Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chaiyavat Chaiyasut | -- | 5149 | 2023-12-01 06:21:55 | | | |
| 2 | Rita Xu | Meta information modification | 5149 | 2023-12-01 06:48:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sivamaruthi, B.S.; Kapoor, D.U.; Kukkar, R.R.; Gaur, M.; Elossaily, G.M.; Prajapati, B.G.; Chaiyasut, C. Mesoporous Silica Nanoparticles for Treatment of Alzheimer’s Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/52258 (accessed on 07 February 2026).
Sivamaruthi BS, Kapoor DU, Kukkar RR, Gaur M, Elossaily GM, Prajapati BG, et al. Mesoporous Silica Nanoparticles for Treatment of Alzheimer’s Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/52258. Accessed February 07, 2026.
Sivamaruthi, Bhagavathi Sundaram, Devesh U. Kapoor, Rajiv R. Kukkar, Mansi Gaur, Gehan M. Elossaily, Bhupendra G. Prajapati, Chaiyavat Chaiyasut. "Mesoporous Silica Nanoparticles for Treatment of Alzheimer’s Disease" Encyclopedia, https://encyclopedia.pub/entry/52258 (accessed February 07, 2026).
Sivamaruthi, B.S., Kapoor, D.U., Kukkar, R.R., Gaur, M., Elossaily, G.M., Prajapati, B.G., & Chaiyasut, C. (2023, December 01). Mesoporous Silica Nanoparticles for Treatment of Alzheimer’s Disease. In Encyclopedia. https://encyclopedia.pub/entry/52258
Sivamaruthi, Bhagavathi Sundaram, et al. "Mesoporous Silica Nanoparticles for Treatment of Alzheimer’s Disease." Encyclopedia. Web. 01 December, 2023.
Copy Citation
Globally, many individuals struggle with Alzheimer’s disease (AD), an unrelenting and incapacitating neurodegenerative condition. Despite notable research endeavors, effective remedies for AD remain constrained, prompting the exploration of innovative therapeutic avenues. Within this context, silica-based nanoplatforms have emerged with pronounced potential due to their unique attributes like expansive surface area, customizable pore dimensions, and compatibility with living systems. These nanoplatforms hold promise as prospective interventions for AD.
silica nanoparticles
Alzheimer’s disease
drug delivery
1. Introduction
Alzheimer’s disease (AD) is the most common type of dementia, which takes its name from the German psychiatrist Alois Alzheimer. It is a slow-progressing neurodegenerative condition that affects the brain, leading to cognitive decline and memory loss. The hallmark features of AD include the presence of neuritic (also known as senile) plaques and neurofibrillary tangles. Alois Alzheimer, a pioneering German neurologist, made a significant observation while studying the brain of his first patient, who exhibited symptoms of memory loss and personality changes before passing away. Upon examination, Alzheimer noticed the presence of abnormal protein deposits known as amyloid plaques and a substantial loss of nerve cells (neurons) in the patient’s cerebral cortex, the brain’s outer layer responsible for complex cognitive functions [1]. Emil Kraepelin is credited with coining the term “Alzheimer’s disease” for the first time in his textbook on psychiatry [2]. AD affects approximately fifty million individuals worldwide, expected to double every 5 years. By 2050, it is projected to reach a staggering 152 million cases. Its far-reaching effects extend beyond individual suffering, affecting emotional, financial, and societal aspects. The assessed annual global cost of AD is about USD 1 trillion. This widespread prevalence and economic burden highlight the urgent need for effective treatments and interventions to alleviate the suffering caused by this devastating neurodegenerative disorder [3][4]. AD is a complex and multifactorial condition influenced by various risk factors depicted in Figure 1.
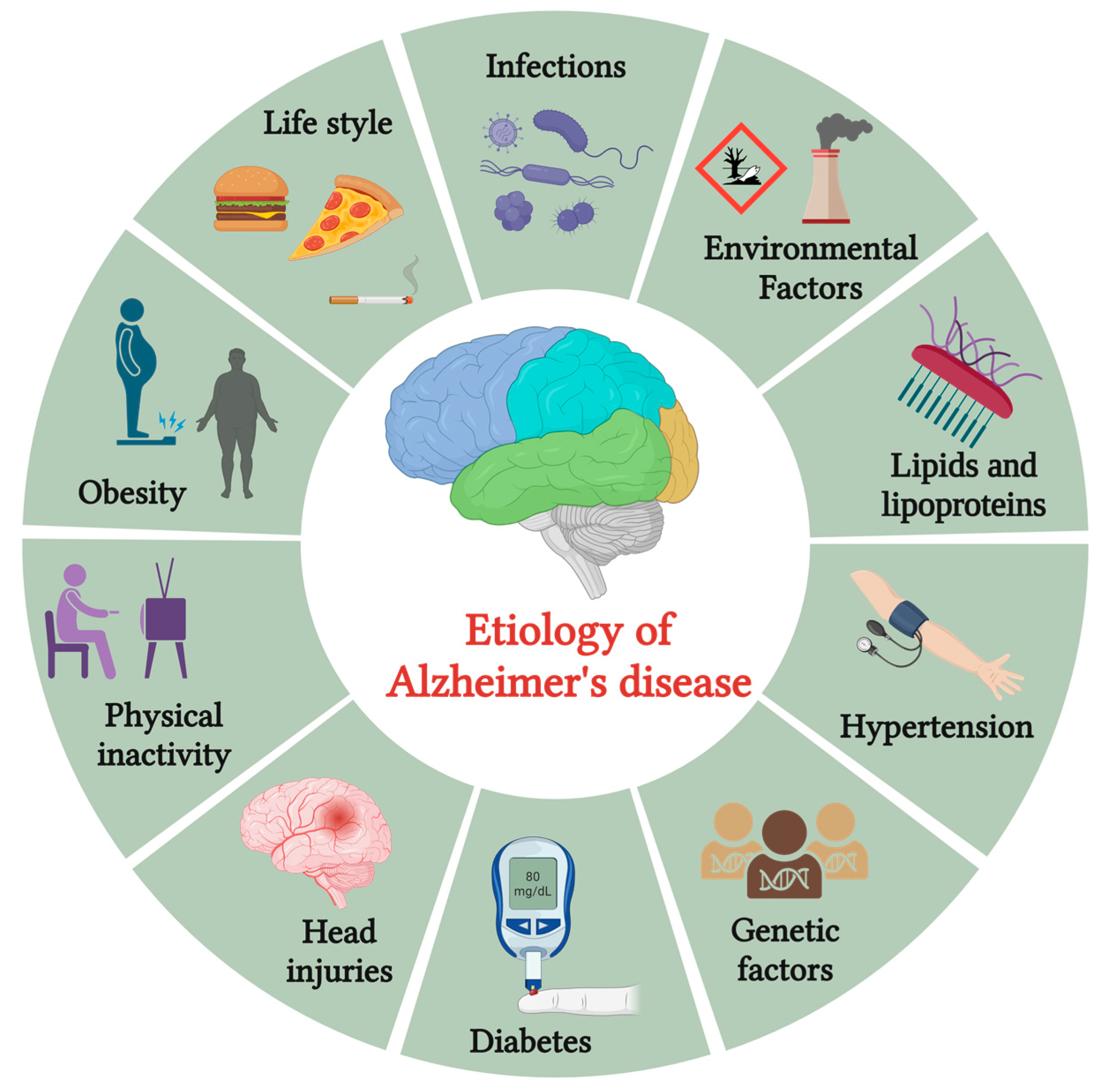
Figure 1. The etiology of Alzheimer’s disease.
These factors encompass various contributors, including advancing age, genetic predisposition, vascular disorders, infections, and environmental influences such as exposure to heavy metals or trace elements. As individuals age, the risk of developing AD increases, making age a significant risk factor. Genetic factors play a crucial role, with certain gene variants associated with a higher susceptibility to the disease. Infections and inflammation have been implicated as potential triggers, with chronic infections or immune responses potentially influencing AD development.
As we age, cognitive decline can result from several factors, including cerebral disorders like AD, non-neurological causes like intoxications, circulatory issues affecting brain oxygen supply, and nutritional deficiencies (e.g., lack of vitamin B12). Additionally, medical conditions such as tumors may also impact cognitive abilities. Taking care of our brain health through regular exercise, a balanced diet, and mental stimulation is crucial to maintaining cognitive function as we age [5][6]. AD causes cognitive impairment and memory loss due to abnormal protein deposits called neurotic plaques and neurofibrillary tangles (NFTs) in the brain. Although extensively studied, the exact reasons behind these changes, such as amyloid-beta (Aβ) plaques and NFTs formation, remain unclear. Researchers continue their efforts to fully comprehend the precise mechanism triggering these brain changes, which is essential for developing effective treatments for AD [7][8].
Several medications (approved by regulatory agencies, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA)) can help to tackle symptoms and diminish the progression of AD for some individuals [9]. Cholinesterase inhibitors such as donepezil, rivastigmine, galantamine, and N-methyl-D-aspartate (NMDA) receptor antagonists, including memantine, are the most employed therapeutics for AD management [10]. Nanomedicine has revolutionized the administration of diagnostics and medications, leading to remarkable AD detection and treatment enhancements.
Hasan et al. (2023) extensively examined many nanomedicines that can scavenge reactive oxygen species (ROS), effectively mitigating oxidative stress in AD [11]. Liu et al. (2022) comprehensively explored several small molecular fluorescent probes with favorable characteristics, including water solubility, blood–brain barrier (BBB) permeability, and adaptability for fine-tuning photophysical and biological attributes. Their research focuses on advancing the field of AD diagnosis by developing these versatile probes [12].
2. Mesoporous Silica Nanoparticles (MSNs)
A wide array of nanomaterials, such as liposomes, dendrimers, carbon nanotubes, gold and iron oxide nanoparticles, titanium dioxide, and MSNs, are employed in various applications. These nanomaterials offer unique properties that enable tailored functionalities in fields like drug delivery, imaging, and environmental remediation [13][14]. Among the various nano-systems, MSNs are one of the useful materials with many mesopores. This unique configuration of MSNs holds several advantages, including significant surface area, large pore volume, pore diameter, chemical and thermal stability, biocompatibility, and biodegradability. The exceptional properties of MSNs make them an attractive candidate for diverse fields, including drug delivery, catalysis, and biomedical applications [15][16].
The evolution of MSNs can be described over three generations. The first generation comprised pioneering MSNs like MCM-41 and SBA-15, demonstrating mesoporous materials. The second generation involved the development of MSNs with nanosized pores and adjustable compositions, setting benchmark in vitro and in vivo evaluations. Finally, the third generation represents multifunctional MSNs, where nanoparticles have multiple functionalities for enhanced performance in various applications. This progressive advancement in MSNs showcases the continuous refinement and diversification of these nanoparticles, expanding their potential in diverse fields of research and technology [17].
2.1. Types of MSNs
MSNs have been classified according to their surface area, pore size, particle size, and preparation method (Figure 2).
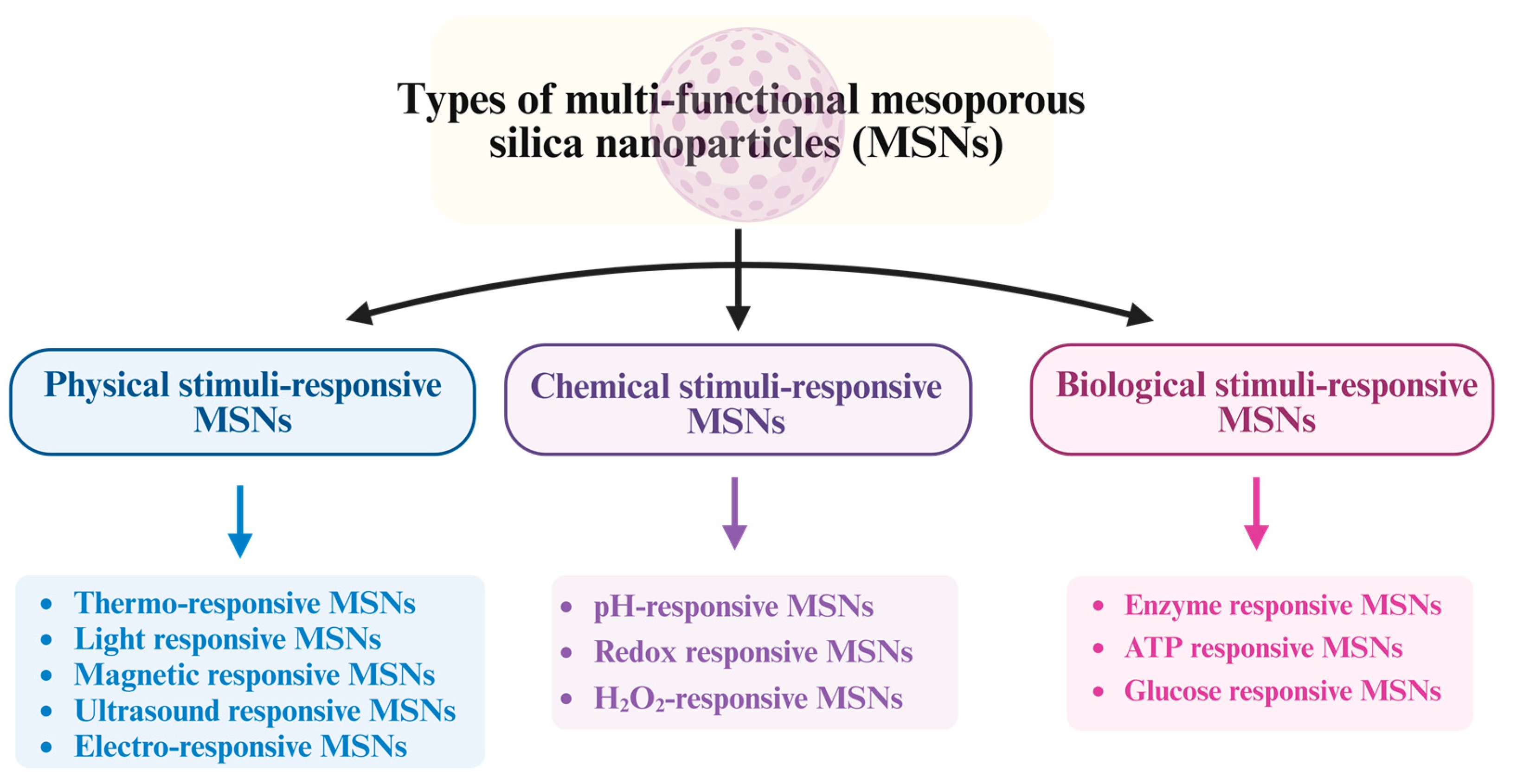
Figure 2. Different types of MSNs.
2.1.1. Physical Stimuli-Responsive MSNs
Temperature is a widely employed and straightforward stimulus that effectively controls the response of MSNs delivery systems in various biomedical applications. Temperature-sensitive MSNs delivery systems are engineered to maintain an inactive state at the body’s physiological temperature (37 °C) to ensure safe circulation within the body. This design allows them to remain stable and inert until they reach the target site, where a temperature change triggers drug release for precise and controlled therapeutic delivery. Upon reaching tumor-targeted sites, these delivery systems can be activated at higher temperatures, particularly when combined with hyperthermia therapy, enabling controlled drug release. This activation is triggered by the conformational change of conjugated polymeric ligands [18]. Poly (N-isopropyl acrylamide) (PNIPAM) is one of the widely recognized and extensively used temperature-sensitive polymers in scientific research [19]. Its conjugation to MSNs has been explored to enhance the thermo-responsive characteristics of the nanoplatforms. PNIPAM-conjugated MSNs hold immense potential in biomedical applications where they can respond to localized hyperthermia conditions or other temperature changes within a tumor microenvironment [20].
Thermo-responsive MSNs: Thermo-responsive systems, also known as temperature-sensitive delivery systems, are an extensively studied group of physical stimuli-responsive technology. These systems can respond to changes in temperature, allowing for controlled drug release and targeted therapies in various biomedical applications. These systems can be customized to function effectively within the body’s typical temperature range while also exhibiting sensitivity to specific tumor-targeted temperatures, like hyperthermia (42 °C). This capability holds significant potential for developing targeted therapies, providing a promising approach to treating various medical conditions [21][22].
Researchers have explored hybrid nanocarriers, combining MSNs with supported lipid bilayers (SLB), as an innovative drug delivery system. Zhang and his colleagues successfully created biocompatible SLB-MSN with high drug loading capacity. Following the synthesis of MSNs through the sol-gel method, a temperature-responsive SLB was applied to the MSNs using sonication to seal the mesopores fully. This step ensures controlled drug release and enhances nanoparticle stability and functionality. The effective coating of MSNs with SLB forms spherical nanoscale particles. Notably, the release test demonstrated a significant 30–40% increase in drug release at 47 °C compared to 37 °C, indicating the temperature-sensitive characteristics of the coated nanoparticles. This temperature-responsive behavior is attributed to the lipid bilayer’s transition temperature (Tm), enhancing permeability beyond this critical point [23]. Among physical stimuli, thermo-responsive strategies are commonly used. However, there are concerns about ensuring the safety and sensitivity of novel thermo-responsive nanomaterials for effective functionality in a physiological environment.
Light-responsive MSNs: Light has emerged as an appealing stimulus for precise and controlled drug release, offering remote activation with high spatial and temporal accuracy. Its versatile control parameters, including wavelength, intensity, exposure duration, and beam size, make it a promising tool for applications related to targeted drug delivery. In recent times, a notable surge of interest has emerged in the realm of advancing light-responsive systems. Many of these innovative systems leverage light-sensitive chromophores such as azobenzene and spiropyran. These chromophores enable precise and regulated reactions to light-based stimuli [24][25][26]. Wu et al. (2023) developed an inventive light-responsive drug carrier, β-CD-MSN. This carrier’s characterization involved various techniques, including SEM, FTIR, UV, BET, XRD, and TGA. To probe the loading and subsequent release capabilities of β-CD-MSN, quercetin (QCT) was chosen as a representative drug surrogate. In vitro drug release testing demonstrated that QCT-β-CD-MSN exhibited favorable characteristics, with slow and light-controlled drug release capabilities. The CCK8 tests revealed that β-CD-MSN effectively reduced the toxicity of QCT, showcasing the potential of this light-responsive drug delivery system for promising applications [24].
Yang et al. (2022) engineered a red light-responsive, self-destructive carrier by incorporating PEG modification and diselenide-bridged MSNs. This innovative carrier system shows promising potential for targeted drug delivery and can release therapeutic agents upon exposure to red light. The carrier is designed to carry the chemotherapy drug doxorubicin and the photosensitizer methylene blue, enabling a combination of chemo-photodynamic therapy for enhanced effectiveness. During photodynamic therapy (PDT) with low-dose red light irradiation, ROS triggers the cleavage of diselenide bonds, leading to the degradation of the organosilica matrix and facilitating the simultaneous release of two drugs. This process enhances the therapeutic efficacy of drug delivery and precision for effective treatment outcomes [25].
Magnetic responsive MSNs: Innovative magnetically guided and responsive delivery strategies are promising in enhancing drug and biologically active molecule therapies. These approaches enable targeted distribution of carriers to the desired site, controlled release, and minimized off-target interactions, leading to improved therapeutic profiles and treatment efficacy [26]. Jia et al. (2020) successfully synthesized Fe3O4 nanoparticles with precise size and uniformity, followed by a coating process involving mesoporous silica and polydopamine to enhance their properties. The distinctive core-shell architecture enhances the drug loading capacity and photothermal conversion efficiency of magnetic nanomaterials, highlighting the promising potential for advanced therapeutic applications. Polydopamine’s reducibility enables the conversion of Fe3+ to Fe2+, triggering the generation of hydroxyl radicals through the Fenton reaction, effectively inducing tumor cell death. Magnetic nanomaterials offer the potential for a dual approach, combining photothermal and chemodynamic therapies, to target and treat tumors in the future. This integrated strategy holds promise in advancing cancer treatment with enhanced precision and effectiveness [27].
Ultrasound responsive MSNs: Ultrasound refers to pressure waves in a medium with frequencies below 20,000 Hz, which are too low for human hearing perception. Ultrasound’s favorable properties, like low absorption by water and tissue, enable non-invasive imaging with deep penetration and controllable frequencies. These advantages enhance its potential for drug delivery applications [28]. Du et al. (2020) introduced a pioneering approach by merging magnetic mesoporous silica nanoparticles with microbubbles (MAG-MSN-MBs). This innovative system enables ultrasound-mediated imaging and gene transfection, offering promising applications in biomedical research and therapies and opening new possibilities in medical applications. Plasmid DNA (pDNA) is efficiently encapsulated inside the pores of MAG-MSN. These pDNA-loaded MAG-MSN were subsequently incorporated into lipid microbubbles, creating a promising platform for gene delivery applications. The gene vector exhibited outstanding biocompatibility, maintaining stable DNA binding while displaying efficient ultrasound imaging capabilities and responsive magnetic properties. It is a promising and versatile tool for various biomedical applications. The introduction of polyethyleneimine (PEI) modification to MAG-MSN displayed effective protection of the loaded pDNA from enzyme degradation, ensuring its stability and effectiveness in gene delivery applications. Encapsulating M-MSNs in lipid microbubbles significantly reduced their cytotoxicity. A magnetic field attracted MAG-MSN-MBs to the tumor area, demonstrating their potential as targeted drug delivery carriers. Ultrasound-targeted microbubble destruction (UTMD) is a novel technique that enhances pDNA delivery efficiency by releasing loaded MAG-MSN and promoting their delivery to tumor tissue. UTMD opens the blood–tumor barrier, increases cytomembrane permeability, and improves the overall therapeutic efficacy of the treatment [29].
Electro-responsive MSNs: Electric fields have proven effective in triggering controlled drug release either in pulsed or sustained manners, owing to their precise dosage control. Various complementary strategies are often employed to optimize drug loading in electro-responsive systems [30]. Zhao’s research group designed a surface coating for titanium implants by incorporating ibuprofen (IBP)-loaded MSN into a chitosan hydrogel to enhance the implant’s performance and improve post-operative recovery and tissue integration. Chitosan hydrogel served as an efficient sealing agent, enabling a controlled and sustained release of the encapsulated drug. The process involved dispersing IBP-loaded MSNs in a chitosan solution, creating a chitosan/MSN complex hydrogel. This hydrogel was then electro-deposited onto a titanium plate with the application of a cathodic voltage, leading to a pH increase and forming a thin opaque surface coat containing IBP-MSN after voltage removal. An in vitro release test revealed the electro-responsive behavior of the system, with the application of a cathodic voltage (−5.0 V) to the titanium plate, leading to a rapid burst release of IBP. Around 95% of the enclosed IBP was released within the first three hours, displaying its potential for controlled drug delivery applications [31].
Recently, Fernandez and his researchers developed conducting polymers with gated MSNs capable of delivering cargo in response to electrochemical stimuli. The MSNs contained surface-modified rhodamine B (Rh B) dye and conductive polymer poly (3,4-ethylene dioxythiophene) (PEDOT) doped with poly [4-styrenesulfonic acid)-co-(maleic acid)]. This functionalization enhanced their optical properties and conductivity, making them potential candidates for applications in imaging and sensing. A bipyridinium derivative was integrated, and heparin (P3) effectively capped the pores through electrostatic interactions. P3 released the confined cargo with the application of a voltage of −640 mV relative to the saturated calomel electrode (SCE). P3 films have biocompatibility with HeLa cells, indicating that they are well-tolerated and compatible with the cells. Additionally, applying a voltage of −600 mV vs. Ag-AgCl triggered the release of rhodamine B from grafted MSN, which was then taken up by HeLa cells [32].
2.1.2. Chemical Stimuli-Responsive MSNs
pH-responsive MSNs: pH plays a vital role in biological systems, including blood circulation and healthy tissues, usually maintained at a steady level of 7.4 [33]. However, several factors can lead to alterations in pH levels. The pH levels in certain cellular compartments, such as cytosols (pH 6.5 to 5.5) and lysosomes (pH 5.5 to 4.5), are notably lower compared to the physiological pH of the body [34]. Researchers have extensively explored the use of pH-responsive strategies to manipulate the behavior and enhance the performance of functionalized MSNs. The development of MSNs can respond to specific pH environments, enabling controlled drug release or targeted delivery of therapeutic agents to acidic cellular regions [18].
Pan et al. (2018) developed a pH-responsive drug delivery system using carboxylated mesoporous silica (COOH-MSN) as a platform. A zeolitic imidazole framework-8 (ZIF-8) film a few nanometers thick was synthesized in situ on the surface of the COOH-MSN. ZIF-8 film acted as a pore blocker, enabling efficient loading of small interfering RNAs (siRNAs) onto the nanoparticles. The remarkably thin ZIF-8 film showcased an exceptional trait related to its capacity to break down within the endo-lysosome’s acidic environment. This served as a trigger for the liberation of enclosed siRNAs and chemotherapeutic agents within the cellular interior. This innovative approach is an advancement, markedly enhancing the efficacy of chemotherapy, especially in the confrontation of drug-resistant cancer cells such as MCF-7/ADR and SKOV-3/ADR cells. The system’s sensitivity to pH fluctuations further contributes to its prowess; it orchestrates a meticulously controlled dispensation of drugs. Specifically, the siRNAs were liberated in response to the distinct pH conditions characteristic of the designated target site [35].
Moorthy et al. (2017) reported synthesized SLN. The surface of nanoparticles was coated with capping units, specifically tetration-maleimide (TTM), to enhance their drug delivery capabilities and responsiveness to changes in pH. The capping units were attached using a “host-guest” complexation mechanism. This modification aimed to create pH-responsive nanoparticles with potential applications in targeting human breast cancer cells, specifically MDA-MB-231 cancer cells. The nanosystem exhibited efficient uptake into the cells, safeguarding the encapsulated cargo molecule (rhodamine B) within its pores and preventing untimely release. This pH-responsive drug delivery system holds significant potential for targeted and controlled release of therapeutic agents, offering promising applications in medicine and pharmaceuticals [36].
Redox-responsive MSNs: Creating redox-responsive vehicles is one of the highly effective approaches for precise and controlled drug delivery, allowing targeted release in response to specific cellular conditions [37]. Redox-responsive drug delivery systems capitalize on the higher concentration of the reducing agent glutathione (GSH) found in tumor cells compared to healthy cells, allowing for targeted drug release in an intracellular environment. This difference enables targeted drug release within tumor cells, enhancing treatment effectiveness while minimizing side effects [38]. Researchers have engineered a pH and redox dual-responsive drug delivery system using MSN-sulfur (SUL)-chitosan (CHS) for controlled release in cancer therapy. This system allows precise drug delivery to cancer cells based on the tumor microenvironment, enhancing treatment efficacy while minimizing off-target effects. The system utilizes an amide reaction to connect dithiodipropionic acid with amino functional groups that can be attached to the surfaces of MSNs and CHS, enabling targeted drug release in response to specific physiological conditions [39]. A model drug, salicylic acid (SA), was used to prepare SA-SUL-MSN-CHS using an impregnation method. The drug loading rate and encapsulation efficiency were calculated as 9.15% and 58.58%, respectively. The in vitro drug release rate was 22.89% under glutathione stimulation, with a significant increase observed as pH decreased [39].
H2O2-responsive MSNs: Geng and his research group reported the utilization of hydrogen peroxide (H2O2)-responsive controlled-release nanoparticles for delivering a therapeutic metal chelator in Alzheimer’s disease treatment [40]. The metal chelator’s delivery is achieved with the elevation of H2O2 levels, showing potential for promising in vivo biomedical applications. This approach holds great prospects for targeted therapies in the future. Xu and his team introduced an innovative microneedle delivery device, which they paired with insulin-loaded and H2O2-sensitive MSNs for efficient and painless administration. The MSNs nanocarriers were created via surface modification using 4-(Imidazole carbamate) phenylboronic acid pinacol ester (ICBE), and subsequently forming a host–guest complex between ICBE and α-cyclodextrin (α-CD). This approach enhances the functionality of nanocarriers for drug delivery and other biomedical applications. Researchers successfully loaded insulin and glucose oxidase (GOX) into the MSNs. GOX’s enzymatic activity converted glucose into gluconic acid, generating H2O2 that led to the disintegration of the ICBE-α-CD complex, resulting in the controlled release of insulin. This method enhances the nanocarriers’ capabilities for targeted and controlled drug delivery, holding great promise for various biomedical applications [41].
2.1.3. Biological Stimuli-Responsive MSNs
Enzyme-responsive MSNs: Enzymes acting as triggers in controlled release have sparked significant interest due to their many advantages, including mild reaction conditions, high specificity, and negligible adverse effects. This approach is promising in developing precise and targeted drug delivery systems with improved therapeutic outcomes [42]. A novel nano platform responsive to enzymes has been created to address implant-related bacterial infections and stimulate tissue regeneration in living organisms. In the first step, silver nanoparticles were incorporated into MSNs using a one-pot method. Subsequently, a layer-by-layer assembly approach was applied to create a composite structure, PG-PAH-Ag MSN, sequentially assembling poly-L-glutamic acid (PG) and polyallylamine hydrochloride (PAH) onto Ag-MSN. This process resulted in forming the desired multifunctional composite [43]. The PG-PAH-Ag MSN was applied to polydopamine-modified Ti substrates for surface deposition [40]. In vitro antibacterial experiments demonstrated that Ti substrates coated with a PG-PAH-Ag MSN exhibited outstanding antibacterial efficacy [43]. In a rat model with bacterium-infected femur defects, the modified Ti implants exhibited successful treatment of bacterial infection, as observed in the animal experiment, showcasing their potential for future clinical applications. The results, obtained through micro-CT, hematoxylin-eosin staining, and Masson’s trichrome staining, demonstrated a significant improvement in forming new bone tissue around the modified Ti implants after a 4-week implantation period. These findings suggest the potential of modified implants to enhance bone regeneration and integration in the body. These promising results highlight the potential benefits of the modified implants for bone regeneration applications [43].
ATP-responsive MSNs: Adenosine-5-triphosphate (ATP) serves as the primary energy currency in all living organisms, powering essential biological processes such as muscle contraction, cellular functions, and the synthesis and breakdown of crucial cellular components like DNA, RNA, and histones. Additionally, ATP facilitates membrane transport, ensuring efficient nutrient uptake and waste removal within cells [44][45]. Elevated ATP expression is associated with various pathological processes. Therefore, the demand for ATP-sensitive controlled-release systems in biomedical applications is crucial [46][47]. Zhang et al. (2022) have created a nano platform called MGMs, consisting of metformin (MTF) and glucose oxidase (GOX) co-loaded into manganese silicon nanoparticles (MSNPs). This nano platform delivers dual-inhibited therapy, targeting the tumor microenvironment (TMIE) to starve cancer cells and enhance chemo-dynamic therapy through ATP modulation. In the mildly acidic conditions of the TMIE, the nanoplatforms MGM undergo decomposition, liberating MTF and GOX. This simultaneous release effectively suppresses ATP production by inhibiting oxidative phosphorylation (OXPHOS) and aerobic glycolysis pathways, offering the potential for targeted cancer therapy [48].
Glucose-responsive MSNs: Glucose-sensitive nanomaterials have become a subject of great interest due to their potential to deliver therapeutic agents effectively. Their unique properties enable precise targeting and controlled drug release, making them promising candidates for future medical applications [49]. Zhao and her team developed a glucose-responsive MSN system capable of dual-controlled insulin delivery and cyclic adenosine monophosphate (cAMP). cAMP activates Ca2+ channels in pancreas beta cells, rapidly increasing insulin secretion when glucose levels rise. This nano-system shows potential for precise and effective glucose-dependent insulin delivery, offering a promising approach for diabetes management [50]. Qin et al. (2021) developed a novel glucose-responsive oral insulin delivery system utilizing polyelectrolyte complexes (PECs). This system aims to regulate postprandial glucose levels effectively, providing a promising approach for managing blood glucose concentrations after meals. The researchers developed negatively charged alginate-g-3-aminophenylboronic acid (ALG-G-APBA) MSN and positively charged chitosan-g-3-fluoro-4-carboxyphenylboronic acid (CS-G-FPBA) MSN by wrapping ALG-G-APBA and CS-G-FPBA on MSN, respectively. The optimized insulin loading capacities were 128 mg/g for ALG-G-APBA-MSN and 298 mg/g for CS-G-FPBA-MSN, showing their potential as effective drug delivery systems. The PECs exhibited a clear insulin release pattern in a laboratory setting in response to varying glucose concentrations. Notably, the release was regulated to switch “on” during hyperglycemic conditions and “off” during normal glucose levels. A CCK-8 (Cell Counting Kit-8) assay revealed that none of the tested nanoparticles, including MSN, ALG-G-APBA-MSN, CS-G-FPBA-MSN, and PECs, exhibited cytotoxic effects on Caco-2 cells. During in vivo testing, the orally administered PECs demonstrated a notable hypoglycemic effect in diabetic rats, maintaining euglycemic levels for around 12 h. These promising results suggest the potential efficacy of the PECs as a therapeutic approach for managing diabetes [51].
2.2. Synthesis/Fabrication Methods
2.2.1. Sol-Gel Method
The cost-effective and straightforward sol-gel method is immensely popular in fabricating MSNs with distinctive surface properties and mesoporous structures. The entire process involves two main stages: hydrolysis and condensation reactions. Specific chemical reactions occur to achieve the desired outcome. Hydrolytic reactions form a colloidal particle solution, which exhibits responsiveness across a broad pH range, including acidic and alkaline conditions. This versatility allows the colloidal particles to be stimulated effectively across a wide spectrum of pH levels.
On the other hand, a condensation reaction takes place at a neutral pH, forming a gel-like structure with three-dimensional networks through crosslinking reactions with siloxane bonds. After the particles undergo a drying procedure, they become receptive to integrating diverse bioactive compounds into the silica gel framework. This unveils intriguing prospects for tailoring nanoparticles to transport distinct therapeutic agents or biomolecules, catering to various medical and biotech applications. Due to the exclusive porous properties and MSNs surface structures, these nanoparticles can effectually control the release of bioactive molecules. This capability enables the preparation of MSNs within a size range of 60–100 nm, offering versatility in various applications [52]. The benefits of this approach are notable. It involves a straightforward 2-step process, saving both time and costs while enabling the production of diverse types of MSNs with precisely controlled mesopore structure and surface properties. Its simplicity and versatility make it attractive for researchers and industries seeking an efficient and customizable synthesis of MSNs for various applications.
Porrang et al. (2021) investigated using natural compounds derived from rice and wheat husks to produce biogenic MSNs through a sol-gel process. These biogenic MSNs loaded with doxorubicin, a chemotherapy drug, demonstrated significant anti-cancer activity against the MCF-7 cell line, indicating their potential as a promising approach for cancer treatment. Using natural compounds to manufacture MSNs highlights an eco-friendly and sustainable method for developing nanocarriers with therapeutic applications [53]. Li et al. (2019) fabricated hollow MSNs using a facile method. They studied the potential of MSNs in improving the solubility, dissolution rate, and bioavailability of Carvedilol (CAR), a poorly water-soluble drug belonging to the Biopharmaceutics Classification System (BSC) type II. Traditional MSNs are commonly prepared using the Stober method, while the fabrication of hollow MSNs with entirely hollow cores involves immersing cetyltrimethylammonium bromide (CTAB) in hot water. The fabricated hollow MSN exhibited remarkable attributes, boasting an expansive surface area measuring 887.76 m2/g, a significant pore volume of 0.82 cm3/g, and a consistent pore size of 2.19 nm. These characteristics facilitated the effective entrapment of the prototype drug CAR within the hollow MSN structure, thereby amplifying their suitability for various drug delivery implementations. This approach enabled a remarkable drug loading of 41.58 ± 0.56%. In vitro experiments confirmed that CAR-hollow MSN showed a sustained drug release profile compared to pure CAR and CAR-MSN synthesized using the Stober approach. These conclusions revealed the potential of CAR-hollow MSN as a drug delivery system with controlled and prolonged release characteristics. A pharmacokinetic study in rats exhibited a noteworthy enhancement in the bioavailability of CAR. The results recommend that CAR-hollow MSN shows enhanced in vivo absorption and distribution, providing a potential benefit over other drug-delivery formulations [54].
2.2.2. Soft Templating Process
Preparing hollow MSNs employs a careful and controlled method to produce different, specialized nanostructures. A surfactant template was utilized during this process to create mesoporous cavities within silica nanoparticles, forming hollow structures, as shown in Figure 3.

Figure 3. Soft templating process of mesoporous silica nanoparticles preparation. CMC: critical micelle concentration; MSN: mesoporous silica nanoparticles.
The ‘soft’ templating method empowers scientists to precisely control factors such as particle dimensions, porosity, and surface characteristics. This approach enables personalized design, proving a versatile and effective way to craft these distinct nanoparticles. These particles hold broad practical utility across various domains, including drug conveyance, catalysis, and sensory applications [55]. The key steps involved in this process are as follows:
Preparation of surfactant solution: a surfactant is combined with a solvent to form a solution. Surfactants consist of components that attract water (hydrophilic) and repel water (hydrophobic).
Formation of surfactant micelles: surfactant molecules autonomously arrange within the solution, creating minuscule micelles with a distinctive core-shell arrangement. The external part of the micelles showcases the hydrophilic ends of the surfactant, while the internal part harbors the hydrophobic tails.
Addition of silica precursors: silica precursors like tetraethyl orthosilicate (TEOS) or tetramethyl orthosilicate (TMOS) are introduced into the solution, encompassing surfactant micelles. These silica precursors then interact with the surfactant template, developing a silica shell around the micelles.
Encapsulation of micelles: the silica building blocks engage with the surfactant micelles, including these micelles within the silica framework.
Hydrolysis and condensation: the initial silica components experience hydrolysis and condensation, yielding silica nanoparticles containing mesoporous cavities that enfold the surfactant micelles. This phase leads to the intended hollow configuration of the nanoparticles. Through skillful management of hydrolysis and condensation reactions, the meticulous control of particle dimensions and porosity becomes feasible, thus establishing an adaptable and promising avenue for fabricating hollow MSNs.
Template removal: this can be attained through solvent extraction or thermal treatment. Once the surfactant template is removed, the desired hollow nanoparticles with well-defined mesoporous structures are achieved [56].
3. MSNs for the Treatment of AD
MSNs have garnered significant interest in drug delivery and biomedicine due to their exceptional attributes, such as substantial surface area and extensive pore volume, facilitating effective drug incorporation and precise release mechanisms. This renders them highly promising for pioneering therapeutic uses. Regarding biomedicine applications, MSNs hold immense potential across various domains, encompassing focused drug administration, gene conveyance, and transportation of imaging agents. Due to their distinctive characteristics, they serve as adaptable platforms for therapeutic and diagnostic applications. The adaptability and ability to enhance treatment results have established MSNs as a promising asset in the progression of medical therapies and individualized medicine methodologies [12]. The different biomedical applications of MSNs are shown in Figure 4.

Figure 4. The illustration represents the representative applications of mesoporous silica nanoparticles.
The specific permeability of the BBB poses a significant obstacle in effectively treating neurodegenerative disorders [57]. This protective barrier restricts the passage of many therapeutic agents into the brain, limiting their efficacy in reaching target areas [58]. Overcoming this challenge is crucial for developing successful treatments for such disorders [59].
Recently, the development of brain-targeted lipid-coated MSNs loaded with berberine (BB) for AD treatment has been reported. Researchers fabricated the Mobil composition of matter-41 (MCM-41) MSN loaded with BB and coated with lipids (MSNs-BB-L). The lipid coating was achieved using the thin film hydration approach. The size of the synthesized MSNs-BB-L was validated to be between 80 and 100 nm. The MSN-based formulation containing BB-L demonstrated significantly higher acetylcholinesterase (AChE) inhibitory activity than other formulations. The results showed a significant reduction in amyloid fibrillation and malondialdehyde levels with MSNs-BB-L treatment. Both pure BB and MSNs-BB-L treatments led to a notable decline in beta-site amyloid precursor protein cleaving enzyme (BACE-1) compared to scopolamine-intoxicated mice. These outcomes highlight the potential therapeutic benefits of MSNs-BB-L in AD treatment (Figure 5) [60].

Figure 5. The formulation of lipid-coated MCM-41 MSNs loaded with berberine improved acetylcholine esterase inhibition and amyloid formation. *** Significant difference; ns: no significant; AChE: acetylcholine esterase; TEOS: tetraethylorthosilicate; AD: Alzheimer’s disease; BACE1: beta-site APP-cleaving enzyme 1; AFM: atomic force microscopy; SEM: scanning electron microscopy; EDX: energy-dispersive X-ray spectroscopy.
Riberio et al. (2022) fabricated a CUR-equipped MSN (CUR-MSN), which defeated the limitations (poor water solubility, low bioavailability, and limited efficacy against the primary causes of AD) related to the clinical use of free CUR. By utilizing MSNs, researchers aim to enhance the delivery and effectiveness of CUR in treating AD, thus providing a potential breakthrough in combating this debilitating neurodegenerative condition. A temperature-responsive hydrogel (HYG) presents an intriguing method to facilitate the delivery of the nanosystem through the nasal route and effectively bypass mucociliary clearance mechanisms. The CUR-MSN was incorporated into the hydrogel matrix. Comprehensive physicochemical analyses successfully characterized the properties of the MSNs and CUR-MSN. A remarkable CUR encapsulation efficiency of 88.90 ± 0.04% was reported. Experiments involving CUR-MSN and HYG-CUR-MSN in ex vivo permeation studies demonstrated significant permeation values in porcine nasal mucosa, with 13.12 ± 1.02 μg cm−2 of CUR for CUR-MSN and 29.15 ± 1.65 μg cm−2 of CUR for HYG-CUR-MSN. Subsequent in vivo investigation conducted in an AD model induced by streptozotocin revealed that HYG-CUR-MSN effectively reversed cognitive deficits in mice. The results highlight the therapeutic potential of HYG-CUR-MSN to treat AD effectively (Figure 6) [61].

Figure 6. The formulation of MSN loaded with curcumin and HYG-CUR-MSN reversed the cognitive deficit in the AD mice model. CUR: curcumin; HYG: thermo-responsive hydrogel; AD: Alzheimer’s disease; FTIR: Fourier-transform infrared spectroscopy; TGA: thermogravimetric analysis; NTA: nanoparticle tracking analysis; DSC: differential scanning calorimeter.
References
- Srivastava, S.; Ahmad, R.; Khare, S.K. Alzheimer’s Disease and Its Treatment by Different Approaches: A Review. Eur. J. Med. Chem. 2021, 216, 113320.
- Cipriani, G.; Dolciotti, C.; Picchi, L.; Bonuccelli, U. Alzheimer and His Disease: A Brief History. Neurol. Sci. 2011, 32, 275–279.
- Chang, C.-P.; Wu, K.-C.; Lin, C.-Y.; Chern, Y. Emerging roles of dysregulated adenosine homeostasis in brain disorders with a specific focus on neurodegenerative diseases. J. Biomed. Sci. 2021, 28, 70.
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446.
- Altieri, M.; Garramone, F.; Santangelo, G. Functional autonomy in dementia of the Alzheimer’s type, mild cognitive impairment, and healthy aging: A meta-analysis. Neurol. Sci. 2021, 42, 1773–1783.
- Raulin, A.C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72.
- Richard, A.A. Risk Factors for Alzheimer’s Disease. Folia Neuropathol. 2019, 57, 87–105.
- Anand, P.; Singh, B. A Review on Cholinesterase Inhibitors for Alzheimer’s Disease. Arch. Pharm. Res. 2013, 36, 375–399.
- Shityakov, S.; Skorb, E.V.; Förster, C.Y.; Dandekar, T. Scaffold Searching of FDA and EMA-Approved Drugs Identifies Lead Candidates for Drug Repurposing in Alzheimer’s Disease. Front. Chem. 2021, 9, 736509.
- Zemek, F.; Drtinova, L.; Nepovimova, E.; Sepsova, V.; Korabecny, J.; Klimes, J.; Kuca, K. Outcomes of Alzheimer’s Disease Therapy with Acetylcholinesterase Inhibitors and Memantine. Expert Opin. Drug Saf. 2014, 13, 759–774.
- Hasan, I.; Guo, B.; Zhang, J.; Chang, C. Advances in Antioxidant Nanomedicines for Imaging and Therapy of Alzheimer’s Disease. Antioxid. Redox Signal. 2023.
- Liu, Y.; Zhuang, D.; Wang, J.; Huang, H.; Li, R.; Wu, C.; Deng, Y.; Hu, G.; Guo, B. Recent advances in small molecular near-infrared fluorescence probes for a targeted diagnosis of the Alzheimer disease. Analyst 2022, 147, 4701–4723.
- Nguyen, T.L.; Nguyen, T.H.; Nguyen, D.H. Development, and in vitro Evaluation of Liposomes Using Soy Lecithin to Encapsulate Paclitaxel. Int. J. Biomater. 2017, 2017, 8234712.
- Nguyen, C.K.; Tran, N.Q.; Nguyen, T.P.; Nguyen, D.H. Biocompatible Nanomaterials Based on Dendrimers, Hydrogels, and Hydrogel Nanocomposites for Use in Biomedicine. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 015001.
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118.
- Patel, R.J.; Pandey, P.; Patel, A.A.; Prajapati, B.G.; Alexander, A.; Pandya, V.; Trivedi, N.; Shah, S.; Patel, V. Ordered mesoporous silica nanocarriers: An innovative paradigm and a promising therapeutic efficient carrier for delivery of drugs. J. Drug Deliv. Sci. Technol. 2023, 82, 104306.
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M. Towards Multifunctional, Targeted Drug Delivery Systems Using Mesoporous Silica Nanoparticles-Opportunities & Challenges. Nanoscale 2010, 2, 1870–1883.
- Hoang Thi, T.T.; Cao, V.D.; Nguyen, T.N.Q.; Hoang, D.T.; Ngo, V.C.; Nguyen, D.H. Functionalized Mesoporous Silica Nanoparticles and Biomedical Applications. Mater. Sci. Eng. C 2019, 99, 631–656.
- Guo, F.; Li, G.; Ma, S.; Zhou, H.; Chen, X. Multi-responsive Nanocarriers Based on Β-CD-PNIPAM Star Polymer Coated MSN-SS-Fc Composite Particles. Polymers 2019, 11, 1716.
- Tian, Z.; Yu, X.; Ruan, Z.; Zhu, M.; Zhu, Y.; Hanagata, N.J.M. Magnetic Mesoporous Silica Nanoparticles Coated with Thermo-Responsive Copolymer for Potential Chemo-and Magnetic Hyperthermia Therapy. Microporous Mesoporous Mater. 2018, 256, 1–9.
- Taghizadeh, B.; Taranejoo, S.; Monemian, S.A.; Salehi Moghaddam, Z.; Daliri, K.; Derakhshankhah, H.; Derakhshani, Z. Classification of Stimuli-Responsive Polymers as Anticancer Drug Delivery Systems. Drug Deliv. 2015, 22, 145–155.
- Lopes, J.R.; Santos, G.; Barata, P.; Oliveira, R.; Lopes, C.M. Physical and Chemical Stimuli-Responsive Drug Delivery Systems: Targeted Delivery and Main Routes of Administration. Curr. Pharm. Des. 2013, 19, 7169–7184.
- Zhang, Q.; Chen, X.; Shi, H.; Dong, G.; Zhou, M.; Wang, T.; Xin, H. Thermo-responsive Mesoporous Silica/Lipid Bilayer Hybrid Nanoparticles for Doxorubicin on-Demand Delivery and Reduced Premature Release. Colloids Surf. B Biointerfaces 2017, 160, 527–534.
- Wu, Y.; Shang, H.; Lai, S.; Di, Y.; Sun, X.; Qiao, N.; Han, L.; Zhao, Z.; Lu, Y. Preparation and Evaluation of Controllable Drug Delivery System: A Light Responsive Nanosphere Based on Β-Cyclodextrin/Mesoporous Silica. Chin. J. Chem. Eng. 2023, 62, 159–167.
- Yang, Y.; Chen, F.; Xu, N.; Yao, Q.; Wang, R.; Xie, X.; Zhang, F.; He, Y.; Shao, D.; Dong, W.F.; et al. Red-light-triggered Self-Destructive Mesoporous Silica Nanoparticles for Cascade-Amplifying Chemo-Photodynamic Therapy Favoring Antitumor Immune Responses. Biomaterials 2022, 281, 121368.
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive Polymers and Their Applications in Nanomedicine. Biointerphases 2012, 7, 9.
- Jia, C.; Wu, H.; Luo, K.; Hao, W.; Wang, S.; Huang, M. Magnetic Silica Nanosystems with NIR-Responsive and Redox Reaction Capacity for Drug Delivery and Tumor Therapy. Front. Chem. 2020, 8, 567652.
- Entzian, K.; Aigner, A. Drug Delivery by Ultrasound-Responsive Nanocarriers for Cancer Treatment. Pharmaceutics 2021, 13, 1135.
- Du, M.; Chen, Y.; Tu, J.; Liufu, C.; Yu, J.; Yuan, Z.; Gong, X.; Chen, Z. Ultrasound Responsive Magnetic Mesoporous Silica Nanoparticle-Loaded Microbubbles for Efficient Gene Delivery. ACS Biomater. Sci. Eng. 2020, 6, 2904–2912.
- Ha, J.H.; Shin, H.H.; Choi, H.W.; Lim, J.H.; Mo, S.J.; Ahrberg, C.D.; Lee, J.M.; Chung, B.G. Electro-responsive Hydrogel-Based Microfluidic Actuator Platform for Photothermal Therapy. Lab Chip 2020, 20, 3354–3364.
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A Study of Chitosan Hydrogel with Embedded Mesoporous Silica Nanoparticles Loaded By Ibuprofen as a Dual Stimuli-Responsive Drug Release System for Surface Coating of Titanium Implants. Colloids Surf. B Biointerfaces 2014, 123, 657–663.
- García-Fernández, A.; Lozano-Torres, B.; Blandez, J.F.; Monreal-Trigo, J.; Soto, J.; Collazos-Castro, J.E.; Alcañiz, M.; Marcos, M.D.; Sancenón, F.; Martínez-Máñez, R. Electro-responsive Films Containing Voltage-Responsive Gated Mesoporous Silica Nanoparticles Grafted onto PEDOT-based Conducting Polymer. J. Control. Release 2020, 323, 421–430.
- Zhou, J.; Yu, Q.; Song, J.; Li, S.; Li, X.L.; Kang, B.; Chen, H.Y.; Xu, J.J. Photothermally Triggered Copper Payload Release for Cuproptosis-Promoted Cancer Synergistic Therapy. Angew. Chem. 2023, 62, e202213922.
- Mo, R.; Sun, Q.; Xue, J.; Li, N.; Li, W.; Zhang, C.; Ping, Q. Multistage pH-Responsive Liposomes for Mitochondrial-Targeted Anticancer Drug Delivery. Adv. Mater. 2012, 24, 3659–3665.
- Pan, Q.S.; Chen, T.T.; Nie, C.P.; Yi, J.T.; Liu, C.; Hu, Y.L.; Chu, X. In Situ Synthesis of Ultrathin ZIF-8 Film-Coated MSNs for Co-delivering Bcl2 siRNA and Doxorubicin to Enhance Chemotherapeutic Efficacy in Drug-Resistant Cancer Cells. ACS Appl. Mater. Interfaces 2018, 10, 33070–33077.
- Moorthy, M.S.; Bharathiraja, S.; Manivasagan, P.; Lee, K.D.; Oh, J. Synthesis of Surface Capped Mesoporous Silica Nanoparticles for pH-Stimuli Responsive Drug Delivery Applications. Medchemcomm 2017, 8, 1797–1805.
- Mollazadeh, S.; Mackiewicz, M.; Yazdimamaghani, M. Recent Advances in the Redox-Responsive Drug Delivery Nanoplatforms: A Chemical Structure and Physical Property Perspective. Mater. Sci. Eng. C 2021, 118, 111536.
- Cui, Y.; Dong, H.; Cai, X.; Wang, D.; Li, Y. Mesoporous Silica Nanoparticles Capped with Disulfide-Linked PEG Gatekeepers for Glutathione-Mediated Controlled Release. ACS Appl. Mater. Interfaces 2012, 4, 3177–3183.
- Xu, Y.; Xiao, L.; Chang, Y.; Cao, Y.; Chen, C.; Wang, D. pH and Redox Dual-Responsive MSN-S-S-CS as a Drug Delivery System in Cancer Therapy. Materials 2020, 13, 1279.
- Geng, J.; Li, M.; Wu, L.; Chen, C.; Qu, X. Mesoporous Silica Nanoparticle-Based H2O2 Responsive Controlled-Release System Used for Alzheimer’s Disease Treatment. Adv. Healthc. Mater. 2012, 1, 332–336.
- Xu, B.; Jiang, G.; Yu, W.; Liu, D.; Zhang, Y.; Zhou, J.; Sun, S.; Liu, Y. H2O2-Responsive Mesoporous Silica Nanoparticles Integrated with Microneedle Patches for the Glucose-Monitored Transdermal Delivery of Insulin. J. Mater. Chem. B 2017, 5, 8200–8208.
- Cheng, Y.J.; Luo, G.F.; Zhu, J.Y.; Xu, X.D.; Zeng, X.; Cheng, D.B.; Li, Y.M.; Wu, Y.; Zhang, X.Z.; Zhuo, R.X.; et al. Enzyme-induced and Tumor-Targeted Drug Delivery System Based on Multifunctional Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 9078–9087.
- Ding, Y.; Hao, Y.; Yuan, Z.; Tao, B.; Chen, M.; Lin, C.; Liu, P.; Cai, K. A Dual-Functional Implant with an Enzyme-Responsive Effect for Bacterial Infection Therapy and Tissue Regeneration. Biomater. Sci. 2020, 8, 1840–1854.
- Mo, R.; Jiang, T.; DiSanto, R.; Tai, W.; Gu, Z. ATP-Triggered Anticancer Drug Delivery. Nat. Commun. 2014, 5, 3364.
- Zhou, Y.; Tozzi, F.; Chen, J.; Fan, F.; Xia, L.; Wang, J.; Gao, G.; Zhang, A.; Xia, X.; Brasher, H.; et al. Intracellular ATP levels are a Pivotal Determinant of Chemoresistance in Colon Cancer Cells. Cancer Res. 2012, 72, 304–314.
- Lai, J.; Shah, B.P.; Zhang, Y.; Yang, L.; Lee, K.B. Real-Time Monitoring of ATP-Responsive Drug Release Using Mesoporous-Silica-Coated Multicolor Upconversion Nanoparticles. ACS Nano 2015, 9, 5234–5245.
- Jiang, Y.; Liu, N.; Guo, W.; Xia, F.; Jiang, L. Highly Efficient Gating of Solid-State Nanochannels by DNA Super Sandwich Structure Containing ATP Aptamers: A Nanofluidic IMPLICATION Logic Device. J. Am. Chem. Soc. 2012, 134, 15395–15401.
- Zhang, J.; Liang, C.; Wei, Z.; Yang, W.; Ge, W.; Qu, X.; Si, W.; Wang, W.; Mou, X.; Dong, X. TME-Triggered MnSiO3@Met@GOx Nanosystem for ATP Dual-Inhibited Starvation/Chemodynamic Synergistic Therapy. Biomaterials 2022, 287, 121682.
- Webber, M.J.; Anderson, D.G. Smart Approaches to Glucose-Responsive Drug Delivery. J. Drug Target. 2015, 23, 651–655.
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400.
- Qin, T.; Yan, L.; Wang, X.; Lin, S.; Zeng, Q. Glucose-Responsive Polyelectrolyte Complexes Based on Dendritic Mesoporous Silica for Oral Insulin Delivery. AAPS PharmSciTech 2021, 22, 226.
- Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.-W.; Chrzanowski, W. Silica-Based Mesoporous Nanoparticles for Controlled Drug Delivery. J. Tissue Eng. 2013, 4, 2041731413503357.
- Porrang, S.; Rahemi, N.; Davaran, S.; Mahdavi, M.; Hassanzadeh, B. Preparation and In-Vitro Evaluation of Mesoporous Biogenic Silica Nanoparticles Obtained From Rice and Wheat Husk as a Biocompatible Carrier for Anti-Cancer Drug Delivery. Eur. J. Pharm. Sci. 2021, 163, 105866.
- Li, T.; Geng, T.; Md, A.; Banerjee, P.; Wang, B. Novel Scheme for Rapid Synthesis of Hollow Mesoporous Silica Nanoparticles (HMSNs) and Their Application as an Efficient Delivery Carrier for Oral Bioavailability Improvement of Poorly Water-Soluble BCS Type II Drugs. Colloids Surf. B Biointerfaces 2019, 176, 185–193.
- Kumar, S.; Malik, M.M.; Purohit, R. Synthesis Methods of Mesoporous Silica Materials. Mater. Today Proc. 2017, 4, 350–357.
- Li, H.; Chen, X.; Shen, D.; Wu, F.; Pleixats, R.; Pan, J. Functionalized Silica Nanoparticles: Classification, Synthetic Approaches and Recent Advances in Adsorption Applications. Nanoscale 2021, 13, 15998–16016.
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217.
- Correia, A.C.; Monteiro, A.R.; Silva, R.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: Crossing or circumventing the blood-brain barrier (BBB) to manage neurological disorders. Adv. Drug Deliv. Rev. 2022, 189, 114485.
- Chen, Y.P.; Chou, C.M.; Chang, T.Y.; Ting, H.; Dembélé, J.; Chu, Y.T.; Liu, T.P.; Changou, C.A.; Liu, C.W.; Chen, C.T. Bridging Size and Charge Effects of Mesoporous Silica Nanoparticles for Crossing the Blood-Brain Barrier. Front. Chem. 2022, 10, 931584.
- Singh, A.K.; Singh, S.S.; Rathore, A.S.; Singh, S.P.; Mishra, G.; Awasthi, R.; Mishra, S.K.; Gautam, V.; Singh, S.K. Lipid-Coated MCM-41 Mesoporous Silica Nanoparticles Loaded with Berberine Improved Inhibition of Acetylcholine Esterase and Amyloid Formation. ACS Biomater. Sci. Eng. 2021, 7, 3737–3753.
- Ribeiro, T.D.; Sábio, R.M.; Luiz, M.T.; de Souza, L.C.; Fonseca-Santos, B.; Cides da Silva, L.C.; Fantini, M.C.; Planeta, C.D.; Chorilli, M. Curcumin-Loaded Mesoporous Silica Nanoparticles Dispersed in Thermo-Responsive Hydrogel as Potential Alzheimer Disease Therapy. Pharmaceutics 2022, 14, 1976.
More
Information
Subjects:
Clinical Neurology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
01 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




