Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Environmental Sciences
沸石基材料被广泛用作净化空气污染物(如 NO)的吸附剂和催化剂x以及由于丰富的孔隙结构、规则的孔隙分布和众多的离子交换位点而产生的 VOC。热处理是去除孔隙中的杂质和促进金属活性物质均匀分散的必要程序,然后应用沸石基吸附剂/催化剂来净化 NOx/挥发性有机化合物。然而,传统的热场处理(即高温煅烧、高温吹扫等)需要大量的能耗。相比之下,非热等离子体等非常规外场处理显示出高效率、低能耗和低污染的显著优势,在许多领域被用于替代传统热处理。
- zeolite
- VOCs
- NOx
- non-thermal plasma
1. 引言
等离子体是一种由放电产生的放热电离气体[25]。通过向反应区域注入能量,电子从原子或分子中释放出来,达到离子、电子和原始物质共存的电中性状态[23]。根据能级、温度和离子密度,等离子体一般分为高温等离子体(核应用)和低温等离子体(包括热等离子体和非热等离子体)[26]。特别是,非热等离子体可以应用于催化剂制备和活化领域,因为相对较低的温度条件(通常为几十到200°C)不会对材料表面造成热损伤[23]。此外,由于真空系统在大气压下工作,因此可以避免真空系统的成本[27]。具体来说,热等离子体的气体温度接近电子温度(约几十电子伏特),而非热等离子体的气体温度可低至室温[28]。
非热等离子体通过在高能物质的蚀刻下产生一些特定的性质或功能,在装饰沸石结构方面显示出巨大的潜力[20]。等离子体活化为沸石主要涉及以下几点:
- (1)
- (2)
-
大量高能的电子和其他活性物质会通过蚀刻和烧蚀作用侵蚀沸石的外表面,形成新的孔隙,从而增加孔隙率。
- (3)
-
高能的电子可以破坏沸石表面的化学键,形成新的活性基团,增加活性位点的数量。
- (4)
-
等离子体放电产生的高能粒子可以促进气体-反应物分子的激发、电离和相互作用,形成新的化学键[30]。
与传统的热处理方法相比,非热等离子体具有快速、清洁、节能、方便、成本相对低等优点[18],同时提高了材料的活性、寿命和选择性[31];因此,它被视为一种理想的治疗方法。目前,非热等离子体通常用于活化吸附剂/催化剂或辅助净化过程,以提高VOCs或NO的去除效率x.用于活化材料的一般非热等离子体技术分别包括介电势垒放电等离子体(DBD)、辉光放电等离子体(GDP)和电晕放电等离子体(CDP)[32]。DBD是在介质中存在强电场的情况下电子穿透的现象,其中电子被电场加速并获得足够的能量。GDP是通过向气体中添加足够的电压以引起原子碰撞,产生电离电子以形成电流引起的。CDP发生在大曲率半径的尖端电极附近的不均匀电场中,是由于局部电场强度超过气体电离场强而导致气体电离和激发引起的。
2. NTP辅助活化材料
非热等离子体可以有效地改变材料的表面性质[25],因为在放电过程中产生的大量高能电子和其他活性物质攻击沸石表面形成新的孔隙和活性基团。同时,沸石载体上的激发物质和酸碱基之间发生化学反应,从而改变载体的酸碱度[19]。此外,碱性金属的活性成分可以通过与酸性位点的相互作用来调节金属的分散性[35]。此外,通过改变沸石的织构特性,最终,非热等离子体可以帮助沸石基材料提高目标产物的反应性、选择性、稳定性和收率[36]。更重要的是,与传统的高温热处理容易破坏沸石骨架相比,等离子体可以避免由于低温而出现这种情况,保持沸石的稳定性[28]。
事实证明,沸石表面会产生羟基等更多的活性基团,伴随着孔隙率的增长和放电后活性位点的相应增加。非热等离子体对材料的活化主要用于辅助VOCs的净化。Liu等[32]发现,Pd/HZSM-5催化剂经非热等离子体处理后,在400 °C下甲烷转化率从25%提高到90%,这是活性位点增加所致[37]。Wang等[38]证明,与未处理的催化剂相比,等离子体处理增强了Pd/Al-MCM-41分子筛催化剂在甲烷燃烧反应中的初始活性(图1 A),这是由于催化剂的酸度较高(图1B)和PdO颗粒活性物质的分散性较好。此外,等离子体处理的钯基催化剂稳定性的提高与氧化钯与沸石载体之间更强的相互作用密切相关。Wang等[39]使用非热等离子体显著增大了孔径,使孔径从0.62 nm增加到0.72 nm,比表面积从222.14增加到618.06 m2/g,孔隙体积为 0.19 至 0.36 cm3/g,同时对萘的吸附量大大增加,达到167%。此外,还归因于放电区产生的大量活性物质(即高能电子、离子、质子、紫外光子和活性自由基)轰击吸附剂的外表面,通过蚀刻效应促进新孔隙的形成和原有孔隙的重建。另一方面,加速电子在电场中的高能量可以破坏化学键并形成新的键,导致活性位点量增加,从而提高分子筛的活性。
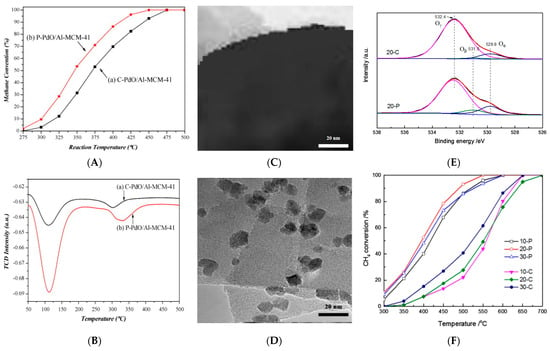
除了改变沸石的孔隙结构和表面性质外,非热等离子体的另一个重要应用是引入一定量的质子酸位点,以提高沸石的酸度和活性[41]。含有高动能的碳、氧、氮的自由基和活性离子很容易与分子筛中的酸性或碱性基团发生反应[42],从而改变分子筛的酸性或碱性[19]。Xia等[43]发现,与常规处理得到的Fe-Mo/HZSM-5分子筛相比,非热等离子体处理分子筛的Brönsted酸度高达265%以上,甲烷转化率从11%显著提高到24%[44]。对于Mo-Fe/HZSM-5分子筛,Zhu等[45]还发现,等离子体处理催化剂的Brönsted酸强度和密度均高于未处理的样品,因此甲烷转化率提高。Liu等[32]证实,非热等离子体处理显著提高了Pd/HZSM-5分子筛的Brönsted和Lewis酸含量。两个酸性位点的浓度分别是新鲜样品的1.13倍和1.21倍。结果,处理后的沸石在甲烷燃烧中表现出比新鲜剂高50%的催化活性和更强的稳定性[37]。除了上述等离子体辅助甲烷脱除的应用外,竹内等[46]证明,对于异丁烷催化裂化,等离子体处理5 min比常规600 °C煅烧2 h处理可使活性提高10%,这归因于等离子体处理后HY分子筛中可以有效形成Lewis酸位点。
The non-thermal plasma treatment can also increase the dispersion and stability of the metal active components loaded on the materials, thus providing more reactive centers [19]. Liu et al. [32] also found that after treatment with the non-thermal plasma, the particle size of the PdO on the surface of the Pd/HZSM-5 catalyst decreased from 21.0 to 16.0 nm and the Pd dispersion increased by 2.5 times, effectively avoiding the aggregation of PdO particles, and blocking the pores of ZSM-5. Thereby, the catalyst showed higher catalytic activity and better stability than that without the plasma treatment in the methane combustion. It was concluded that the plasma treatment could make the PdO cluster (alkaline oxide) better attracted to the increased Brönsted and Lewis acidic sites, facilitating the PdO dispersion on the zeolite. In addition, the enhanced interaction between the PdO and acidic sites made the PdO more stable during the reaction, significantly improving the stability of the catalyst [37]. Cao et al. [40] found that compared to the calcination, the plasma treatment could promote the formation and dispersion of small-size Co3O4 on Co3O4/H-ZSM-5 (TEM shown in Figure 1C,D). Moreover, it was obvious that the content of the adsorbed oxygen species Oβ for 20-P (5.2) was more than 7 times higher than that for 20-C (0.7), which indicated that plasma could improve the number of lattice oxygen of Co3O4 on the catalyst surface (XPS spectra shown in Figure 1E). Therefore, the catalytic activity of methane was simultaneously improved (the conversion rate of 100% at 550 °C) (Figure 1F). Wang et al. [47] reported a high performance of the plasma-enhanced Zn/MCM-41 catalyst for acetylene hydration, showed that plasma treatment could effectively enhance the interaction between the Zn active sites and MCM-41 carrier, promote the dispersion of the Zn active component, and inhibit the loss of Zn species.
There is also some research on the activation materials by non-thermal plasma to assist NOx purification, although are not as widespread as the VOCs removal. Yoon et al. [48] found that the active sites on the NaY catalyst increased via non-thermal plasma treatment, which greatly improved the NOx conversion rate of the catalyst from 20% to 55% at 225 °C in the lean NOx reduction [49]. Zhang et al. [50] proved that the dispersion of the Pd active component on the surface of the Pt/NaZSM-5 catalyst increased markedly from 4.2% to 63.3% after plasma treatment. Correspondingly, the particle size decreased significantly from 27.0 to 1.8 nm, and the NO redox activity enhanced observably from almost zero to 61.3% at 400 °C.
3. NTP Cooperative Catalysis Process
In addition to the above-mentioned activation level of material modification, during the process of activating zeolite materials by non-thermal plasma, reaction gas can be accelerated to activated when injected in the discharge region, NOx/VOCs can be cooperatively removed, and the conversion rate can be improved. The basic principle of this technology is that a high-intensity electric field provides energy for the electrons so that electrons can obtain high kinetic energy. Then the high-speed moving electrons transfer energy to molecules through collision, so that some gas molecules in the gas are excited and ionized. Through direct generation or ionization, many active particles such as ions, excited atoms, molecules, electrons, and free radicals are generated, and these active particles undergo a series of complex physicochemical reactions with the NOx or VOCs molecules to achieve the purpose of removal [51]. The energy of the active particles in the plasma is higher than that of most chemical bonds, which indicates that these extremely high-energy active particles can completely promote the fracture of the old bonds of reactants, thus generating new chemical bonds [30].
The NTP has been widely applied for purifying the NOx via the routes of direct decomposition [52], oxidation combined with absorption [53], adsorption combined with in-situ decomposition [4], selective catalytic reduction [54], etc., the core roles of which in above actions are mainly decomposition, oxidation, and reduction. The NO decomposition by the NTP is realized through colliding and making N-O bonds broken by the electrons or high-energy species generated from the NTP to produce N2 and O2 [52]. The NO oxidation by the NTP is to use the oxidizing species O, O3, or OH generated by NTP to oxidize NO to NO2 or nitrate [53]. NO reduction by NTP is to use the N atom generated by NPT to react with NO to produce N2 and O2; or introduce NH3, HC, or CxHy into the reaction gas to reduce NO to N2 and O2 [54]. The specific reaction process can be seen in Equations (1)–(3). The possible ways of combining the discharge plasma with the catalyst are shown schematically in Figure 2A,B [55]. The single-stage method is also known as the plasma-driven catalyst (PDC) system [19]. In this method, the catalyst is placed directly in the plasma reactor. The PDC system will induce both the gas-phase chemical reaction and the catalytic reaction on the catalyst surface. On the other hand, in the two-stage method, the plasma reactor is followed by the catalyst bed. This method is referred to as plasma-enhanced selective catalyst reduction (PE-SCR) method. In this case, NOx is oxidized by plasma and then reduced by the catalyst.
NTP decomposition of NO: 2NO + e→N2 + O2
NTP oxidation of NO: NO + O+M→NO2 + M, NO + O3→NO2 + O2
NTP reduction of NO: NO + N→N2 + O
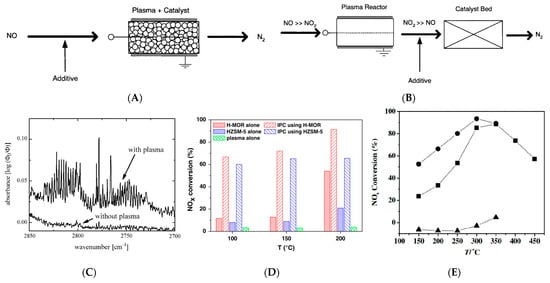
Figure 2. Combination of plasma with a catalyst: (A) single−stage (PDC) system; (B) two−stage (PE−SCR) system. (C) The infrared spectrum of the exhaust is not treated and treated with plasma [56]. (D) NOx conversions for the plasma alone, catalyst alone, and in−plasma catalysis (IPC) cases [57]. (E) NOx conversion as functions of temperature over (■) Co−HZSM5 catalyst alone, (●) discharge over Co−HZSM-5 catalyst, and (▲) discharge over quartz pellets [58].
It has been demonstrated that the main pathway of plasma to deal with NOx is HC-SCR, NH3-SCR, CH4-SCR, etc. by producing electrons or free radicals. Coupling the NTP technology with the NH3-SCR system by Byun et al. [59], the low-temperature reaction activity of NOx was greatly improved under the synergistic effect, and the reaction temperature window was also widened from 300–400 °C to 100–500 °C. This tendency could be attributed to the fact that the OH and H radicals generated from the NTP will react rapidly to decompose NH3 at low temperatures to produce NH2 radicals, followed by selective non-catalytic reduction through the reaction of NO with NH2 to produce N2. The NTP in collaboration with the NH3-SCR technology was also used by Schmidt et al. to remove NOx from diesel exhaust [56]. It was regarded that the conversion of NO is mainly triggered by the electron impact dissociation of molecular oxygen and molecular nitrogen. By comparing the infrared spectra of the gas mixture before and after being treated with plasma, it was found that the byproducts are probably formed due to the action of the plasma. With this method, the species produced by plasma can be found since new absorption lines would occur in the spectrum as depicted in Figure 2C. It has been reported by Singh et al. [60] that the reaction system cooperated the NTP with NH3-SCR could produce some long-lived and short-lived active substances, such as the gas-phase electronic excited states of NOx, hydrocarbon fragments, some oxygen-containing derivatives, and vibration-excited substances. These active species, which exist in gas or surface phases, are a key factor for the significant synergistic effect of the NTP and SCR catalysts. Fan et al. [57] studied the removal of NOx by C2H2-SCR on an H-MOR catalyst in the DBD plasma. The results showed that the synergistic effect of the catalyst and DBD significantly increased the low-temperature catalytic activity of NOx from 54.0% to 91.4% (Figure 2D). A common understanding about the mechanism of C2H2-SCR is plenty of HCN by-products, derived from key surface intermediate species, such as -CN, -NCO, -NCaObHc, which would react with NOx and O2 to produce N2. By introducing hydrocarbon, Niu et al. [58] studied the synergistic effect of the non-thermal plasma on the denitrification efficiency of the Co/ZSM-5 catalyst in HC-SCR and found that when 500 ppm NOx was introduced, the NOx removal rate of Co-HZSM-5 catalyst applied plasma discharge was better than Co-HZSM5 catalyst alone, which increased by 30% at most in the temperature range of 150–450 °C. The synergistic effect showed that the excess oxygen generated by NTP led to the massive conversion of NO to NO2, which is then effectively reduced to N2 by hydrocarbons (Figure 2E).
Similar to the NOx removal via the NTP technique, the VOCs molecules are excited and ionized by the oxidizing substances produced by NTP (such as atomic oxygen, ozone, hydroxyl radicals, active ions, or high-energy electrons, etc.), so that they can be broken down into small molecular substances, even harmless substances such as CO2 and H2O. Previous studies strongly suggested that the removal of VOCs proceeds mainly on the surface of the metal-loaded zeolites. During the discharge process, dissociative chemisorption of O2 onto metal-loaded zeolites produces reactive surface species (such as O2− and O−) [61], which lead to the oxidative removal of pollutants, which was shown in Equations (4) and (5). Here the subscripts (g) and (s) indicate the species in the gas phase and surface, respectively. When plasma is applied to the catalyst, reactive surface oxygen species can be also generated from the lattice oxygen [62] and direct interaction with O3(g) [63], or O(g) radicals as well [64], as shown in Equations (6)–(8). The NTP can also promote the desorption of reaction products, which helps keep the catalytic activity, shown as in Figure 3A [65].
O2(g) + Catalyst*→O2(s)− + Catalyst
O2(s)− +e→O(s)− + O−(s)
Lattice-O(s) + plasma→O(s)− + Catalyst
O3(g) + Catalyst*→O(s)− + O2(s) + Catalyst
O(g) + Catalyst*→O(s)− + Catalyst
The following examples all show that the plasma discharge will produce active substances consisting of reactive short-lived intermediates and long-lived radicals that participate in induced VOCs decomposition reactions. Wallis et al. [66] studied the non-thermal plasma-assisted catalysis for the destruction of dichloro-methane (CH2Cl2), demonstrating that the combination of the plasma and HZSM-5 catalyst improved dichloro-methane conversion (36%) compared to plasma processing alone (27%), which is attributed to the synergistic action of zeolite surface with oxygen active species dissociated in the plasma. The schematic diagram of the reaction mechanism for CH2Cl2 decomposition in gaseous air has been given in Figure 3B. In a combined zeolite plasma reactor, Oh et al. [67] proved that toluene was decomposed not only by direct plasma decomposition in the plasma region but also by oxidation with O3 on the zeolite outside the plasma region. With increasing reactor temperature, toluene decomposition increased, and the formation of CO was promoted owing to the enhanced decomposition of HCOOH. The reaction mechanism of the zeolite-plasma compound system for toluene decomposition was also studied by Teramoto et al. [68]. It was found that O3 directly decomposed toluene adsorbed in the inner of the zeolite micropores because it had a long lifetime, while the contributions of short-lived radicals and fast electrons to the decomposition of adsorbed toluene were low. Trinh et al. [69] proposed and described the main reaction pathways responsible for the formation of gaseous byproducts in Figure 3C. The gaseous and adsorbed acetone molecules are first dissociated into CH3, CH3CO, and atomic hydrogen (H) radicals by plasma-induced energetic species. Subsequently, the recombination of these fragments in the gas phase leads to the formation of CH3CHO, HCHO, and CH4. Meanwhile, further oxidations of the intermediates by the oxidizing agents such as O atomic, OH radicals, and O2 finally lead to the formation of COx through reactions. About other VOCs, Francke et al. [70] described the synergistic application of plasma treatment and NH4-mordinite zeolite for the catalytic removal of butyl acetate and dichloroethene as typical VOCs oxidized to CO2 at 100 °C. This synergy was partly due to the catalytic oxidation with ozone produced in the discharge. In addition, a plausible mechanism for the plasma-driven catalysis of the VOCs was summarized by Kim et al. [71] in Figure 3D. The models explaining the catalytic reactions are the Langmuir–Hinshelwood (L–H) model and the Eley–Rideal (E–R) model. In the L–H model, both of the reactants need to be absorbed on the surface and followed by migration to the active site. In the E–R model, only one reactant is adsorbed on the surface and the other exists in the gas phase. The adsorbed surface species can also migrate from one site to the other and eventually disappear via recombination and chemical reactions.
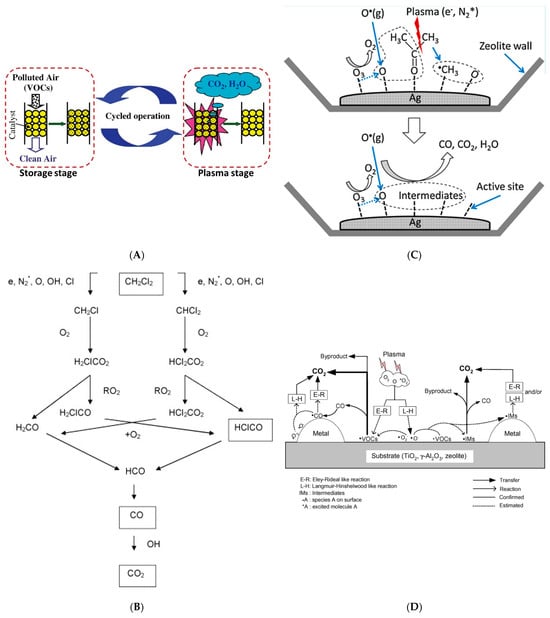
Figure 3. (A) The working principle of the cyclic adsorption−plasma catalytic process for VOC removal [65]. (B) A schematic diagram of the reaction pathways for plasma destruction of CH2Cl2 in air [66]. (C) Reaction pathways for the oxidation of acetone on the Ag surface [69]. (D) A plausible mechanism for the plasma-driven catalysis of VOCs [71].
4. Influence Parameters of NTP
Whether in the modification process of material structure or the reaction process of pollutant gas, to maximize the performance and simultaneously reduce the energy consumption of non-thermal plasma, it is essential to determine the appropriate parameters such as discharge power, voltage, and time. Above all, the input power is one of the most important parameters affecting the adsorption/catalysis capacity of the plasma-modified materials. With the growth of power, the reactivity of the zeolites will climb up and then decline. Li et al. [72] found that with the enhancement of the plasma power, the NOx conversion of NaY zeolite first increased and then decreased, reaching the maximum at 7.6 W owing to the maximum number of high-energy particles produced. However, when the power was too strong, the side reaction of the NOx synthesis from substantial N2 and O2 might occur, which would reduce the NOx conversion rate. Xiang et al. [73] investigated the change of the hexanal removal rate of MnOx/SBA-15 with the discharge voltage under the coordination of the NTP and proved that the higher the discharge voltage, the higher the energy density, and the higher the concentration of active particles and O3 produced by the plasma field, which could promote the complete oxidation of hexanal. However, when the discharge voltage was greater than 8 kv, the hexanal removal rate of the MnOx/SBA-15 catalyst no longer changed significantly (Figure 4A).
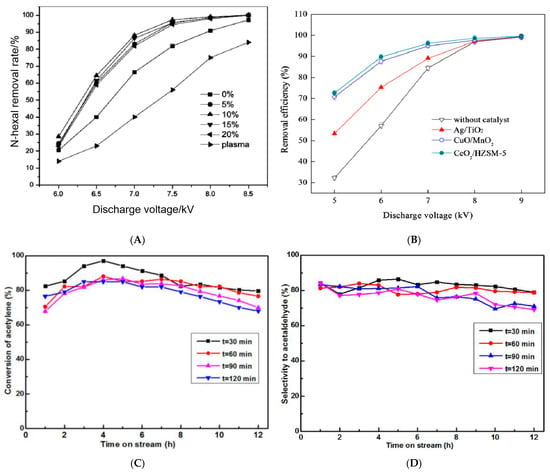
电压是影响等离子体催化系统目标的一个影响因素,以低能源成本实现可接受的去除效率。Zhu等[74]发现,NTP和催化剂之间的协同作用使转化效率提高了18-40%,特别是在5 kV的放电电压下。在低电压下,高能电子和反应物质的数量相对较低。此外,氧气的浓度由O产生3分解率低,紫外线辐射太弱,无法有效活化反应器中的催化剂。因此,催化剂表面的O原子浓度较低。当放电电压增加时,气体放电变得更加强烈,催化活性增加,从而产生更高的去除效率(图4B)。
非热等离子体的放电时间对反应也表现出重要影响,具体而言,最佳放电时间不仅可以提高沸石的反应性,还可以降低能耗。Wang[47]研究了不同氧等离子体处理次数的Zn基催化剂对乙炔水合的催化性能。催化剂的活性和选择性如图4C,D所示。当t=30 min时,反应12 h后乙炔转化率最高,对乙醛的选择性约为80%。与t = 30 min相比,反应12 h后,催化剂的转化率和选择性降低,当t = 60、90 120 min时。可以解释为,等离子体处理的长周期可能导致活性组分的团聚或催化剂结构的破坏,从而阻碍了催化剂实现更好的催化性能。Wang等[39]证实,随着等离子体放电时间的增加,NaY分子筛的萘吸附能力迅速增加,在25 min时达到最大值,与未改性的NaY相比提高了56%。可归因于产生丰富的酸位点和表面化学官能团,可促进萘吸附到沸石的外表面。然而,当放电时间超过25 min时,NaY的吸附能力没有明显变化,这主要是由于活性位点的数量达到饱和,不再增加。
This entry is adapted from the peer-reviewed paper 10.3390/catal13121461
This entry is offline, you can click here to edit this entry!
