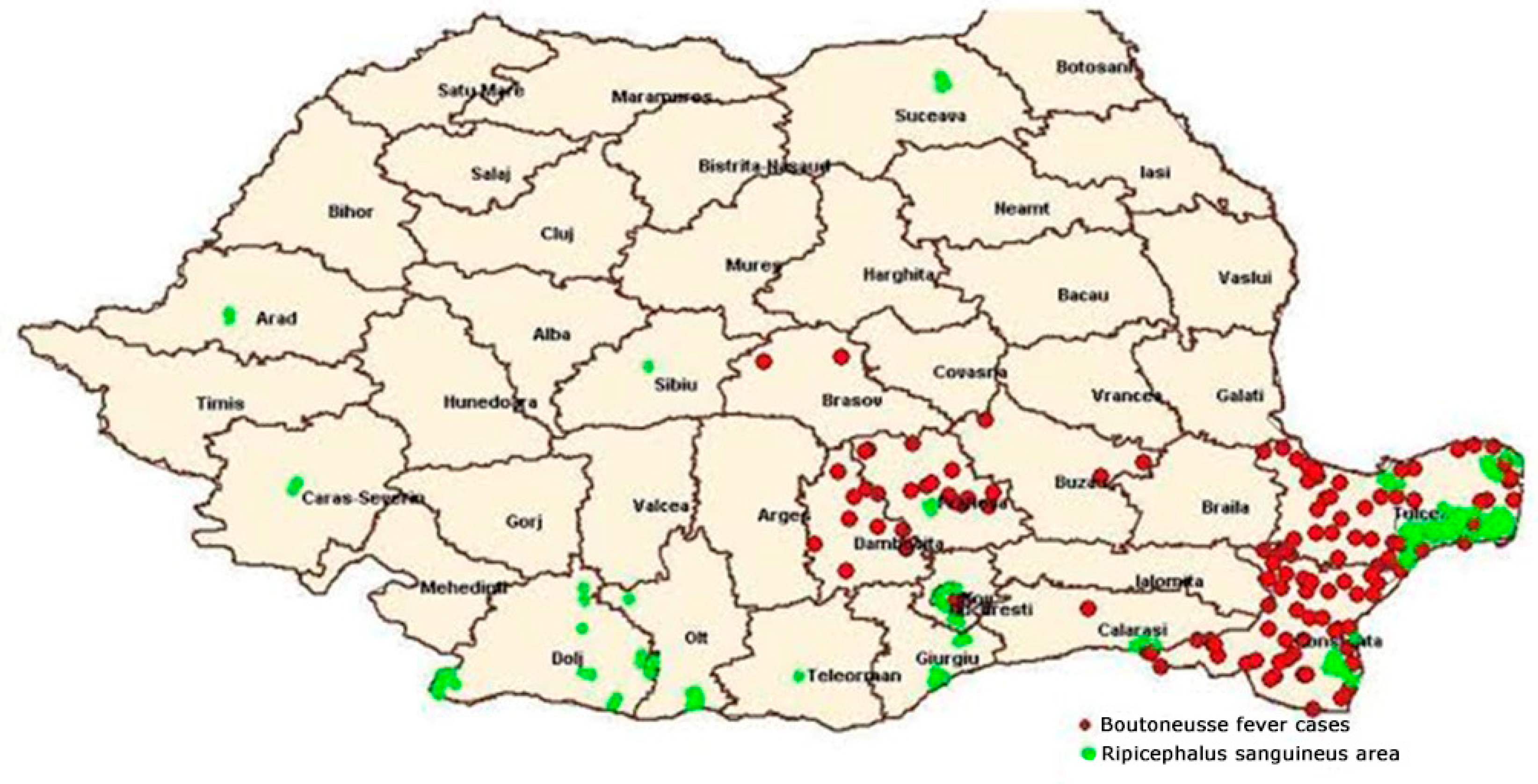
Figure 1. Distribution of BF in correlation with the spread area of R. sanguineus—Romania in 2014 (according to data from the National Institute of Public Health and National Center for Surveillance and Control of Infectious Diseases) [7].
Recently, the study of Ivan and contributors [14] showed, for the first time, the discovery of Rickettsia hoogstraalii in Romania and also R. rossicus as ticks. In this study, five species of rickettsia were identified. This new species of R. hoogstraalii was described for the first time in Croatia in 2006, and its pathogenicity is currently not well known. Also, the detection of Rickettsia raoultii and Rickettsia monacensis in unfed Haemaphysalis punctata larvae supports the hypothesis of rickettsial transmission from female ticks to larvae. Considering this last aspect, the bite of larvae could present a transmission risk for this disease, which should be studied more in the future [14].
R. conorii is a Gram-negative, intracellular bacterium, which retains fuchsin by the Gimenez technique [15]. More recently, the species has been classified into four groups: a spotted fever group (i.e., Rickettsia rickettsii, R. conorii and others); a typhus group (i.e., Rickettsia prowazekii and Rickettsia typhi); an ancestral group; and the recently formed transitional group [16].
Even if it is a long or short journey, all age groups are at risk for rickettsial infections during visits to endemic areas. The transmission risk increases with time spent engaging in outdoor activities, especially during the lifecycle activity for the vector. In many parts of the world, however, rickettsial infections occur year-round. The commonly diagnosed rickettsial diseases in travelers are in the spotted fever groups [17].
Interestingly, genetic techniques have made it possible to dig much deeper into the rickettsial field by unraveling the rickettsial taxonomy [18]. Some authors showed that R. conorii strains could be divided into other subspecies like Rickettsia conorii caspia, Rickettsia conorii israelensis, Rickettsia conorii indica and Rickettsia conorii conorii [19].
After rickettsiae infect the host, they multiply in organs including fluids of the tick which transmit the disease by using rostra [20]. The vector of R. conorii is the dog tick R. sanguineus, first named by Durand and Conseil in 1930 [10]. It was shown that the ticks have a more important role in the rickettsia lifecycle [21].
Other species like R. conorii israelensis have been discovered in different areas including sub-Saharan Africa, India, Greece, Turkey, Bulgaria and Ukraine. Although all these species are different in genetic morphology, they present a similar clinical aspect [22].
3. Pathophysiology
The main target of R. conorii is the endothelial cells of small and medium blood vessels, but also macrophages and hepatocytes [23]. After the infection of human endothelial cells, vascular permeability increases, as well as inflammation, but also the infiltration of immune cells through mechanisms not yet fully clarified [24]. During rickettsial infection, several vasoactive mediators are produced by endothelial cells [25]. The transcriptional activation of cyclooxygenase-2 occurs, leading to robust prostaglandin secretion. In the context of rickettsioses, the damage of the endothelial cells is mediated by oxidants. This is also supported by the severe evolution of the disease in patients with glucose-6-phosphate dehydrogenase deficiency. During endothelial activation, two major signaling cascades, nuclear factor kB and mitogen-activated protein kinase, are activated to produce proinflammatory cytokines [25]. These cytokines increase the expression of cells’ adhesion molecules that allow the recruitment of leukocytes to the inflammation site [26].
The host’s response to R. conorii is the production of interferon beta (IFN-β) by infected endothelial cells. IFN-β causes the activation of the transducer and activator of transcription protein families, which subsequently interfere with rickettsial replication in host cells [27]. Mechanisms involved in the intracellular destruction of Rickettsia spp. are nitric oxide synthesis, hydrogen peroxide production and tryptophan degradation. Macrophages, natural killer (NK) cells and T lymphocytes produce IFN-γ and tumor necrosis factor alpha which act synergically to induce nitric oxide production in endothelial cells [28]. In human macrophages, the eradication of bacteria is achieved by the production of the enzyme indoleamine-pyrrole 2,3-dioxygenase, which, through degradation, limits the availability of tryptophan, leading to the starvation of the bacteria [14]. Dendritic cells (DCs) play an important role in the immune response against rickettsial infections by increasing CD4+, CD8+, NK and IFN-γ cell production [29].
Although much is known about the pathogenesis of this disease, many other aspects of R. conorii infection remain obscure [10].
References
- de Sousa, R.; Nobrega, S.D.; Bacellar, F.; Torgal, J. Mediterranean spotted fever in Portugal: Risk factors for fatal outcome in 105 hospitalized patients. Ann. N. Y. Acad. Sci. 2008, 990, 285–294.
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-Borne Rickettsioses around the World: Emerging Diseases Challenging Old Concepts. Clin. Microbiol. Rev. 2005, 18, 719–756.
- Florea, V. Updates in Boutonneuse Fever; Ovidius University Press: Constanta, Romania, 1998; ISBN 973-9367-22-4. (In Romanian)
- Louly, C.C.B.; Fonseca, I.N.; de Oliveira, V.F.; Borges, L.M.F. Ocorrência de Rhipicephalus sanguineus em trabalhadores de clínicas veterinárias e canis, no município de Goiânia, GO. Cienc. Anim. Bras. 2006, 7, 103–106.
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites Vectors 2010, 3, 26.
- Sandor, A.D.; Dumitrache, M.O.; D’Amico, G.; Kiss, B.J.; Mihalca, A.D. Rhipicephalus rossicus and not R. sanguineus is the dominant tick species of dogs in the wetlands of the Danube Delta, Romania. Vet. Parasitol. 2014, 204, 430–432.
- INSB; CNSCBT. Analiza Evoluției Bolilor Transmisibile Aflate în Supraveghere Raport Pentru Anul 2014. Available online: https://www.cnscbt.ro/index.php/rapoarte-anuale/548-analiza-evolutiei-bolilor-transmisibile-aflate-in-supraveghere-raport-pentru-anul-2014/file (accessed on 10 August 2023).
- Brouqui, P.; Bacellar, F.; Baranton, G.; Birtles, R.; Bjoërsdorff, A.; Blanco, J.; Caruso, G.; Cinco, M.; Fournier, P.; Francavilla, E.; et al. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 2004, 10, 1108–1132.
- Conor, A.; Bruch, A. Une fièvre éruptive observée en Tunisie. Bull. Soc. Pathol. Exot. Fil. 1910, 8, 492–496.
- Rovery, C.; Brouqui, P.; Raoult, D. Questions on Mediterranean Spotted Fever a Century after Its Discovery. Emerg. Infect. Dis. 2008, 14, 1360–1367.
- Combiesco, D. Sur une épidémie de fièvre boutonneuse observée à Constantza-Roumanie. Arch. Roum. Pathol. Exp. Microbiol. 1948, 14, 99–112. (In French)
- Combiescu, D.; Dumitrescu, N.; Russ, M.; Dinculescu, M. Epidemiological considerations of some cases of boutonneuse fever in the last 41 years. Cultivation of Rickettsia conori and characteristics of the strain isolated from an autochthonous outbreak of bou-tonneuse fever. Stud. Cercet. Inframicrobiol. Microbiol. Parazitol. 1953, IV, 99–107. (In Romanian)
- Cambrea, S.C.; Petcu, L.C.; Iliescu, D.M. Relations of environmental factors and evolution of boutonneusse fever in the county of Constanta–Romania. JEPE 2018, 19, 914–922.
- Ivan, T.; Matei, I.A.; Novac, C.Ș.; Kalmár, Z.; Borșan, S.D.; Panait, L.C.; Gherman, C.M.; Ionică, A.M.; Papuc, I.; Mihalca, A.D. Spotted Fever Group Rickettsia spp. Diversity in Ticks and the First Report of Rickettsia hoogstraalii in Romania. Vet. Sci. 2022, 9, 343.
- Spernovasilis, N.; Markaki, I.; Papadakis, M.; Mazonakis, N.; Ierodiakonou, D. Mediterranean Spotted Fever: Current Knowledge and Recent Advances. Trop. Med. Infect. Dis. 2021, 6, 172.
- El Karkouri, K.; Ghigo, E.; Raoult, D.; Fournier, P.-E. Genomic evolution and adaptation of arthropod-associated Rickettsia. Sci. Rep. 2022, 12, 3807.
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; et al. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis—United States: A practical guide for health care and public health professionals. MMWR Recomm. Rep. 2016, 65, 1–44.
- Klein, D.; Beth-Din, A.; Cohen, R.; Lazar, S.; Glinert, I.; Zayyad, H.; Atiya-Nasagi, Y. New Spotted Fever Group Rickettsia Isolate, Identified by Sequence Analysis of Conserved Genomic Regions. Pathogens 2019, 9, 11.
- Dasch, G.A.; Jackson, L.M. Genetic Analysis of Isolates of the Spotted Fever Group of Rickettsiae Belonging to the R. conorii Complex. Ann. N. Y. Acad. Sci. 2006, 849, 11–20.
- Niu, H.; Xiong, X. Editorial: New insights on the transmission and pathogenicity of rickettsiae. Front. Cell. Infect. Microbiol. 2023, 13, 1183558.
- Gilot, B.; Laforge, M.L.; Pichot, J.; Raoult, D. Relationships between the Rhipicephalus sanguineus complex ecology and mediterranean spotted fever epidemiology in France. Eur. J. Epidemiol. 1990, 6, 357–362.
- MacConnachie, K.; Tishkowski, K. Boutonneuse Fever. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Walker, D.H.; Gear, J.H. Correlation of the distribution of Rickettsia conorii, microscopic lesions, and clinical features in South African tick bite fever. Am. J. Trop. Med. Hyg. 1985, 34, 361–371.
- Osterloh, A. Immune response against rickettsiae: Lessons from murine infection models. Med. Microbiol. Immunol. 2017, 206, 403–417.
- Rydkina, E.; Sahni, A.; Baggs, R.B.; Silverman, D.J.; Sahni, S.K. Infection of Human Endothelial Cells with Spotted Fever Group Rickettsiae Stimulates Cyclooxygenase 2 Expression and Release of Vasoactive Prostaglandins. Infect. Immun. 2006, 74, 5067–5074.
- Kaplanski, G.; Teysseire, N.; Farnarier, C.; Kaplanski, S.; Lissitzky, J.C.; Durand, J.M.; Soubeyrand, J.; Dinarello, C.A.; Bongrand, P. IL-6 and IL-8 production from cultured human endothelial cells stimulated by infection with Rickettsia conorii via a cell-associated IL-1 alpha-dependent pathway. J. Clin. Investig. 1995, 96, 2839–2844.
- Colonne, P.M.; Eremeeva, M.E.; Sahni, S.K. Beta interferon-mediated activation of signal transducer and activator of transcription protein 1 interferes with Rickettsia conorii replication in human endothelial cells. Infect. Immun. 2011, 79, 3733–3743.
- Feng, H.M.; Popov, V.L.; Walker, D.H. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: Impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect. Immun. 1994, 62, 1952–1960.
- Jordan, J.M.; Woods, M.E.; Feng, H.; Soong, L.; Walker, D.H. Rickettsiae-Stimulated Dendritic Cells Mediate Protection against Lethal Rickettsial Challenge in an Animal Model of Spotted Fever Rickettsiosis. J. Infect. Dis. 2007, 196, 629–638.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
488
Revisions:
2 times
(View History)
Update Date:
06 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




