Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laura Zaccaro | -- | 2081 | 2023-11-30 12:05:25 | | | |
| 2 | Wendy Huang | Meta information modification | 2081 | 2023-11-30 12:41:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Giorgio, A.; Del Gatto, A.; Pennacchio, S.; Saviano, M.; Zaccaro, L. Radiolabeled Peptoids and Peptoid/Peptide Hybrids for Cancer-Targeted Imaging. Encyclopedia. Available online: https://encyclopedia.pub/entry/52229 (accessed on 03 March 2026).
Giorgio A, Del Gatto A, Pennacchio S, Saviano M, Zaccaro L. Radiolabeled Peptoids and Peptoid/Peptide Hybrids for Cancer-Targeted Imaging. Encyclopedia. Available at: https://encyclopedia.pub/entry/52229. Accessed March 03, 2026.
Giorgio, Anna, Annarita Del Gatto, Simone Pennacchio, Michele Saviano, Laura Zaccaro. "Radiolabeled Peptoids and Peptoid/Peptide Hybrids for Cancer-Targeted Imaging" Encyclopedia, https://encyclopedia.pub/entry/52229 (accessed March 03, 2026).
Giorgio, A., Del Gatto, A., Pennacchio, S., Saviano, M., & Zaccaro, L. (2023, November 30). Radiolabeled Peptoids and Peptoid/Peptide Hybrids for Cancer-Targeted Imaging. In Encyclopedia. https://encyclopedia.pub/entry/52229
Giorgio, Anna, et al. "Radiolabeled Peptoids and Peptoid/Peptide Hybrids for Cancer-Targeted Imaging." Encyclopedia. Web. 30 November, 2023.
Copy Citation
Peptoids (N-substituted glycine oligomers) are a relatively new class of peptidomimetics, being highly versatile and capable of mimicking the architectures and the activities of the peptides but with a marked resistance to proteases and a propensity to cross the cellular membranes over the peptides themselves. For these properties, they have gained an ever greater interest in applications in bioengineering and biomedical fields.
peptoids
diagnosis
targeted imaging
cancer
VEGFR2
NTS1/NTS2
biomarker
1. Introduction
The early and accurate diagnosis of deadly and disabling diseases such as cancers and neurological syndromes is constantly a challenge for the research community. The identification of early biomarkers and the development of related molecular probes are crucial to classify those patients who could best benefit from a specified therapeutic regimen, dose, or duration of the drug prolonging their survival and quality of life. Recently, different methodologies for the identification of specific, smart, and selective probes have been proposed. The design of peptide-based systems has been one of the most successful approaches for the discovery of new probes. Thanks to the advantages of using short synthetic peptides over recombinant proteins and small molecules [1], such as high selectivity, specificity, non-immunogenicity, low cost of production, and high degree of chemical diversity, all features tightly correlated to peptide architectures. Unfortunately, their acceptance in diagnostics has very often been hindered by the low resistance to proteolytic degradation. Peptidomimetics are a worthy alternative to peptides for circumventing such complications, and some of them have entered clinical trials as drugs [2][3]. Peptoids (N-substituted glycine oligomers) represent one of the most promising classes of peptidomimetics, with a backbone like a polyglycine, featured with the side-chains covalently linked to the amide nitrogen on the peptoid.
Zuckermann and coworkers in 1992 [4], published the invention of the sub-monomer solid-phase synthesis method for peptoids preparation (Figure 1). This strategy has greatly increased the chemical diversity, the synthetic efficiency and yield, reducing time and costs.
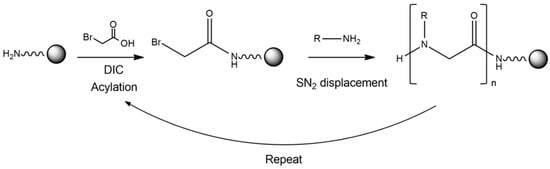
Figure 1. Schematic representation of the synthetic strategy used to obtain a peptoid. In particular, a primary amine on the resin is acylated via an activated haloacetic acid, such as bromoacetic acid, with N,N-diisopropylcarbodiimide (DIC). Then, the bromine is displaced via a primary amine during a SN2 reaction.
The shift of the side chain on the amide nitrogen improves the enzyme resistance (Figure 2) and their chemical and thermal stability. The enhanced stability of peptoids is reasoned to prevent premature drug activity caused via enzymatic degradation in vivo.
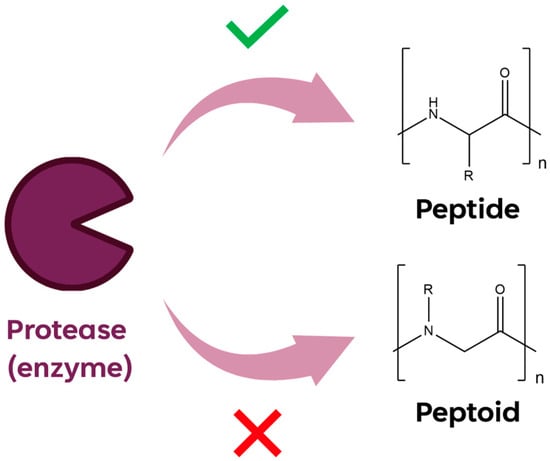
Figure 2. Graphic representation of enzymatic proteases cleavage on peptide and peptoid sequences.
In the last decade, many research studies have demonstrated different applications of peptoids in biomedicine and material science [5]. Some of them act as molecular modulators, e.g., ligands for GPCRs [6] and protease inhibitors [7], novel bio-inspired nanomaterials [8][9], potent antiviral [10] and anti-cancer agents [11], and notably, antimicrobials [12][13][14][15][16].
Nowadays, pathology diagnosis relies mainly on targeted molecular imaging techniques, such as nuclear medicine modalities PET and SPECT, which use radioligands and provide detailed metabolic and functional information and accurate data, thus revealing disease progression. PET and SPECT imaging are mainly employed in oncology and neurology, and are characterized by a good sensitivity, spatial resolution, and unlimited penetration depth, leading to their wide use for preclinical and clinical studies. The use of specific contrast agents, such as peptides and peptoids for an array of disease-related receptors or surrogate biomarkers, has contributed to improving the targeting efficiency of the detection by eliminating misinterpretation of false-positive outcomes. Nevertheless, PET and SPECT imaging applications are entirely dependent on the availability of radiotracers whose development is the result of different steps. These steps include selection of suitable biological targets, design and identification of promising targeting molecules, optimization of the radiolabeling strategies, in vitro and in vivo preclinical assessment of candidate radiotracers [17].
The success of an accurate and reliable diagnosis is correlated with the identification of biomarkers associated with a given pathology. The detection of well-researched and widely accepted biomarkers using tailored probes is fundamental for effective imaging targeted approaches, reducing the frequency of false positive diagnoses and bringing down overall healthcare costs.
To illustrate the advances made in cancer-targeted imaging, the peptoid oligomers and peptide/peptoid hybrids known to date are shown below (Figure 3). These oligomers are useful for the targeted imaging of different biomarkers, such as VEGFR2 and NTS1/NTS2.
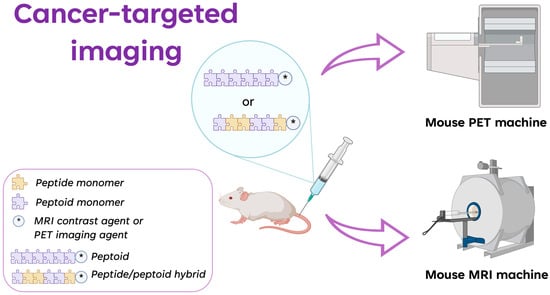
Figure 3. Graphic representation of the application of peptoids and peptide/peptoid hybrids in cancer diagnosis by using PET and MRI as imaging techniques. * is the contrast agent in MRI and the imaging agent in PET (created with Biorender.Com).
2. VEGFR2 Biomarker
Several angiogenesis-related biomarkers have been discovered and used as imaging targets so far. Vascular endothelial growth factors receptor 2 (VEGFR2) plays a fundamental role in promoting tumor angiogenesis and tumor growth. Recently, a VEGFR2 binding peptoid sequence GU40C (9 mer) was selected via a large library screening [18], but the molecule shows a low binding affinity for the target limiting its development as receptor-based imaging reagent. The same peptoid synthesized as dimer (GU40C4) was developed and proven to have a high affinity against the VEGFR2 activation and an effect in inhibiting angiogenesis and tumor growth in vivo [18]. In 2010, De Leòn-Rodrìgues et al. [19] created targeted agents for MRI made up of GU40C4 and a poly(DOTA-lysine) dendron (Gd8 dendron) with a lysine linker (Table 1). A Cys residue was added to the C-terminus of the peptoid to maintain the conjugation site away from the central pharmacophore and allow a site-specific coupling with DOTA through the maleimide-thiol coupling chemistry. DOTA was chosen as Gd3+ chelating agent because it forms complexes with a high level of thermodynamic and kinetic stability, eliminating issues with the metal release, especially for in vivo use. Two conjugates with different linkers between GU40C4 and Gd8-dendron were investigated, and the best of them (conjugate 2 reported in De León-Rodríguez et al. [19], Table 1) was selected for the binding affinity to the receptor and the molecular r1 relaxivity value. Considering the interesting MR imaging results obtained on porcine aortic endothelial cells expressing VEGFR2, the MRI properties of the compound were also evaluated in vivo by using MDA-MB-231 tumor xenografts known to express VEGFR2 [20]. Tumor uptake in mice treated with the peptoid-Gd8 dendron was maximally enhanced at ∼4 h post-injection, while the image intensity after injection of a scrambled peptoid Gd8-dendron conjugate reverted to baseline levels by 4 h. The kidneys intensity indicated the compound does not accumulate in these organs until much later in the study, but it is localized elsewhere during the early time points. At 48–72 h after injection, the kidney signal intensity in mice treated with the specific and the scrambled peptoid was restored to pre-treatment levels. All the results showed that this platform can give a promising T1 probe for MRI imaging of VEGFR2.
In 2011, Hao et al. [21] evaluated the potential of the dimeric peptoid GU40C4 labeled with positron emitter 64Cu to be used for tumor visualization in a prostate cancer mouse model highly expressing VEGFR2, exploiting the relatively small size of the molecule, the high in vivo stability and binding affinity to the receptor. The tumor uptake level of 64Cu-DOTA-GU40C4 was relatively low when compared to other reported VEGFR2-targeted imaging agents. This is likely due to different tumor models expressing dissimilar levels of VEGFR2 and non-specific tumor uptake effects which can occur. Nevertheless, the steady tumor uptake retention and effective clearance from non-target organs within the 20 h period of 64Cu-labeled homodimer allowed for superior tumor imaging contrast. This can be attributed to the peptoid cell permeability, provided by its positive charge [22] and the rapid in vivo kinetics which are correlated with its low molecular weight. Unfortunately, as the most reported PET probes for VEGFR2 imaging, GU40C4 binds to both VEGFR1 and VEGFR2 with a similar affinity [23] and thus, the discovery of novel peptoids with higher binding selectivity is required for this purpose.
3. NTS1/NTS2 Biomarker
The neurotensin receptor 1 (NTSR1) is an attractive target for the development of diagnostic and therapeutic drugs since its role in the onset and progression of many tumors such as breast, prostate, and colorectal [24] is well documented [25].
The neurotensin peptide (NT) shows a short half-life in blood and is not suitable for direct radiolabeling and application [26][27]. Accordingly, most advancements in neurotensin receptor imaging have been addressed at enhancing NT serum stability and NTSR1 specificity [28].
Maschauer et al. [29] reported a successful strategy to produce 18F-glycopeptoid derivatives of NT as PET imaging agents, combining the 18F-labeling with the glycosylation required for the improvement of the biokinetic and in vivo clearance properties. In detail, they synthesized some glycopeptide/peptoid hybrid analogs of NT 8–13, which is the highest active C-terminal hexapeptide of the endogenous NT, by using the CuACC click chemistry for the introduction of the glycosyl moiety at the N-terminus of the peptide. According to previous studies on the effects of the ligand conformation and peptide backbone modifications on affinity changes for several NT 8–13 analogs [30], metabolic stabilization was predicted to be achieved by the replacement of the N-terminal Arg-Arg by the peptoid-like residue N-(4-aminobutyl) Gly-Lys (NLys-Lys). The stabilized derivative [18F]FGlc-NT4 (Table 1) showed specific binding in HT29 cells expressing a high level of NTSR. Rat brain slices used for in vitro autoradiography further evidenced the [18F]FGlc-NT4 selective binding to NTSR-rich regions, which were fully blocked in the presence of NT. The biokinetics behavior of [18F]FGlc-NT4 in HT29 xenografted nude mice was also evaluated assessing metabolic stability in vivo and an appropriate signal-to-noise ratio for PET imaging at the earliest time points after injection. Although the results obtained are promising, further studies will focus on improving kidney clearance by changing the glycosyl moiety of the 18F-glycopeptoids to promote their use as tracers.
The same authors also investigated the properties of the peptide/peptoid hybrid NT4, radiolabeled with 68Ga for the visualization of NTSR1-expressing tumors by PET [31] since one of the major obstacles to using 18F is the high-cost production of the radionuclide by the cyclotron; the short-lived 68Ga is provided by the 68Ge/68Ga generator, which allows frequent and easy access to the isotope. Because elongation of the N-terminus of NT 8–13 is usually well-tolerated with respect to NTSR recognition, the DOTA chelator was inserted at the N-terminal ending. NT receptor binding studies using hNTS1-expressing CHO cells indicated that the Ga-DOTA, conjugate [68Ga]3 (Table 1) showed a lower affinity in comparison with NT 8-13 and the mimetic [18F]FGlc-NT4. Nevertheless, the lower affinity was balanced by the ability of the molecule to internalize into tumor cells via NTSR1, a key factor for in vivo tumor visualization. Biodistribution studies in HT29 xenografted nude mice revealed metabolic stability in vivo, fast blood clearance, high uptake in kidneys, and low uptake in the liver. Most importantly, the uptake of 68Ga-3 in the HT29 tumor was comparable to the 18F-labeled glycopeptide/peptoid hybrid, and the kidney uptake and clearance was improved respect to [18F]FGlc-NT4. The in vivo specificity and affinity of NTSR1-mediated uptake of [68Ga]3 was demonstrated in animals co-injected with [68Ga]3 and NT4.
A few publications have shown a correlation between tumor progression and expression of the NTSR2 subtype [32] indicating this receptor is also an interesting biomarker for personalized (precision) medicine of cancer. In 2015, Maschauer et al. [33] focused on the synthesis and in vivo evaluation of 18F4 (Table 1). This is a peptide/peptoid hybrid derived from NT 8-13 that was identified by the selection of a series of hybrids [34][35][36]. The molecule was significantly labeled with 18F isotope applying the strategy previously reported for [18F]FGlc-NT4 [29]. In vitro studies revealed the proteolytic stability of the derivative, and the binding affinity studies evidenced the outstanding subtype selectivity over NTSR1. In vitro autoradiography analyses of rat brain slices confirmed the ability of the molecule to discriminate between NTSR1 and NTSR2, differently distributed in the brain. Biodistribution studies using PC3 and HT29 xenografted nude mice showed successful retention of the tracer in the tumor, high accumulation in the kidneys, and moderate to low uptake in all other organs. Nevertheless, stability studies in vivo indicated a fast degradation of the compound in mouse blood in contrast to the high proteolytic resistance observed in vitro and further optimization of the compound is required.
Table 1. Radiolabeled peptoid and peptide/peptoid hybrid sequences for cancer-targeted imaging.
| Name | Sequence | Ref. |
|---|---|---|
| Gd8-dendron-GU40C4 | GU40C * -Ahx-bAla-Lys(GU40C *)-Cys[linker-(Gd8-dendron) **]-NH2 | [19] |
| 64Cu-DOTA-GU40C4 | GU40C *-Ahx-bAla-Lys(GU40C *)-Cys(64Cu-maleimide-monoamide-DOTA derivative)-NH2 | [21] |
| [18F]FGlc-NT4 | Pra *** (218FGlc)-NLys-Lys-Pro-Tyr-Tle-Leu-OH | [29] |
| [68Ga]3 | 68Ga-DOTA-NLys-Lys-Pro-Tyr-Tle-Leu-OH | [31] |
| 18F-4 | Pra *** (618FGlc)-NMeArg-Arg-Pro-NhomoTyr-Ile-Leu-OH | [33] |
* GU40C = NLys-NLeu-NLys-Nmba-Npip-NLys-NLeu-NLys-NLys. ** (Gd8-dendron) = poly(GdDOTA)lysine dendrimer scaffold. *** Pra: propargyl Glycine to allow 18F-fluoroglycosylation via CuAAC click chemistry.
References
- Pandey, S.; Malviya, G.; Chottova Dvorakova, M. Role of Peptides in Diagnostics. Int. J. Mol. Sci. 2021, 22, 8828.
- Gomari, M.M.; Abkhiz, S.; Pour, T.G.; Lotfi, E.; Rostami, N.; Monfared, F.N.; Ghobari, B.; Mosavi, M.; Alipour, B.; Dokholyan, N.V. Peptidomimetics in Cancer Targeting. Mol. Med. 2022, 28, 146.
- Qvit, N.; Rubin, S.J.S.; Urban, T.J.; Mochly-Rosen, D.; Gross, E.R. Peptidomimetic Therapeutics: Scientific Approaches and Opportunities. Drug Discov. Today 2017, 22, 454–462.
- Zuckermann, R.N.; Kerr, J.M.; Kent, S.B.; Moos, W.H. Efficient Method for the Preparation of Peptoids by Submonomer Solid-Phase Synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647.
- Sun, J.; Li, Z. Peptoid Applications in Biomedicine and Nanotechnology. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 183–213.
- Zuckermann, R.N.; Martin, E.J.; Spellmeyer, D.C.; Stauber, G.B.; Shoemaker, K.R.; Kerr, J.M.; Figliozzi, G.M.; Goff, D.A.; Siani, M.A.; Simon, R.J.; et al. Discovery of Nanomolar Ligands for 7-Transmembrane G-Protein-Coupled Receptors from a Diverse N-(Substituted)Glycine Peptoid Library. J. Med. Chem. 1994, 37, 2678–2685.
- Mazaleyrat, J.-P.; Rage, I.; Mouna, A.M.; Šavrda, J.; Wakselman, M.; Boulay, R.; Lelièvre, Y. Peptoid Mimics of a C2-Symmetric Inhibitor of the HIV-1 Protease. Bioorganic Med. Chem. Lett. 1994, 4, 1281–1284.
- Sun, J.; Zuckermann, R.N. Peptoid Polymers: A Highly Designable Bioinspired Material. ACS Nano 2013, 7, 4715–4732.
- Tran, H.; Gael, S.L.; Connolly, M.D.; Zuckermann, R.N. Solid-Phase Submonomer Synthesis of Peptoid Polymers and Their Self-Assembly into Highly-Ordered Nanosheets. JoVE (J. Vis. Exp.) 2011, 57, e3373.
- Diamond, G.; Molchanova, N.; Herlan, C.; Fortkort, J.A.; Lin, J.S.; Figgins, E.; Bopp, N.; Ryan, L.K.; Chung, D.; Adcock, R.S. Potent Antiviral Activity against HSV-1 and SARS-CoV-2 by Antimicrobial Peptoids. Pharmaceuticals 2021, 14, 304.
- Huang, W.; Seo, J.; Willingham, S.B.; Czyzewski, A.M.; Gonzalgo, M.L.; Weissman, I.L.; Barron, A.E. Learning from Host-Defense Peptides: Cationic, Amphipathic Peptoids with Potent Anticancer Activity. PLoS ONE 2014, 9, e90397.
- Chongsiriwatana, N.P.; Patch, J.A.; Czyzewski, A.M.; Dohm, M.T.; Ivankin, A.; Gidalevitz, D.; Zuckermann, R.N.; Barron, A.E. Peptoids That Mimic the Structure, Function, and Mechanism of Helical Antimicrobial Peptides. Proc. Natl. Acad. Sci. USA 2008, 105, 2794–2799.
- Kapoor, R.; Wadman, M.W.; Dohm, M.T.; Czyzewski, A.M.; Spormann, A.M.; Barron, A.E. Antimicrobial Peptoids Are Effective against Pseudomonas Aeruginosa Biofilms. Antimicrob. Agents Chemother. 2011, 55, 3054–3057.
- Mojsoska, B.; Zuckermann, R.N.; Jenssen, H. Structure-Activity Relationship Study of Novel Peptoids That Mimic the Structure of Antimicrobial Peptides. Antimicrob. Agents Chemother. 2015, 59, 4112–4120.
- Mojsoska, B.; Carretero, G.; Larsen, S.; Mateiu, R.V.; Jenssen, H. Peptoids Successfully Inhibit the Growth of Gram Negative E. Coli Causing Substantial Membrane Damage. Sci. Rep. 2017, 7, 42332.
- Nyembe, P.L.; Ntombela, T.; Makatini, M.M. Structure-Activity Relationship of Antimicrobial Peptoids. Pharmaceutics 2023, 15, 1506.
- Gee, A.D.; Herth, M.M.; James, M.L.; Korde, A.; Scott, P.J.; Vasdev, N. Radionuclide Imaging for Neuroscience: Current Opinion and Future Directions. Mol. Imaging 2020, 19, 1536012120936397.
- Zhu, W.; Okollie, B.; Bhujwalla, Z.M.; Artemov, D. PAMAM Dendrimer-Based Contrast Agents for MR Imaging of Her-2/Neu Receptors by a Three-Step Pretargeting Approach. Magn. Reson. Med. 2008, 59, 679–685.
- De León-Rodríguez, L.M.; Lubag, A.; Udugamasooriya, D.G.; Proneth, B.; Brekken, R.A.; Sun, X.; Kodadek, T.; Dean Sherry, A. MRI Detection of VEGFR2 in Vivo Using a Low Molecular Weight Peptoid−(Gd)8-Dendron for Targeting. J. Am. Chem. Soc. 2010, 132, 12829–12831.
- Roland, C.L.; Dineen, S.P.; Lynn, K.D.; Sullivan, L.A.; Dellinger, M.T.; Sadegh, L.; Sullivan, J.P.; Shames, D.S.; Brekken, R.A. Inhibition of Vascular Endothelial Growth Factor Reduces Angiogenesis and Modulates Immune Cell Infiltration of Orthotopic Breast Cancer Xenografts. Mol. Cancer Ther. 2009, 8, 1761–1771.
- Hao, G.; Hajibeigi, A.; León-Rodríguez, L.M.D.; Öz, O.K.; Sun, X. Peptoid-Based PET Imaging of Vascular Endothelial Growth Factor Receptor (VEGFR) Expression. Am. J. Nucl. Med. Mol. Imaging 2011, 1, 65.
- Kwon, Y.-U.; Kodadek, T. Quantitative Evaluation of the Relative Cell Permeability of Peptoids and Peptides. J. Am. Chem. Soc. 2007, 129, 1508–1509.
- Udugamasooriya, D.G.; Dineen, S.P.; Brekken, R.A.; Kodadek, T. A Peptoid “Antibody Surrogate” That Antagonizes VEGF Receptor 2 Activity. J. Am. Chem. Soc. 2008, 130, 5744–5752.
- Myers, R.M.; Shearman, J.W.; Kitching, M.O.; Ramos-Montoya, A.; Neal, D.E.; Ley, S.V. Cancer, Chemistry, and the Cell: Molecules That Interact with the Neurotensin Receptors. ACS Chem. Biol. 2009, 4, 503–525.
- Ouyang, Q.; Zhou, J.; Yang, W.; Cui, H.; Xu, M.; Yi, L. Oncogenic Role of Neurotensin and Neurotensin Receptors in Various Cancers. Clin. Exp. Pharmacol. Physiol. 2017, 44, 841–846.
- Kokko, K.P.; Hadden, M.K.; Orwig, K.S.; Mazella, J.; Dix, T.A. In Vitro Analysis of Stable, Receptor-Selective Neurotensin Analogues. J. Med. Chem. 2003, 46, 4141–4148.
- Previti, S.; Vivancos, M.; Rémond, E.; Beaulieu, S.; Longpré, J.-M.; Ballet, S.; Sarret, P.; Cavelier, F. Insightful Backbone Modifications Preventing Proteolytic Degradation of Neurotensin Analogs Improve NT S1-Induced Protective Hypothermia. Front. Chem. 2020, 8, 406.
- Maschauer, S.; Prante, O. Radiopharmaceuticals for Imaging and Endoradiotherapy of Neurotensin Receptor-positive Tumors. J. Label. Compd. Radiopharm. 2018, 61, 309–325.
- Maschauer, S.; Einsiedel, J.; Haubner, R.; Hocke, C.; Ocker, M.; Hübner, H.; Kuwert, T.; Gmeiner, P.; Prante, O. Labeling and Glycosylation of Peptides Using Click Chemistry: A General Approach to 18F-Glycopeptides as Effective Imaging Probes for Positron Emission Tomography. Angew. Chem. Int. Ed. 2010, 49, 976–979.
- Einsiedel, J.; Hübner, H.; Hervet, M.; Härterich, S.; Koschatzky, S.; Gmeiner, P. Peptide Backbone Modifications on the C-Terminal Hexapeptide of Neurotensin. Bioorganic Med. Chem. Lett. 2008, 18, 2013–2018.
- Maschauer, S.; Einsiedel, J.; Hocke, C.; Hübner, H.; Kuwert, T.; Gmeiner, P.; Prante, O. Synthesis of a 68Ga-Labeled Peptoid−Peptide Hybrid for Imaging of Neurotensin Receptor Expression in Vivo. ACS Med. Chem. Lett. 2010, 1, 224–228.
- Swift, S.L.; Burns, J.E.; Maitland, N.J. Altered Expression of Neurotensin Receptors Is Associated with the Differentiation State of Prostate Cancer. Cancer Res. 2010, 70, 347–356.
- Maschauer, S.; Greff, C.; Einsiedel, J.; Ott, J.; Tripal, P.; Hübner, H.; Gmeiner, P.; Prante, O. Improved Radiosynthesis and Preliminary in Vivo Evaluation of a 18F-Labeled Glycopeptide–Peptoid Hybrid for PET Imaging of Neurotensin Receptor 2. Bioorganic Med. Chem. 2015, 23, 4026–4033.
- Held, C.; Plomer, M.; Hübner, H.; Meltretter, J.; Pischetsrieder, M.; Gmeiner, P. Development of a Metabolically Stable Neurotensin Receptor 2 (NTS2) Ligand. ChemMedChem 2013, 8, 75–81.
- Held, C.; Hübner, H.; Kling, R.; Nagel, Y.A.; Wennemers, H.; Gmeiner, P. Impact of the Proline Residue on Ligand Binding of Neurotensin Receptor 2 (NTS2)-Selective Peptide–Peptoid Hybrids. ChemMedChem 2013, 8, 772–778.
- Einsiedel, J.; Held, C.; Hervet, M.; Plomer, M.; Tschammer, N.; Hübner, H.; Gmeiner, P. Discovery of Highly Potent and Neurotensin Receptor 2 Selective Neurotensin Mimetics. J. Med. Chem. 2011, 54, 2915–2923.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
511
Revisions:
2 times
(View History)
Update Date:
30 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





