1. Introduction
Plasma is an exothermic ionized gas produced by electrical discharge
[1]. By injecting energy into the reaction region, electrons are released from the atoms or molecules to achieve an electrically neutral state where ions, electrons, and the original species coexist
[2]. According to the energy level, temperature, and ionic density, plasma is generally classified into high-temperature plasma (for nuclear applications) and low-temperature plasma (including thermal plasma and non-thermal plasma)
[3]. Particularly, the non-thermal plasma can be applied in the fields of catalyst preparation and activation, because the relatively low-temperature condition (usually tens to 200 °C) will not cause thermal damage to the material surface
[2]. Additionally, since it works at atmospheric pressure, the cost of the vacuum system can be avoided
[4]. Specifically, the gas temperature of thermal plasma is close to the electron temperature (about tens of electron volts), while that of non-thermal plasma can be as low as room temperature
[5].
The non-thermal plasma shows great potential in decorating zeolitic structures by generating some specific properties or functions under the etch of energetic species
[6]. The activation of plasma to zeolites mainly involves the following points:
- (1)
-
During the discharge process, the molecules in the gas atmosphere will be ionized to generate many high-energy electrons, which will collide with each other to convert electric energy into internal energy and kinetic energy of particles, thus providing the activation energy of chemical reactions
[7], which can process materials that cannot be activated by the conventional thermal field
[8].
- (2)
-
Numerous electrons and other active substances with high energy will attack the external surface of zeolites to form new pores through etching and ablation effects, thus increasing the porosity.
- (3)
-
The electrons with high energy can break the chemical bonds on the zeolite surface to form new active groups and increase the active sites amount.
- (4)
-
The high-energy particles produced by plasma discharge can promote the excitation, ionization, and interaction of gas-reactant molecules to form new chemical bonds
[9].
Compared to the traditional thermal treatment method, non-thermal plasma is fast, clean, energy-saving, convenient, and relatively low-cost
[8] despite improving the activity, life, and selectivity of materials
[10]; therefore, it is viewed as an ideal treatment method. At present, the non-thermal plasma is generally applied for activating the adsorbents/catalysts or assisting the purification process for enhancing the removal efficiency of the VOCs or NO
x. The general non-thermal plasma technologies used for activating materials include dielectric barrier discharge plasma (DBD), glow discharge plasma (GDP), and corona discharge plasma (CDP)
[11], respectively. DBD is the phenomenon of electron penetration in the presence of a strong electric field in the medium, where the electrons are accelerated by the electric field and obtain enough energy. The GDP is caused by adding enough voltage to the gas to cause the collision of atoms, producing ionized electrons to form an electric current. The CDP, occurring in an uneven electric field near a tip electrode with a large radius of curvature, is caused by the ionization and excitation of the gas due to the local electric field strength exceeding the gas ionization field strength.
2. NTP Assisted Activation of Materials
Non-thermal plasma could effectively modify the surface properties of materials
[1] due to the large number of energetic electrons and other active substances produced during the discharge process attacking the zeolite surface to form new pores and active groups. Meanwhile, chemical reactions take place between the excited species and acid-base groups on the zeolite carriers, thus changing the acidity and alkalinity of the carriers
[12]. Further, the alkaline metal's active components can regulate the metal dispersion through the interaction with acid sites
[13]. In addition, by modifying the textural properties of zeolites, ultimately, non-thermal plasma can assist the zeolite-based materials to improve the reactivity, selectivity, stability, and yield of the target products
[14]. More significantly, compared to the traditional thermal treatment at high temperatures that easily destroy the zeolite framework, the plasma can avoid such a situation due to the low temperature, maintaining the stability of zeolites
[5].
It has been proved that more active groups such as hydroxyl will be generated on the surface of the zeolites, accompanied by the growth of porosity and the corresponding increase of active sites after the electric discharge. Material activation by non-thermal plasma is mainly applied in assisting the purification of the VOCs. Liu et al.
[11] found that the methane conversion rate of the Pd/HZSM-5 catalyst grew from 25% to 90% at 400 °C after being treated with non-thermal plasma, which resulted from the increase of active sites
[15]. Wang et al.
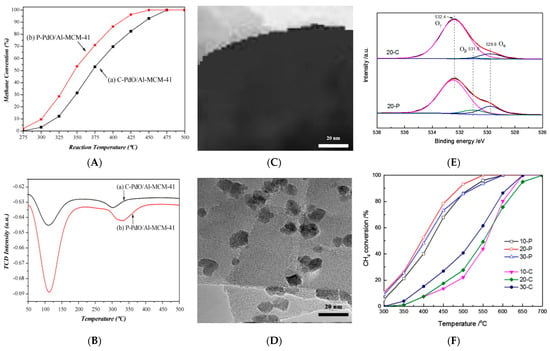
[16] proved that the plasma treatment enhanced the initial activity of the Pd/Al-MCM-41 zeolite catalyst in the methane combustion reaction compared to the untreated catalyst (
Figure 1A), which was caused by the higher acidity of the catalyst (
Figure 1B) and the better dispersion of the PdO particles active species. Moreover, the improved stability of the plasma-treated Pd-based catalyst was proven to be closely related to the stronger interaction between the palladium oxide and the zeolite support. Using the non-thermal plasma, Wang et al.
[17] significantly increased the pore size from 0.62 to 0.72 nm, the specific surface area from 222.14 to 618.06 m
2/g, and the pore volume from 0.19 to 0.36 cm
3/g, respectively, accompanied with the greatly increasing adsorption capacity of 167% for naphthalene. It was also ascribed to those abundant active substances (i.e., energetic electrons, ions, protons, ultraviolet photons, and active radicals) produced in the discharge region bombarded the outer surface of the adsorbents to promote the formation of new pores and reconstruction of the original pores by etching effect. On the other hand, the high energy of accelerating electrons in the electric field can break chemical bonds and form new bonds, resulting in an increase in the active site amount, thus improving the activity of the zeolites.
Figure 1. (
A) Initial activity for methane combustion and (
B) The NH
3−TPD profiles of PdO/Al−MCM−41 catalysts: (a) C−PdO/Al−MCM−41 treated conventionally and (b) P−PdO/Al−MCM−41 treated with the plasma treatment
[16]. The TEM images of Co
3O
4/HZSM−5 catalysts treated by (
C) calcination and (
D) plasma; (
E) The XPS spectra of O 1 s (alfa oxygen is the lattice oxygen concentration of surface Co
3O
4, beta oxygen is the concentration of surface adsorbed oxygen, and gamma oxygen is the lattice oxygen concentration of HZSM−5 support); (
F) Methane conversion of Co
3O
4/HZSM−5 catalysts treated by calcination and plasma (“C” in the image stands for calcination and “P” for plasma)
[18].
In addition to changing the pore structure and surface properties of zeolites, another important application of non-thermal plasma is to introduce a certain amount of protonic acid sites to improve the acidity and activity of zeolites
[19]. Free radicals and active ions containing carbon, oxygen, and nitrogen possessing high kinetic energy can easily react with acidic or alkaline groups in the zeolites
[20], thus changing the acidic or alkaline properties of the zeolites
[12]. Xia et al.
[21] found that compared to the Fe-Mo/HZSM-5 zeolite obtained by the conventional treatment, the Brönsted acidity of the non-thermal plasma-treated zeolite was up to more than 265%, which significantly enhanced methane conversion from 11% to 24%
[22]. For the Mo-Fe/HZSM-5 zeolite, Zhu et al.
[23] also found that the Brönsted acid intensity and density of the plasma-treated catalyst were higher than that of the untreated sample, and thus the methane conversion was increased. Liu et al.
[11] confirmed that the non-thermal plasma treatment significantly increased both the Brönsted and Lewis acids contents of the Pd/HZSM-5 zeolite. The concentration of both the acidic sites was 1.13 times and 1.21 times higher than that of the fresh sample, respectively. As a result, the treated zeolite showed 50% higher catalytic activity and stronger stability than the fresh agent in methane combustion
[15]. In addition to the above applications for plasma-assisted methane removal, Takeuchi et al.
[24] proved that for the iso-butane catalytic cracking, the plasma treatment for 5 min could enhance the activity by 10% compared to the treatment by conventional calcination at 600 °C for 2 h, which was attributed to the fact that the Lewis acid sites could be effectively formed in the HY zeolite after plasma treatment.
The non-thermal plasma treatment can also increase the dispersion and stability of the metal active components loaded on the materials, thus providing more reactive centers
[12]. Liu et al.
[11] also found that after treatment with the non-thermal plasma, the particle size of the PdO on the surface of the Pd/HZSM-5 catalyst decreased from 21.0 to 16.0 nm and the Pd dispersion increased by 2.5 times, effectively avoiding the aggregation of PdO particles, and blocking the pores of ZSM-5. Thereby, the catalyst showed higher catalytic activity and better stability than that without the plasma treatment in the methane combustion. It was concluded that the plasma treatment could make the PdO cluster (alkaline oxide) better attracted to the increased Brönsted and Lewis acidic sites, facilitating the PdO dispersion on the zeolite. In addition, the enhanced interaction between the PdO and acidic sites made the PdO more stable during the reaction, significantly improving the stability of the catalyst
[15]. Cao et al.
[18] found that compared to the calcination, the plasma treatment could promote the formation and dispersion of small-size Co
3O
4 on Co
3O
4/H-ZSM-5 (TEM shown in
Figure 1C,D). Moreover, it was obvious that the content of the adsorbed oxygen species O
β for 20-P (5.2) was more than 7 times higher than that for 20-C (0.7), which indicated that plasma could improve the number of lattice oxygen of Co
3O
4 on the catalyst surface (XPS spectra shown in
Figure 1E). Therefore, the catalytic activity of methane was simultaneously improved (the conversion rate of 100% at 550 °C) (
Figure 1F). Wang et al.
[25] reported a high performance of the plasma-enhanced Zn/MCM-41 catalyst for acetylene hydration, showed that plasma treatment could effectively enhance the interaction between the Zn active sites and MCM-41 carrier, promote the dispersion of the Zn active component, and inhibit the loss of Zn species.
There is also some research on the activation materials by non-thermal plasma to assist NO
x purification, although are not as widespread as the VOCs removal. Yoon et al.
[26] found that the active sites on the NaY catalyst increased via non-thermal plasma treatment, which greatly improved the NO
x conversion rate of the catalyst from 20% to 55% at 225 °C in the lean NO
x reduction
[27]. Zhang et al.
[28] proved that the dispersion of the Pd active component on the surface of the Pt/NaZSM-5 catalyst increased markedly from 4.2% to 63.3% after plasma treatment. Correspondingly, the particle size decreased significantly from 27.0 to 1.8 nm, and the NO redox activity enhanced observably from almost zero to 61.3% at 400 °C.
3. NTP Cooperative Catalysis Process
In addition to the above-mentioned activation level of material modification, during the process of activating zeolite materials by non-thermal plasma, reaction gas can be accelerated to activated when injected in the discharge region, NO
x/VOCs can be cooperatively removed, and the conversion rate can be improved. The basic principle of this technology is that a high-intensity electric field provides energy for the electrons so that electrons can obtain high kinetic energy. Then the high-speed moving electrons transfer energy to molecules through collision, so that some gas molecules in the gas are excited and ionized. Through direct generation or ionization, many active particles such as ions, excited atoms, molecules, electrons, and free radicals are generated, and these active particles undergo a series of complex physicochemical reactions with the NO
x or VOCs molecules to achieve the purpose of removal
[29]. The energy of the active particles in the plasma is higher than that of most chemical bonds, which indicates that these extremely high-energy active particles can completely promote the fracture of the old bonds of reactants, thus generating new chemical bonds
[9].
The NTP has been widely applied for purifying the NO
x via the routes of direct decomposition
[30], oxidation combined with absorption
[31], adsorption combined with in-situ decomposition
[32], selective catalytic reduction
[33], etc., the core roles of which in above actions are mainly decomposition, oxidation, and reduction. The NO decomposition by the NTP is realized through colliding and making N-O bonds broken by the electrons or high-energy species generated from the NTP to produce N
2 and O
2 [30]. The NO oxidation by the NTP is to use the oxidizing species O, O
3, or OH generated by NTP to oxidize NO to NO
2 or nitrate
[31]. NO reduction by NTP is to use the N atom generated by NPT to react with NO to produce N
2 and O
2; or introduce NH
3, HC, or C
xH
y into the reaction gas to reduce NO to N
2 and O
2 [33]. The specific reaction process can be seen in Equations (1)–(3). The possible ways of combining the discharge plasma with the catalyst are shown schematically in
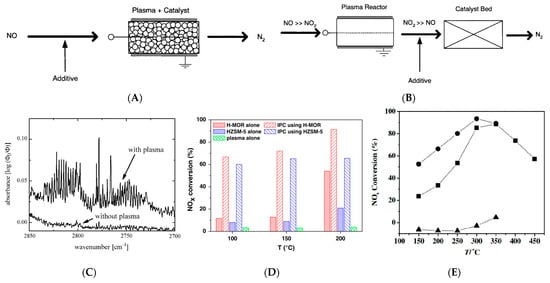
Figure 2A,B
[34]. The single-stage method is also known as the plasma-driven catalyst (PDC) system
[12]. In this method, the catalyst is placed directly in the plasma reactor. The PDC system will induce both the gas-phase chemical reaction and the catalytic reaction on the catalyst surface. On the other hand, in the two-stage method, the plasma reactor is followed by the catalyst bed. This method is referred to as plasma-enhanced selective catalyst reduction (PE-SCR) method. In this case, NO
x is oxidized by plasma and then reduced by the catalyst.
Figure 2. Combination of plasma with a catalyst: (
A) single−stage (PDC) system; (
B) two−stage (PE−SCR) system. (
C) The infrared spectrum of the exhaust is not treated and treated with plasma
[35]. (
D) NO
x conversions for the plasma alone, catalyst alone, and in−plasma catalysis (IPC) cases
[36]. (
E) NO
x conversion as functions of temperature over (■) Co−HZSM5 catalyst alone, (●) discharge over Co−HZSM-5 catalyst, and (▲) discharge over quartz pellets
[37].
It has been demonstrated that the main pathway of plasma to deal with NOx is HC-SCR, NH
3-SCR, CH
4-SCR, etc. by producing electrons or free radicals. Coupling the NTP technology with the NH
3-SCR system by Byun et al.
[38], the low-temperature reaction activity of NO
x was greatly improved under the synergistic effect, and the reaction temperature window was also widened from 300–400 °C to 100–500 °C. This tendency could be attributed to the fact that the OH and H radicals generated from the NTP will react rapidly to decompose NH
3 at low temperatures to produce NH
2 radicals, followed by selective non-catalytic reduction through the reaction of NO with NH
2 to produce N
2. The NTP in collaboration with the NH
3-SCR technology was also used by Schmidt et al. to remove NO
x from diesel exhaust
[35]. It was regarded that the conversion of NO is mainly triggered by the electron impact dissociation of molecular oxygen and molecular nitrogen. By comparing the infrared spectra of the gas mixture before and after being treated with plasma, it was found that the byproducts are probably formed due to the action of the plasma. With this method, the species produced by plasma can be found since new absorption lines would occur in the spectrum as depicted in
Figure 2C. It has been reported by Singh et al.
[39] that the reaction system cooperated the NTP with NH
3-SCR could produce some long-lived and short-lived active substances, such as the gas-phase electronic excited states of NO
x, hydrocarbon fragments, some oxygen-containing derivatives, and vibration-excited substances. These active species, which exist in gas or surface phases, are a key factor for the significant synergistic effect of the NTP and SCR catalysts. Fan et al.
[36] studied the removal of NO
x by C
2H
2-SCR on an H-MOR catalyst in the DBD plasma. The results showed that the synergistic effect of the catalyst and DBD significantly increased the low-temperature catalytic activity of NO
x from 54.0% to 91.4% (
Figure 2D). A common understanding about the mechanism of C
2H
2-SCR is plenty of HCN by-products, derived from key surface intermediate species, such as -CN, -NCO, -NC
aO
bH
c, which would react with NO
x and O
2 to produce N
2. By introducing hydrocarbon, Niu et al.
[37] studied the synergistic effect of the non-thermal plasma on the denitrification efficiency of the Co/ZSM-5 catalyst in HC-SCR and found that when 500 ppm NO
x was introduced, the NO
x removal rate of Co-HZSM-5 catalyst applied plasma discharge was better than Co-HZSM5 catalyst alone, which increased by 30% at most in the temperature range of 150–450 °C. The synergistic effect showed that the excess oxygen generated by NTP led to the massive conversion of NO to NO
2, which is then effectively reduced to N
2 by hydrocarbons (
Figure 2E).
Similar to the NO
x removal via the NTP technique, the VOCs molecules are excited and ionized by the oxidizing substances produced by NTP (such as atomic oxygen, ozone, hydroxyl radicals, active ions, or high-energy electrons, etc.), so that they can be broken down into small molecular substances, even harmless substances such as CO
2 and H
2O. Previous studies strongly suggested that the removal of VOCs proceeds mainly on the surface of the metal-loaded zeolites. During the discharge process, dissociative chemisorption of O
2 onto metal-loaded zeolites produces reactive surface species (such as O
2− and O
−)
[40], which lead to the oxidative removal of pollutants, which was shown in Equations (4) and (5). Here the subscripts (g) and (s) indicate the species in the gas phase and surface, respectively. When plasma is applied to the catalyst, reactive surface oxygen species can be also generated from the lattice oxygen
[41] and direct interaction with O
3(g) [42], or O
(g) radicals as well
[43], as shown in Equations (6)–(8). The NTP can also promote the desorption of reaction products, which helps keep the catalytic activity, shown as in
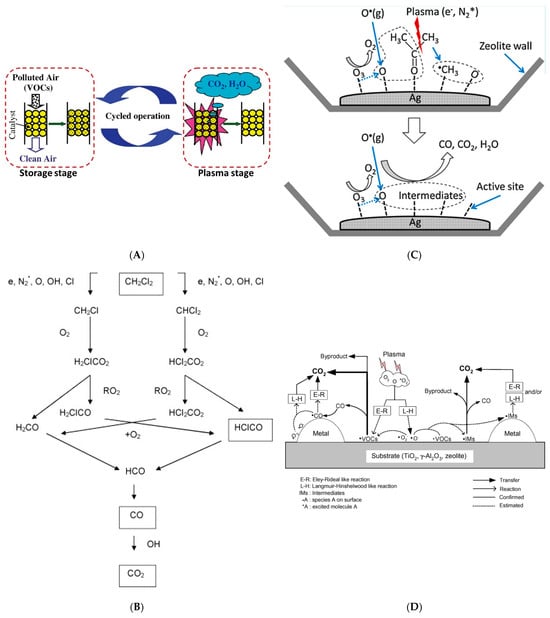
Figure 3A
[44].
The following examples all show that the plasma discharge will produce active substances consisting of reactive short-lived intermediates and long-lived radicals that participate in induced VOCs decomposition reactions. Wallis et al.
[45] studied the non-thermal plasma-assisted catalysis for the destruction of dichloro-methane (CH
2Cl
2), demonstrating that the combination of the plasma and HZSM-5 catalyst improved dichloro-methane conversion (36%) compared to plasma processing alone (27%), which is attributed to the synergistic action of zeolite surface with oxygen active species dissociated in the plasma. The schematic diagram of the reaction mechanism for CH
2Cl
2 decomposition in gaseous air has been given in
Figure 3B. In a combined zeolite plasma reactor, Oh et al.
[46] proved that toluene was decomposed not only by direct plasma decomposition in the plasma region but also by oxidation with O
3 on the zeolite outside the plasma region. With increasing reactor temperature, toluene decomposition increased, and the formation of CO was promoted owing to the enhanced decomposition of HCOOH. The reaction mechanism of the zeolite-plasma compound system for toluene decomposition was also studied by Teramoto et al.
[47]. It was found that O
3 directly decomposed toluene adsorbed in the inner of the zeolite micropores because it had a long lifetime, while the contributions of short-lived radicals and fast electrons to the decomposition of adsorbed toluene were low. Trinh et al.
[48] proposed and described the main reaction pathways responsible for the formation of gaseous byproducts in
Figure 3C. The gaseous and adsorbed acetone molecules are first dissociated into CH
3, CH
3CO, and atomic hydrogen (H) radicals by plasma-induced energetic species. Subsequently, the recombination of these fragments in the gas phase leads to the formation of CH
3CHO, HCHO, and CH
4. Meanwhile, further oxidations of the intermediates by the oxidizing agents such as O atomic, OH radicals, and O
2 finally lead to the formation of CO
x through reactions. About other VOCs, Francke et al.
[49] described the synergistic application of plasma treatment and NH
4-mordinite zeolite for the catalytic removal of butyl acetate and dichloroethene as typical VOCs oxidized to CO
2 at 100 °C. This synergy was partly due to the catalytic oxidation with ozone produced in the discharge. In addition, a plausible mechanism for the plasma-driven catalysis of the VOCs was summarized by Kim et al.
[50] in
Figure 3D. The models explaining the catalytic reactions are the Langmuir–Hinshelwood (L–H) model and the Eley–Rideal (E–R) model. In the L–H model, both of the reactants need to be absorbed on the surface and followed by migration to the active site. In the E–R model, only one reactant is adsorbed on the surface and the other exists in the gas phase. The adsorbed surface species can also migrate from one site to the other and eventually disappear via recombination and chemical reactions.
Figure 3. (
A) The working principle of the cyclic adsorption−plasma catalytic process for VOC removal
[44]. (
B) A schematic diagram of the reaction pathways for plasma destruction of CH
2Cl
2 in air
[45]. (
C) Reaction pathways for the oxidation of acetone on the Ag surface
[48]. (
D) A plausible mechanism for the plasma-driven catalysis of VOCs
[50].
4. Influence Parameters of NTP
Whether in the modification process of material structure or the reaction process of pollutant gas, to maximize the performance and simultaneously reduce the energy consumption of non-thermal plasma, it is essential to determine the appropriate parameters such as discharge power, voltage, and time. Above all, the input power is one of the most important parameters affecting the adsorption/catalysis capacity of the plasma-modified materials. With the growth of power, the reactivity of the zeolites will climb up and then decline. Li et al.
[51] found that with the enhancement of the plasma power, the NO
x conversion of NaY zeolite first increased and then decreased, reaching the maximum at 7.6 W owing to the maximum number of high-energy particles produced. However, when the power was too strong, the side reaction of the NO
x synthesis from substantial N
2 and O
2 might occur, which would reduce the NO
x conversion rate. Xiang et al.
[52] investigated the change of the hexanal removal rate of MnO
x/SBA-15 with the discharge voltage under the coordination of the NTP and proved that the higher the discharge voltage, the higher the energy density, and the higher the concentration of active particles and O
3 produced by the plasma field, which could promote the complete oxidation of hexanal. However, when the discharge voltage was greater than 8 kv, the hexanal removal rate of the MnO
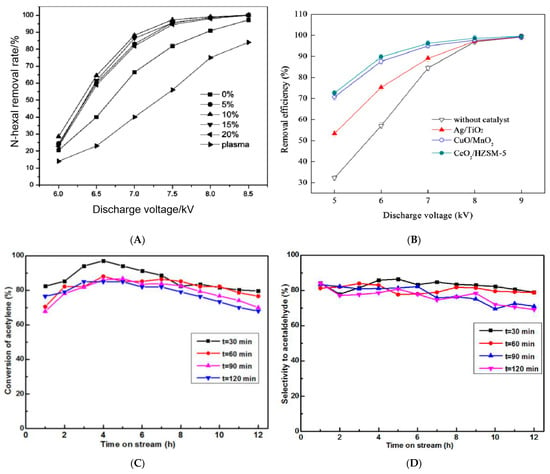
x/SBA-15 catalyst no longer changed significantly (
Figure 4A).
Figure 4. (
A) Relationship between hexanal conversion and applied voltage over different loading catalysts
[52]. (
B) Effect of discharge voltage on chlorobenzene removal efficiency
[53]. Conversion of acetylene (
C) and selectivity to acetaldehyde (
D) in acetylene hydration over Zn−based catalysts with different plasma treatment times
[25].
Voltage is an influential factor that affects the goal of a plasma catalytic system to achieve an acceptable removal efficiency with low energy cost. Zhu et al.
[53] found that synergy between the NTP and catalyst enhanced the conversion efficiency by 18–40%, especially at a discharge voltage of 5 kV. At low applied voltage, the numbers of energetic electrons and reactive species are relatively low. In addition, the concentration of oxygen resulting from O
3 decomposition was low, and UV radiation was too weak to effectively activate the catalyst in the reactor. Consequently, the concentration of O atoms on the catalyst surface was low. When the discharge voltage increased, the gas discharge became more intense, and catalytic activity increased, resulting in higher removal efficiency (
Figure 4B).
The discharge time of non-thermal plasma also exhibits an important effect on the reaction, and specifically, the optimal discharge time can not only improve the reactivity of zeolites but also can reduce energy consumption. The catalytic performance of the Zn-based catalysts with different oxygen plasma treatment times for acetylene hydration was studied by Wang
[25]. The activity and selectivity of the catalysts were shown in
Figure 4C,D. When t = 30 min, it showed that the highest conversion of acetylene was over 95% and selectivity to acetaldehyde was about 80% after 12 h of reaction. The conversion and selectivity of the catalyst were reduced after 12 h of reaction when t = 60, 90 120 min compared to t = 30 min. It may be explained that the long period of the plasma treatment may lead to the agglomeration of the active components or the destruction of the catalyst structure, thus obstructing the better catalytic performance of the catalyst to be achieved. Wang et al.
[17] verified that with the increase of plasma discharge time, the naphthalene adsorption capacity of the NaY zeolites increased rapidly, achieving the maximum at 25 min, which was improved by 56% compared to the unmodified NaY. It could be attributed to the generation of plentiful acid sites and surface chemical functional groups, which can facilitate the adsorption of naphthalene to the external surface of zeolite.