Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Goran Gajski | -- | 2044 | 2023-11-29 15:14:50 | | | |
| 2 | Jessie Wu | Meta information modification | 2044 | 2023-11-30 03:34:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sjakste, N.; Gajski, G. Effects of Biologically Active Compounds of Animal Origin. Encyclopedia. Available online: https://encyclopedia.pub/entry/52196 (accessed on 13 January 2026).
Sjakste N, Gajski G. Effects of Biologically Active Compounds of Animal Origin. Encyclopedia. Available at: https://encyclopedia.pub/entry/52196. Accessed January 13, 2026.
Sjakste, Nikolajs, Goran Gajski. "Effects of Biologically Active Compounds of Animal Origin" Encyclopedia, https://encyclopedia.pub/entry/52196 (accessed January 13, 2026).
Sjakste, N., & Gajski, G. (2023, November 29). Effects of Biologically Active Compounds of Animal Origin. In Encyclopedia. https://encyclopedia.pub/entry/52196
Sjakste, Nikolajs and Goran Gajski. "Effects of Biologically Active Compounds of Animal Origin." Encyclopedia. Web. 29 November, 2023.
Copy Citation
Envenomation by animal venoms remains a serious medical and social problem, especially in tropical countries. On the other hand, animal venoms are widely used as a source of biologically active compounds for the development of novel drugs. Numerous derivatives of animal venoms are already used in clinical practice. Current research data point to the possibility of using animal venoms and their components in the development of various potential therapeutic agents; however, before their possible clinical use the route of injection, molecular target, echanism of action, exact dosage, possible side effects and other fundamental parameters should be her investigated.
venom
invertebrates
vertebrates
genotoxicity
DNA damage
pharmaceuticals
drugs
1. Introduction
Pharmaceuticals derived from animals continuously make a major contribution to health in terms of prevention and treatment of many diseases [1][2][3][4]. Animal venoms and their components, such as those from snakes, scorpions, spiders, bees, wasps, snails, toads, frogs, lizards and sea anemones have long been used in scientific research and are the basis of many products and drugs that are of great use in medicine today [5][6][7][8][9][10][11][12]. Crude venoms are complex bioactive chemicals rich in proteins and peptides with diverse pharmacological actions that are often protease-resistant due to their disulphide-rich structures. For example, in spider venoms the disulphide bridges form “cysteine knots”, where three bridges stabilize antiparallel beta sheets, drastically increasing the stability of the protein [13]. The components of venoms are specific, stable, potent and have the ability to modify molecular targets, thus making good therapeutic candidates. Animal venoms have been used as a traditional medicine to treat a variety of conditions, including arthritis, rheumatism and chronic pain as well as autoimmune, cardiovascular and skin diseases [3][6][14][15][16][17]. Moreover, one of the most promising fields in venom research from the therapeutic aspect is their use in anticancer research. This is driven by the resistance to chemotherapeutics by cancerous cells that is making cancer treatment more complicated, hence, animal venoms have emerged as an alternative strategy for anticancer therapeutics and could also impact the costs related to cancer treatment [15][18][19][20][21][22][23]. The anticancer activities of animal venoms include the inhibition of the proliferation of cancer cells, their invasion, cell cycle arrest, induction of apoptosis or necrosis and the identification of the involved signalling pathways [14][15][24].
Although there are numerous animal venoms that often show good results towards cancerous cells, there are always open questions regarding their potential toxicity towards normal non-target cells and tissues, making this kind of toxicity one of the greatest obstacles for the possibility of an actual remedy [4][14][25][26][27]. Therefore, the possible genotoxic effects of chemical compounds used as medical remedies are intensively studied. There is a vast body of literature concerning both natural and synthetic compounds [28][29]. Much less attention is paid to the genotoxic effects of animal venoms and other compounds of animal origin, although animal venoms per se are in the focus of interest of numerous researchers [4]. However, genotoxic effects can be produced by these compounds due to envenomation or as a side effect of medical remedies.
2. Coelenterata
Venomous jellyfish produce numerous problems for both tourists and fishermen in coastal areas where jellyfish are abundant. The possible genotoxic action of the venom extracted from the Mediterranean jellyfish Pelagia noctiluca nematocysts was studied in green monkey kidney (Vero) and human colon cancer (HCT 116) cells at concentrations of 80, 160, 320 and 640 μg/mL. An alkaline comet assay indicated dose-dependent DNA damage. DNA breakage was observed in both cell cultures. It is presumed that the breaks arose due to the oxidative stress triggered by the venom, whereas later DNA lesions favoured the development of apoptosis [30][31]. When non-fractionated nematocysts were incubated with green monkey Vero cells, dose-dependent DNA damage was also observed with a maximum effect produced by 150,000 nematocysts per mL [30].
3. Sponges (Poryphera)
Sponges possess a very potent secondary metabolism, producing numerous alkaloids, terpenoids and other biologically active compounds. These products compensate for the lack of an immune system in this taxon. Chemical compounds isolated from sponges are tested for various pharmacological effects [32][33]. Although mostly marine sponges are in the focus of interest, freshwater animals also synthesize numerous active compounds. The acetone extract of the freshwater sponge Ochridaspongia rotunda manifested cytotoxic and antiradical activities; however, the extract did not induce DNA breaks [34]. Similarly, the extract of the marine sponge Agelas oroides produced cytotoxic effects and induced reactive oxygen species (ROS) formation and apoptosis but did not induce DNA breaks [35]. On the contrary, aqueous and alcoholic–aqueous extracts from Aplysina fulva, a Brazilian marine sponge, produced DNA breakage in Balb/c 3T3 mouse fibroblasts quantified by the alkaline comet assay compared with untreated cells, whereas the extent of the breakage decreased with the dilution of the extracts (1:2; 1:5; 1:10) [36].
The genotoxic effects of individual compounds isolated from sponges also differ from the genotoxicity point of view. The terpenoid avarol from Dysidea avara induced DNA breaks in a human cancer (HT29) cell line and Friend leukaemia cells assayed by alkaline single-cell electrophoresis [37][38]. The DNA damaging effect was due to the formation of hydroxyl radicals generated in the quinone/hydroquinone (avarol/avarone) cycle, as the compound does not bind DNA covalently [37]. However, avarol appears to bind DNA non-covalently, both via intercalation and minor groove binding with subsequent DNA damage [39].
Another sponge terpenoid, 10-acetylirciformonin B (Figure 1), produced double-strand breaks detected both by the neutral comet assay and histone γ-H2AX expression in human leukaemia (HL 60) cells; the DNA breakage was followed by apoptosis. In cells treated with the compound at concentrations of 1.25 and 2.5 μg/mL, the mean tail moment in the neutral comet assay increased in a dose-dependent manner compared with the untreated control, and the intensity of the γ-H2AX band followed the same trend [40]. Ingenamine G (Figure 1), an alkaloid isolated from the Brazilian marine sponge Pachychalina alcaloidifera (Haplosclerida), induced DNA breaks in human lymphocytes. The DNA damage was evaluated by alkaline comet assay at concentrations of 5, 10, 15 and 20 μg/mL and compared with the untreated control. It was suggested that the compound affects the construction of a mitotic fuse [41]. According to the results of synchrotron beam diffraction on the crystal variolin B (Figure 1), an alkaloid with anticancer activity from the Antarctic sponge Kirckpatrickia variolosa binds DNA via the intercalative mode [42]. Acetylated bile acids from Siphonochalina fortis did not induce DNA breaks in human lymphocytes [43].
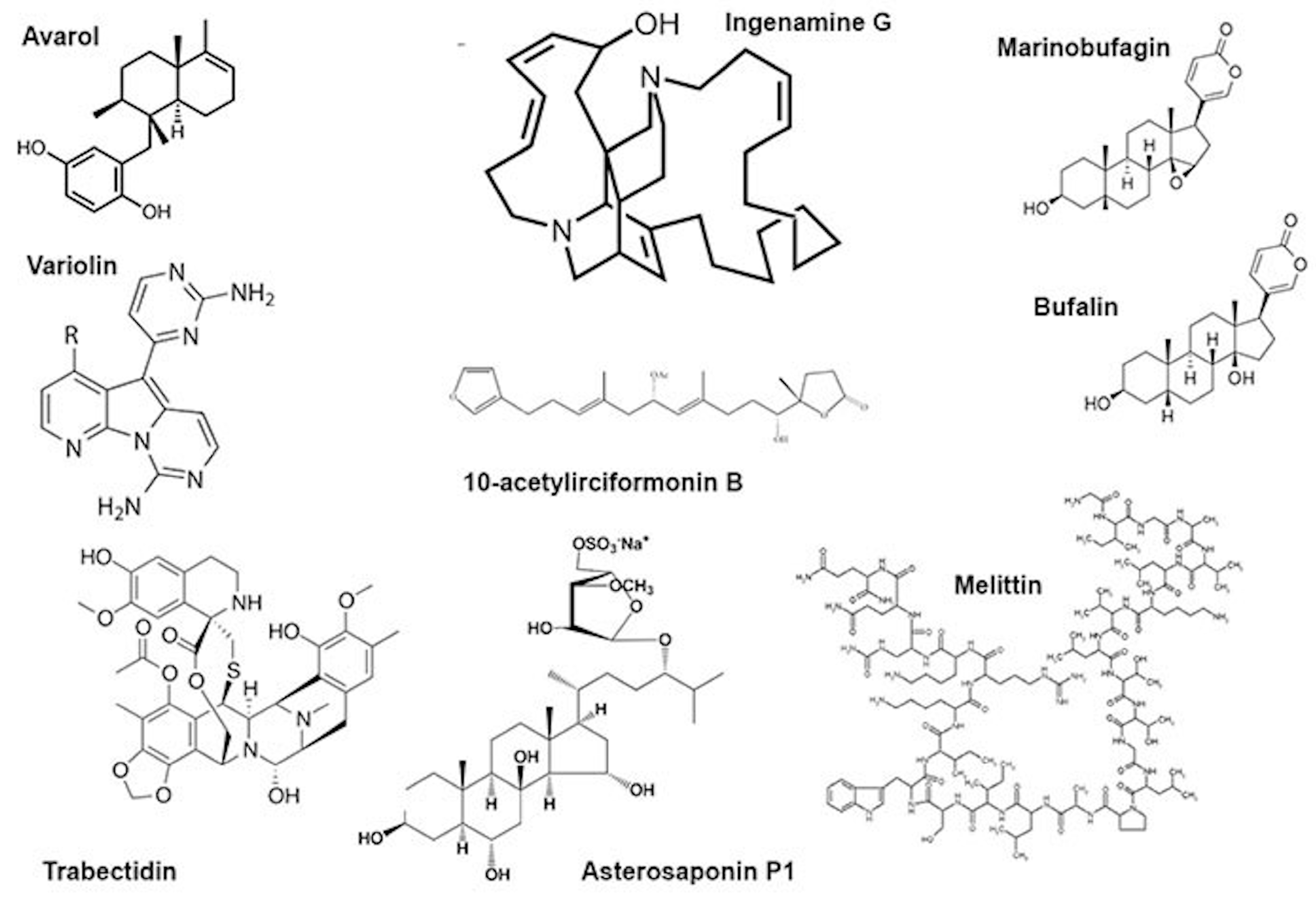
Figure 1. Some low-molecular substances from animal venoms.
4. Annelida
Genotoxic effects were produced by tissue extracts of marine polychaetes. DNA breaks were produced in mussel gills by a skin extract from Hediste diversicolor and Glycera alba jaw proboscis. It was suggested that H. diversicolor secretes toxins via the skin for protection against predators, but G. alba secretes toxins for predation [44].
5. Mollusca
The venom of very dangerous snails of the Conus genus is composed of hundreds of peptides called conotoxins. Some are used as analgesics, whereas other pharmacological applications are sought [45]. Data on the possible genotoxic action of these species are scarce; however, it was shown that the NMDA receptor agonist ω-conotoxin did not produce DNA damage in the spinal cords and blood cells of Wistar rats. The alkaline comet assay demonstrated the protective effect of the CGX-1007, a recombinant analogue of ω-conotoxin, against DNA damage produced by staurospirine in primary cultures of forebrain neurons [46].
6. Arachnida
This taxon is represented by numerous venomous animals, scorpions and spiders. Venoms of Arachnida are complex mixtures of proteins and low-molecular organic compounds and are mainly neurotoxic [9][47].
6.1. Scorpions
The venom of a Brazilian scorpion Tityus stigmurus produces DNA damage in the blood and testicular cells of Swiss mice, probably by triggering oxidative stress [48], consequently resulting in reproductive disorders in animals and humans. The animals received a single dose of the venom’s 1/2 LD50 (0.387 mg/kg), the effects were monitored at different time points up to 48 h, six animals per point. Alkaline comet assays indicated accumulation of DNA breaks, peaking at 2 h for both organs [48]. Similarly, the bolus injection of the venom of the most dangerous Brazilian yellow scorpion (Tityus serrulatus) at a dose corresponding to 1/2 LD50 (0.90 mg/kg) produced DNA damage in several organs (hippocampus, cortex, striatum, blood, heart, lung, liver and kidney) of Swiss mice. DNA damage was monitored by the alkaline comet assay, the effect appeared one hour after injection and remained at the same level for 12 h, 8–10 animals were taken for a time point [49]. The venom of the Indian black scorpion (Heterometrus bengalensis Koch) produced DNA damage in human leukemic cell lines (U937 and K562) leading to apoptosis. Cells were treated with the venom at an IC50 concentration (41.5 μg/mL for U937 and 88.3 μg/mL for K562) and the alkaline comet assay was performed 48 h later. Compared with the control cells, the comet tail length increased in the treated U937 cells by 26.4% and to 80.7% in K562 cells [50].
6.2. Araneae
Despite a great number of venomous spider species, data about the genotoxic effects of these venoms are scarce. There is a report on the genotoxic effects of sphingomyelinase D isolated from venoms of the Loxosceles genus spiders on human (HaCaT) keratinocytes. The enzyme binds the plasmatic membrane of the cells and triggers an increase in the intracellular superoxide level, the radical that produces DNA damage [51]. The venom of the Phoneutria nigriventer spider manifests analgesic effects. It was shown that the Phα1β peptide isolated from this venom induced DNA damage in the spinal cord cells of Wistar rats but not in white blood cells. The Wistar rats were administered native Phα1β toxin intrathecally at 500 pmol/site and its recombinant analogue at 200, 500 and 1000 pmol/site. Positive control animals received hydrogen peroxide, and negative control animals received phosphate-buffered saline; there were five animals per group. The increased micronucleus frequency in bone marrow cells suggested mutagenic effects [52].
7. Insecta
Hymenoptera venoms consist of a complex mixture of chemically or pharmacologically bioactive components including phospholipases, hyaluronidases and mastoparans [53]. Mastoparans, a group of alpha-helical peptides, appear to be the most promising for pharmacological activities [54] since they are able to penetrate into cells and bind DNA [55]. Melectin, an antimicrobial peptide from the venom of the cleptoparasitic bee Melecta albifrons, does not exhibit sequence homology with other wasp venom peptides. Melectin manifests antitumour activity, penetrates membranes and binds DNA [56]. The venom of a parasitoid wasp, Pteromalus puparum, contains a peptide with endonuclease activity (PpENVP), which inhibits gene expression in transfected cells relying on two activation sites [57].
It is believed that Hymenoptera contain substances able to decrease the genotoxic or mutagenic action of other compounds. A protective effect of the Egyptian honeybee (Apis mellifera lamarckii) venom against the damaging action of propionic acid (250 mg/kg 3 days) was observed in the neurons of Sprague–Dawley rat pups. DNA damage was assayed by single-cell electrophoresis in alkaline conditions, 0.5 mg/kg of the venom was administered for 4 weeks, and there were 10 pups in a group. Both DNA damage and markers of oxidative stress were decreased [58]. Moreover, the radioprotective effects of honeybee venom (Apis mellifera) against 915 MHz microwave-radiation-induced DNA damage in Wistar rat lymphocytes was observed in vitro [59]. On the other hand, the A. mellifera venom even at low concentrations (0.1, 0.05 and 0.01μg/mL) did not protect HepG2 cells against the genotoxic action of methyl methanesulfonate [60]. In a similar study conducted with venom from the wasp Polybia paulista, it was shown that higher concentrations of the venom (10, 5 and 1 μg/mL) were genotoxic per se, whereas lower concentrations (1 ng/mL, 100 and 10 pg/mL) were not genotoxic; neither displayed a genoprotective effect. The genotoxic and mutagenic activity of the venom of P. paulista could have been caused by phospholipase, mastoparan and hyaluronidase, as these enzymes disrupt the cell membrane and thereby interact with the genetic material of the cells or even facilitate the entrance of other compounds of the venom that can act on the DNA. Furthermore, venom substances are able to trigger inflammatory process and generate ROS that can interact with the DNA [61].
Bee venom (A. mellifera) and its mayor constituent melittin (Figure 1) are cytotoxic towards human peripheral blood cells and are able to induce morphological changes in the cell membrane, granulation and lysis of the cells. Moreover, they showed increased DNA damage including oxidative DNA damage as well as increased formation of other markers of genomic instability. This genotoxicity coincides with the increased formation of ROS, reduction of glutathione and increased lipid peroxidation as well as phospholipase C activity, indicating the induction of oxidative stress. Melittin itself is also capable of modulating the gene expression patterns of genes involved in the DNA damage response, oxidative stress and apoptosis [25][26][27][62][63][64].
References
- McDermott, A. News Feature: Venom Back in Vogue as a Wellspring for Drug Candidates. Proc. Natl. Acad. Sci. USA 2020, 117, 10100–10104.
- Holford, M.; Daly, M.; King, G.F.; Norton, R.S. Venoms to the Rescue. Science 2018, 361, 842–844.
- King, G.F. Venoms as a Platform for Human Drugs: Translating Toxins into Therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484.
- von Reumont, B.M.; Anderluh, G.; Antunes, A.; Ayvazyan, N.; Beis, D.; Caliskan, F.; Crnković, A.; Damm, M.; Dutertre, S.; Ellgaard, L.; et al. Modern Venomics-Current Insights, Novel Methods, and Future Perspectives in Biological and Applied Animal Venom Research. Gigascience 2022, 11, giac048.
- Abd El-Aziz, T.M.; Garcia Soares, A.; Stockand, J.D. Snake Venoms in Drug Discovery: Valuable Therapeutic Tools for Life Saving. Toxins 2019, 11, 564.
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic Application of Anti-Arthritis, Pain-Releasing, and Anti-Cancer Effects of Bee Venom and Its Constituent Compounds. Pharmacol. Ther. 2007, 115, 246–270.
- Kalita, B.; Saviola, A.J.; Mukherjee, A.K. From Venom to Drugs: A Review and Critical Analysis of Indian Snake Venom Toxins Envisaged as Anticancer Drug Prototypes. Drug Discov. Today 2021, 26, 993–1005.
- Qi, J.; Zulfiker, A.H.M.; Li, C.; Good, D.; Wei, M.Q. The Development of Toad Toxins as Potential Therapeutic Agents. Toxins 2018, 10, 336.
- Saez, N.J.; Senff, S.; Jensen, J.E.; Er, S.Y.; Herzig, V.; Rash, L.D.; King, G.F. Spider-Venom Peptides as Therapeutics. Toxins 2010, 2, 2851–2871.
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325.
- Harvey, A. From Demons to Darlings: Drugs from Venoms. Drug Discov. Today 1998, 3, 531–532.
- Gajski, G.; Čimbora-Zovko, T.; Osmak, M.; Garaj-Vrhovac, V. Bee Venom and Melittin Are Cytotoxic against Different Types of Tumor and Non-Tumor Cell Lines In Vitro. In Advancements in Cancer Research; Viktorsson, K., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 163–178. ISBN 978-1-61470-252-8.
- Herzig, V.; King, G.F. The Cystine Knot Is Responsible for the Exceptional Stability of the Insecticidal Spider Toxin ω-Hexatoxin-Hv1a. Toxins 2015, 7, 4366–4380.
- Gajski, G.; Garaj-Vrhovac, V. Melittin: A Lytic Peptide with Anticancer Properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705.
- Chatterjee, B. Animal Venoms Have Potential to Treat Cancer. Curr. Top. Med. Chem. 2018, 18, 2555–2566.
- Lewis, R.J.; Garcia, M.L. Therapeutic Potential of Venom Peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802.
- Greener, M. The next Generation of Venom-based Drugs. Prescriber 2020, 31, 28–32.
- Gajski, G.; Čimbora-Zovko, T.; Rak, S.; Rožman, M.; Osmak, M.; Garaj-Vrhovac, V. Combined Antitumor Effects of Bee Venom and Cisplatin on Human Cervical and Laryngeal Carcinoma Cells and Their Drug Resistant Sublines. J. Appl. Toxicol. 2014, 34, 1332–1341.
- Gajski, G.; Čimbora-Zovko, T.; Rak, S.; Osmak, M.; Garaj-Vrhovac, V. Antitumour Action on Human Glioblastoma A1235 Cells through Cooperation of Bee Venom and Cisplatin. Cytotechnology 2016, 68, 1197–1205.
- Roy, A.; Bharadvaja, N. Venom-Derived Bioactive Compounds as Potential Anticancer Agents: A Review. Int. J. Pept. Res. Ther. 2021, 27, 129–147.
- Chaisakul, J.; Hodgson, W.C.; Kuruppu, S.; Prasongsook, N. Effects of Animal Venoms and Toxins on Hallmarks of Cancer. J. Cancer 2016, 7, 1571–1578.
- Viegas, S.; Ladeira, C.; Costa-Veiga, A.; Perelman, J.; Gajski, G. Forgotten Public Health Impacts of Cancer—An Overview. Arch. Ind. Hyg. Toxicol. 2017, 68, 287–297.
- Norouzi, P.; Mirmohammadi, M.; Houshdar Tehrani, M.H. Anticancer Peptides Mechanisms, Simple and Complex. Chem. Biol. Interact. 2022, 368, 110194.
- Oršolić, N. Bee Venom in Cancer Therapy. Cancer Metastasis Rev. 2012, 31, 173–194.
- Gajski, G.; Domijan, A.-M.; Žegura, B.; Štern, A.; Gerić, M.; Novak Jovanović, I.; Vrhovac, I.; Madunić, J.; Breljak, D.; Filipič, M.; et al. Melittin Induced Cytogenetic Damage, Oxidative Stress and Changes in Gene Expression in Human Peripheral Blood Lymphocytes. Toxicon 2016, 110, 56–67.
- Gajski, G.; Garaj-Vrhovac, V. Bee Venom Induced Cytogenetic Damage and Decreased Cell Viability in Human White Blood Cells after Treatment in Vitro: A Multi-Biomarker Approach. Environ. Toxicol. Pharmacol. 2011, 32, 201–211.
- Garaj-Vrhovac, V.; Gajski, G. Evaluation of the Cytogenetic Status of Human Lymphocytes after Exposure to a High Concentration of Bee Venom in Vitro. Arch. Hig. Rada Toksikol. 2009, 60, 27–34.
- Sjakste, N.; Djelić, N.; Dzintare, M.; Živković, L. DNA-BINDING and DNA-Protecting Activities of Small Natural Organic Molecules and Food Extracts. Chem. Biol. Interact. 2020, 323, 109030.
- Muhamedejevs, R.; Živković, L.; Dzintare, M.; Sjakste, N. DNA-Binding Activities of Compounds Acting as Enzyme Inhibitors, Ion Channel Blockers and Receptor Binders. Chem. Biol. Interact. 2021, 348, 109638.
- Ayed, Y.; Boussabbeh, M.; Zakhama, W.; Bouaziz, C.; Abid, S.; Bacha, H. Induction of Cytotoxicity of Pelagia Noctiluca Venom Causes Reactive Oxygen Species Generation, Lipid Peroxydation Induction and DNA Damage in Human Colon Cancer Cells. Lipids Health Dis. 2011, 10, 232.
- Ayed, Y.; Bouaziz, C.; Brahmi, D.; Zaid, C.; Abid, S.; Bacha, H. Cell Death in Relation to DNA Damage after Exposure to the Jellyfish Pelagia Noctiluca Nematocysts. Environ. Toxicol. 2014, 29, 337–344.
- Anjum, K.; Abbas, S.Q.; Shah, S.A.A.; Akhter, N.; Batool, S.; Hassan, S.S.U. Marine Sponges as a Drug Treasure. Biomol. Ther. 2016, 24, 347–362.
- Varijakzhan, D.; Loh, J.-Y.; Yap, W.-S.; Yusoff, K.; Seboussi, R.; Lim, S.-H.E.; Lai, K.-S.; Chong, C.-M. Bioactive Compounds from Marine Sponges: Fundamentals and Applications. Mar. Drugs 2021, 19, 246.
- Talevska, A.; Pejin, B.; Kojic, V.; Beric, T.; Stankovic, S. A Contribution to Pharmaceutical Biology of Freshwater Sponges. Nat. Prod. Res. 2018, 32, 568–571.
- Ferretti, C.; Marengo, B.; De Ciucis, C.; Nitti, M.; Pronzato, M.; Marinari, U.; Pronzato, R.; Manconi, R.; Domenicotti, C. Effects of Agelas Oroides and Petrosia Ficiformis Crude Extracts on Human Neuroblastoma Cell Survival. Int. J. Oncol. 2007, 30, 161–169.
- Aiub, C.; Giannerini, A.; Ferreira, F.; Mazzei, J.; Stankevicins, L.; Lobo-Hajdu, G.; Guimarães, P.; Hajdu, E.; Felzenszwalb, I. Genotoxic Evaluation of Extracts from Aplysina Fulva, a Brazilian Marine Sponge. Mutat. Res. 2006, 611, 34–41.
- Sladic, D.; Gasic, M. Reactivity and Biological Activity of the Marine Sesquiterpene Hydroquinone Avarol and Related Compounds from Sponges of the Order Dictyoceratida. Molecules 2006, 11, 1–33.
- Pejin, B.; Iodice, C.; Kojic, V.; Jakimov, D.; Lazovic, M.; Tommonaro, G. In Vitro Evaluation of Cytotoxic and Mutagenic Activity of Avarol. Nat. Prod. Res. 2016, 30, 1293–1296.
- Vujčić, M.T.; Tufegdžić, S.; Novaković, I.; Djikanović, D.; Gašić, M.J.; Sladić, D. Studies on the Interactions of Bioactive Quinone Avarone and Its Methylamino Derivatives with Calf Thymus DNA. Int. J. Biol. Macromol. 2013, 62, 405–410.
- Su, J.-H.; Chang, W.-B.; Chen, H.-M.; El-Shazly, M.; Du, Y.-C.; Kung, T.-H.; Chen, Y.-C.; Sung, P.-J.; Ho, Y.-S.; Kuo, F.-W.; et al. 10-Acetylirciformonin B, A Sponge Furanoterpenoid, Induces DNA Damage and Apoptosis in Leukemia Cells. Molecules 2012, 17, 11839–11848.
- Cavalcanti, B.C.; Sombra, C.M.L.; de Oliveira, J.H.H.L.; de Berlinck, R.G.S.; de Moraes, M.O.; Pessoa, C. Cytotoxicity and Genotoxicity of Ingenamine G Isolated from the Brazilian Marine Sponge Pachychalina Alcaloidifera. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2008, 147, 409–415.
- Canals, A.; Arribas-Bosacoma, R.; Albericio, F.; Álvarez, M.; Aymamí, J.; Coll, M. Intercalative DNA Binding of the Marine Anticancer Drug Variolin B. Sci. Rep. 2017, 7, 39680.
- Patiño Cano, L.P.; Bartolotta, S.A.; Casanova, N.A.; Siless, G.E.; Portmann, E.; Schejter, L.; Palermo, J.A.; Carballo, M.A. Isolation of Acetylated Bile Acids from the Sponge Siphonochalina Fortis and DNA Damage Evaluation by the Comet Assay. Steroids 2013, 78, 982–986.
- D’Ambrosio, M.; Ramos, Í.; Martins, C.; Costa, P.M. An Investigation into the Toxicity of Tissue Extracts from Two Distinct Marine Polychaeta. Toxicon X 2022, 14, 100116.
- Jin, A.-H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.W.A.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Conotoxins: Chemistry and Biology. Chem. Rev. 2019, 119, 11510–11549.
- Dave, J.R.; Williams, A.J.; Moffett, J.R.; Koenig, M.L.; Tortella, F.C. Studies on Neuronal Apoptosis in Primary Forebrain Cultures: Neuroprotective/Anti-Apoptotic Action of NR2B NMDA Antagonists. Neurotox. Res. 2003, 5, 255–264.
- Escoubas, P.; Diochot, S.; Corzo, G. Structure and Pharmacology of Spider Venom Neurotoxins. Biochimie 2000, 82, 893–907.
- Silva, M.A.; Souza, T.G.; Melo, M.E.G.; Silva, J.M.; Lima, J.R.; Lira, A.F.A.; de Aguiar-Júnior, F.C.A.; Martins, R.D.; Jorge, R.J.B.; Chagas, C.A.; et al. Tityus Stigmurus Venom Causes Genetic Damage in Blood and Testicular Cells and Affects the Number and Morphology of Gametogenic Lineage Cells in Mice. Toxicon 2020, 185, 114–119.
- Galvani, N.C.; Vilela, T.C.; Domingos, A.C.; Fagundes, M.Í.; Bosa, L.M.; Della Vechia, I.C.; Scussel, R.; Pereira, M.; Steiner, B.T.; Damiani, A.P.; et al. Genotoxicity Evaluation Induced by Tityus Serrulatus Scorpion Venom in Mice. Toxicon 2017, 140, 132–138.
- Das Gupta, S.; Debnath, A.; Saha, A.; Giri, B.; Tripathi, G.; Vedasiromoni, J.R.; Gomes, A.; Gomes, A. Indian Black Scorpion (Heterometrus Bengalensis Koch) Venom Induced Antiproliferative and Apoptogenic Activity against Human Leukemic Cell Lines U937 and K562. Leuk. Res. 2007, 31, 817–825.
- da Silva, M.S.; Lopes, P.H.; Elias, M.C.; Tambourgi, D. V Cytotoxic and Genotoxic Effects on Human Keratinocytes Triggered by Sphingomyelinase D from Loxosceles Venom. Arch. Toxicol. 2020, 94, 3563–3577.
- de Souza, A.H.; da Rosa, L.G.; Uliano, M.R.; da Silva Prado, L.; Ferraz, A.G.; Conter, L.U.; Grivicich, I.; Dallegrave, E.; Gomez, M.V.; Picada, J.N. Evaluation of DNA Damage in Spinal Cord and Mutagenic Effect of a Phα1β Recombinant Toxin with Analgesic Properties from the Phoneutria Nigriventer Spider. Basic Clin. Pharmacol. Toxicol. 2019, 124, 615–620.
- Luo, L.; Kamau, P.M.; Lai, R. Bioactive Peptides and Proteins from Wasp Venoms. Biomolecules 2022, 12, 527.
- de Santana, C.J.C.; Pires Júnior, O.R.; Fontes, W.; Palma, M.S.; Castro, M.S. Mastoparans: A Group of Multifunctional α-Helical Peptides With Promising Therapeutic Properties. Front. Mol. Biosci. 2022, 9, 331.
- Niidome, T.; Urakawa, M.; Takaji, K.; Matsuo, Y.; Ohmori, N.; Wada, A.; Hirayama, T.; Aoyagi, H. Influence of Lipophilic Groups in Cationic Alpha-Helical Peptides on Their Abilities to Bind with DNA and Deliver Genes into Cells. J. Pept. Res. 1999, 54, 361–367.
- Liang, X.; Yan, J.; Lu, Y.; Liu, S.; Chai, X. The Antimicrobial Peptide Melectin Shows Both Antimicrobial and Antitumor Activity via Membrane Interference and DNA Binding. Drug Des. Devel. Ther. 2021, 15, 1261–1273.
- Wang, J.; Yan, Z.; Xiao, S.; Wang, B.; Fang, Q.; Schlenke, T.; Ye, G. Characterization of a Cell Death-Inducing Endonuclease-like Venom Protein from the Parasitoid Wasp Pteromalus Puparum (Hymenoptera: Pteromalidae). Pest Manag. Sci. 2021, 77, 224–233.
- Khalil, S.R.; Abd-Elhakim, Y.M.; Selim, M.E.; Al-Ayadhi, L.Y. Apitoxin Protects Rat Pups Brain from Propionic Acid-Induced Oxidative Stress: The Expression Pattern of Bcl-2 and Caspase-3 Apoptotic Genes. Neurotoxicology 2015, 49, 121–131.
- Gajski, G.; Garaj-Vrhovac, V. Radioprotective Effects of Honeybee Venom (Apis Mellifera) Against 915-MHz Microwave Radiation-Induced DNA Damage in Wistar Rat Lymphocytes: In Vitro Study. Int. J. Toxicol. 2009, 28, 88–98.
- Hoshina, M.M.; Marin-Morales, M.A. Anti-Genotoxicity and Anti-Mutagenicity of Apis Mellifera Venom. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 762, 43–48.
- Hoshina, M.M.; Santos, L.D.; Palma, M.S.; Marin-Morales, M.A. Cytotoxic, Genotoxic/Antigenotoxic and Mutagenic/Antimutagenic Effects of the Venom of the Wasp Polybia Paulista. Toxicon 2013, 72, 64–70.
- Gajski, G.; Domijan, A.-M.; Garaj-Vrhovac, V. Alterations of GSH and MDA Levels and Their Association with Bee Venom-Induced DNA Damage in Human Peripheral Blood Leukocytes. Environ. Mol. Mutagen. 2012, 53, 469–477.
- Gajski, G.; Garaj-Vrhovac, V. Genotoxic Potential of Bee Venom (Apis Mellifera) on Human Peripheral Blood Lymphocytes in Vitro Using Single Cell Gel Electrophoresis Assay. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2008, 43, 1279–1287.
- Gajski, G.; Garaj-Vrhovac, V. Increased Frequency of Sister Chromatid Exchanges and Decrease in Cell Viability and Proliferation Kinetics in Human Peripheral Blood Lymphocytes after In Vitro Exposure to Whole Bee Venom. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2010, 45, 1654–1659.
More
Information
Subjects:
Toxicology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
562
Revisions:
2 times
(View History)
Update Date:
30 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




