Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xian Zhang | -- | 1952 | 2023-11-28 11:22:09 | | | |
| 2 | Rita Xu | + 647 word(s) | 2599 | 2023-11-29 02:35:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Miao, P.; Wang, Q.; Ren, K.; Zhang, Z.; Xu, T.; Xu, M.; Zhang, X.; Rao, Z. d-Tagatose by l-Arabinose Isomerase. Encyclopedia. Available online: https://encyclopedia.pub/entry/52135 (accessed on 07 February 2026).
Miao P, Wang Q, Ren K, Zhang Z, Xu T, Xu M, et al. d-Tagatose by l-Arabinose Isomerase. Encyclopedia. Available at: https://encyclopedia.pub/entry/52135. Accessed February 07, 2026.
Miao, Peiyu, Qiang Wang, Kexin Ren, Zigang Zhang, Tongtong Xu, Meijuan Xu, Xian Zhang, Zhiming Rao. "d-Tagatose by l-Arabinose Isomerase" Encyclopedia, https://encyclopedia.pub/entry/52135 (accessed February 07, 2026).
Miao, P., Wang, Q., Ren, K., Zhang, Z., Xu, T., Xu, M., Zhang, X., & Rao, Z. (2023, November 28). d-Tagatose by l-Arabinose Isomerase. In Encyclopedia. https://encyclopedia.pub/entry/52135
Miao, Peiyu, et al. "d-Tagatose by l-Arabinose Isomerase." Encyclopedia. Web. 28 November, 2023.
Copy Citation
d-tagatose is a low-calorie alternative to sucrose natural monosaccharide that is nearly as sweet. As a ketohexose, d-tagatose has disease-relieving and health-promoting properties. Due to its scarcity in nature, d-tagatose is mainly produced through chemical and biological methods.
d-tagatose
production
l-arabinose isomerase
1. Introduction
In recent years, traditional high-absorption and high-calorie sugars have gradually been replaced by low-calorie and low-absorption rare sugars. As a representative of such rare sugars, d-tagatose is a naturally occurring monosaccharide with 92% of the sweetness of sucrose but only one-third of the calories.
d-tagatose, with the molecular formula C6H12O6, is the ketose form of d-galactose and the epimer of d-fructose at the C-4 position (Figure 1). d-tagatose is very rare in nature and small amounts of d-tagatose are found to be naturally present in cheese, yogurt, hot cocoa, sterilized powder milk, and other dairy-derived products [1].
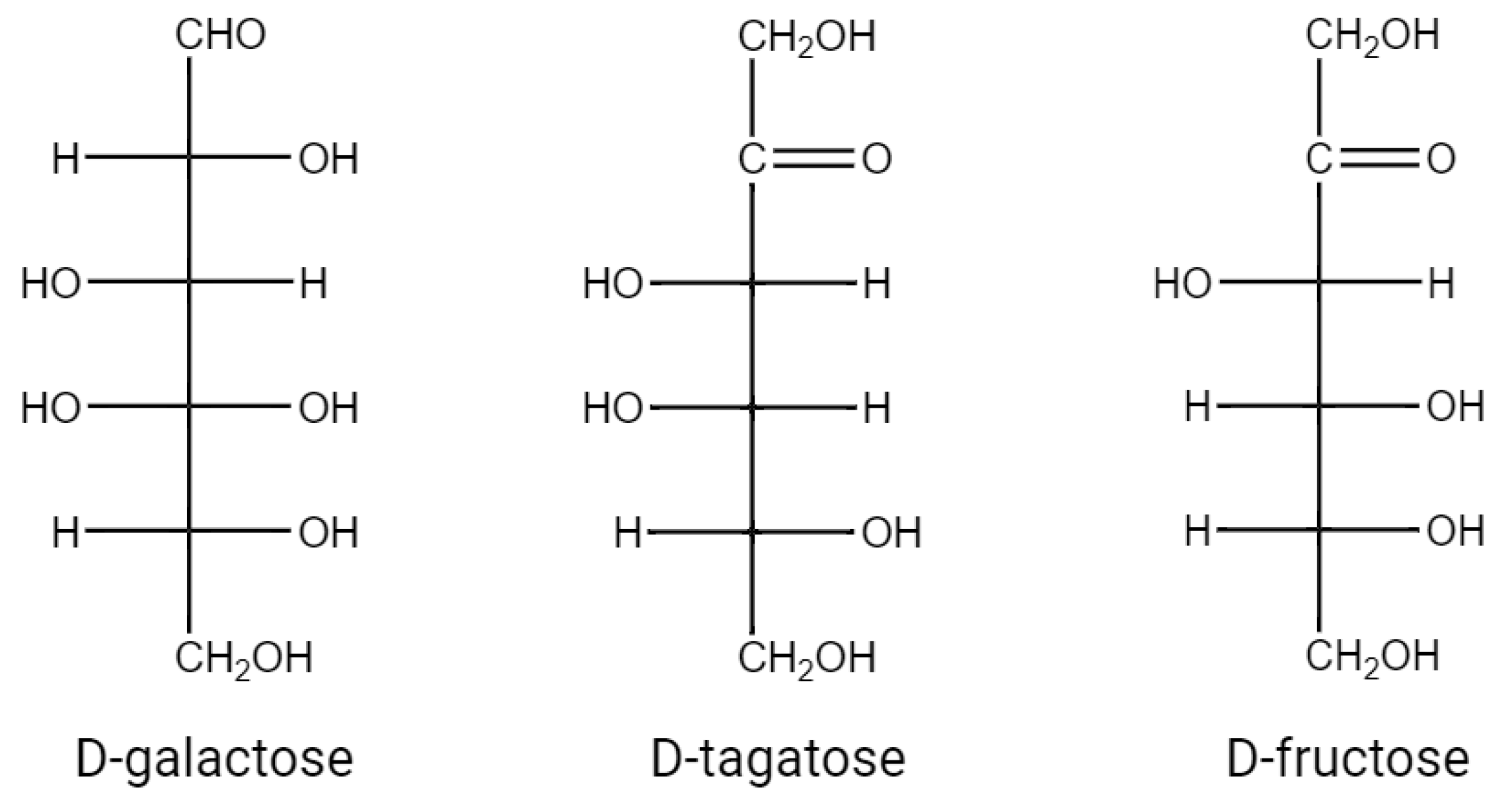
Figure 1. The structural formula of d-galactose, d-tagatose, d-fructose.
The functions of d-tagatose have been extensively evaluated for disease amelioration (obesity control, antidiabetes, and regulation of blood metabolites) and health promotion (anti-aging, antioxidant, and prebiotic properties) (Figure 2) [2]. Diabetic patients have been shown to experience gradual weight loss through long-term treatment with d-tagatose [3]. This is because d-tagatose does not lead to fat deposition. Research by Buemann et al. [4] showed that osmotic effects caused by unabsorbed d-tagatose lead to intestinal distension. This would potentially mediate an acute appetite suppressant effect and help to reduce energy intake; d-tagatose holds potential in blood sugar control with a glycemic index (GI) value of 3 [5]. When individuals consume d-tagatose before meals, approximately 20% of it is absorbed by the body, converting blood glucose into glycogen and slowing down the rate of decomposition of glycogen into glucose [6]. The reason for this may be due to its liver metabolism process, which is similar to that of d-fructose. d-tagatose is phosphorylated to tagatose-1-phosphate by fructokinase. Its slower rate of breakdown results in the accumulation of tagatose-1-phosphate. Tagatose-1-phosphate stimulates glucokinase and promotes the conversion of glucose to glucose-6-phosphate. Glucose-6-phosphate stimulates liver glycogen synthase to accelerate glycogen formation. The remaining 80% is not absorbed and may compete with or partially inhibit glucose transporters in the small intestine, thereby inhibiting and preventing glucose absorption [7][8]. d-tagatose helps improve blood health [9] by strengthening key blood factors, such as the red blood cell count, prothrombin time, and activated partial thromboplastin time, as well as increasing coagulation factors. Therefore, it is a candidate for the treatment of anemia and hemophilia. d-tagatose also has the potential to prevent oral diseases [10]. Mayumi et al. [11] found that it selectively inhibits the growth of oral pathogens by affecting their glycolysis and downstream metabolism. Hasibul et al. [12] discovered that d-tagatose prevents the cariogenic species Streptococcus mutans from growing and forming biofilms. Dietary restriction through d-tagatose maintains lower blood sugar and insulin levels in the body, thereby delaying age-related disease development and further extending the lifespan of those who practice it [13][14]; Additionally, d-tagatose is an excellent prebiotic. Research has shown [15] that unabsorbed d-tagatose will enter the large intestine, where it is selectively fermented by microbial flora. This fermentation process promotes the proliferation of beneficial flora while inhibiting the growth of harmful flora, thereby improving the gut microbiota. At the same time, the fermentation of d-tagatose yields large amounts of beneficial short-chain fatty acids such as butyric acid [16], which is a good source of energy for colon epithelial cells.
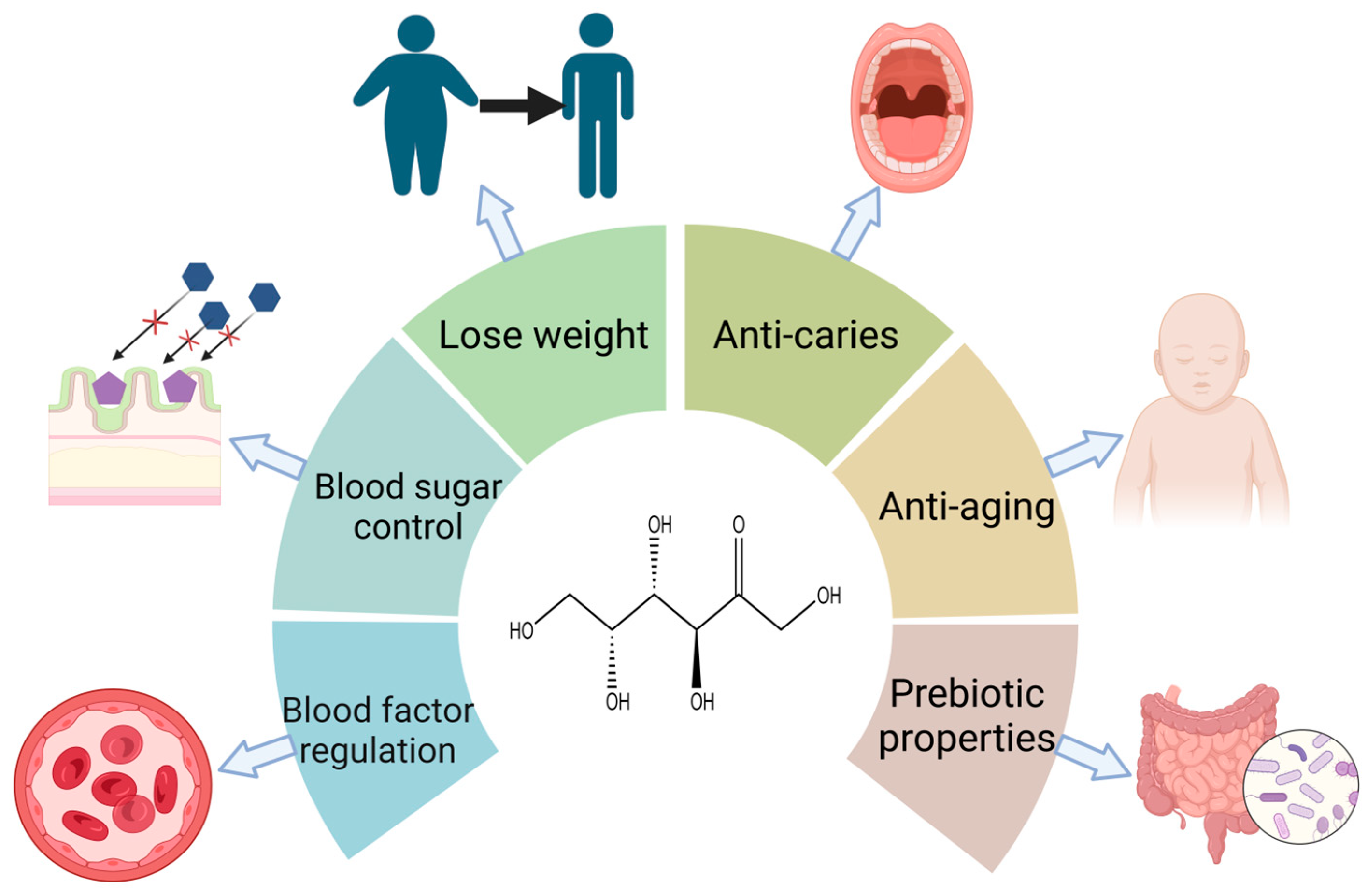
Figure 2. Various physiological functions of d-tagatose on the human body.
d-tagatose is an emerging low-calorie sweetener with potential to replace sucrose [17]. It can provide sweetness in a wide range of concentrations without adding an undesirable taste. The addition of d-tagatose to low-sugar products can provide them with desirable organoleptic properties, which means it can be beneficial to health without changing the flavor of the product [18][19]. Since d-tagatose has been approved as “generally recognized as safe” (GRAS), it can be used in confectionery, beverages, nutraceuticals, and dietary products. d-tagatose can also be used as an additive in prescription medications and as a sweetener in toothpaste, mouthwash, and cosmetics.
d-tagatose was discovered in the gum of Sterculia setigera as early as 1949 [20]. However, its scarcity makes it difficult to extract d-tagatose directly from nature on a large scale. In order to make d-tagatose production more economical and efficient, chemical and biological methods are usually used (Figure 3).
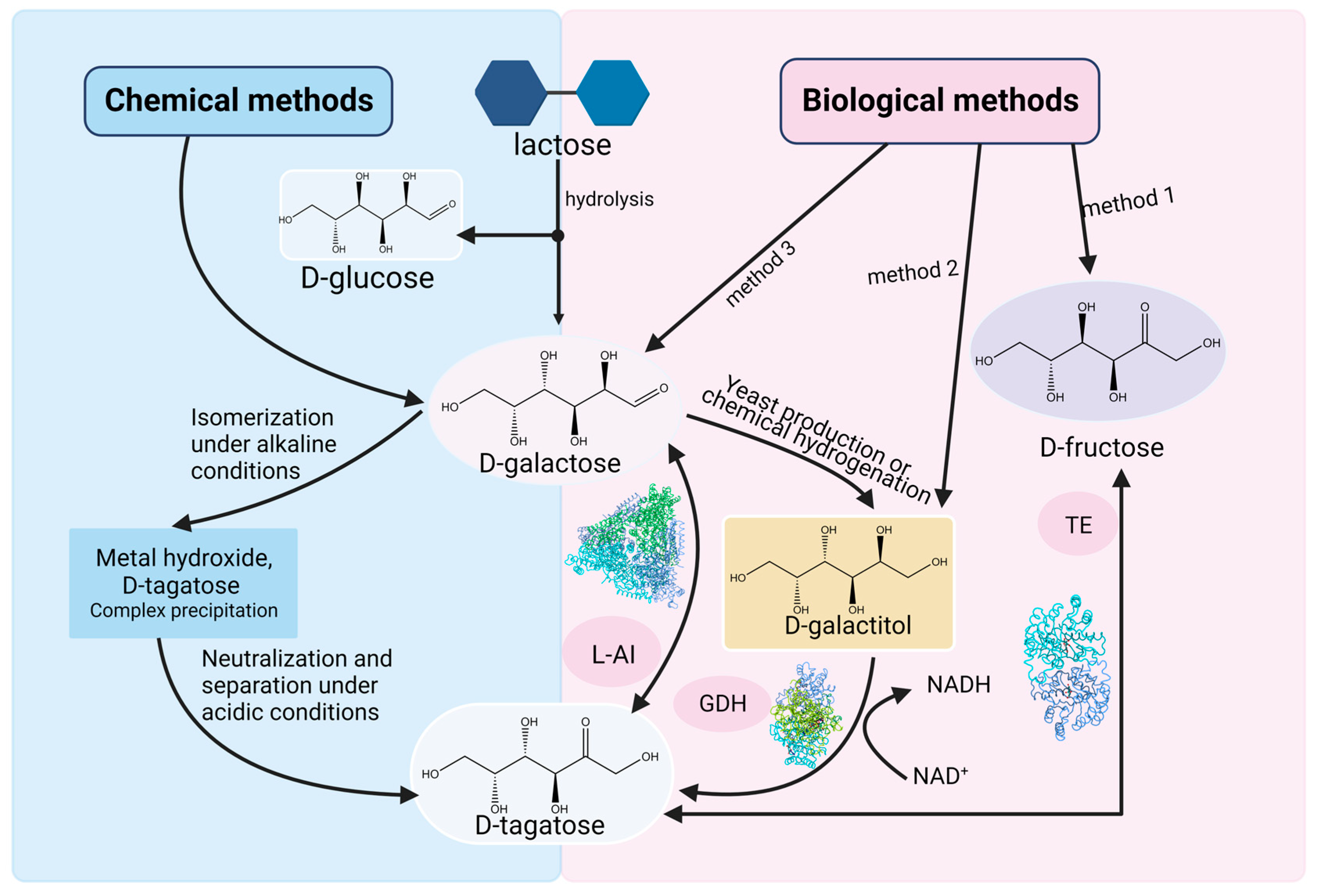
Figure 3. Current methods for producing d-tagatose via feasible chemical or biological routes. L-AI: l-arabinose isomerase, GDH: Galactitol dehydrogenase, TE: Tagatose 4-epimerase.
The typically used chemical method, also known as non-enzymatic isomerization [21], uses metal hydroxides as catalysts. This method consists of three steps: firstly, an insoluble d-tagatose complex is generated when d-galactose interacts with metal hydroxides, which are stable under alkaline conditions; in the second stage, the insoluble d-tagatose complex is neutralized with an acid to produce an insoluble salt; finally, filtering is used to separate d-tagatose from the insoluble salt. During the isomerization, the metal hydroxides perform two functions: isomerizing d-galactose to d-tagatose and degrading d-galactose into dicarbonyl compounds and acidic substances. This method has a high yield (>70%) but includes the following disadvantages: severe d-galactose degradation leads to a reduction in the yield of d-tagatose, while the decrease in the quality of the syrup makes the production of crystalline d-tagatose difficult; the removal of the degradation products requires complex extraction steps; and a large number of metal hydroxides and acids are consumed in the reaction, which ultimately has many negative impacts on the cost and the environment. There are also other methods such as supercritical fluid (<24%) [22], triethylamine (<34%), arginine (<16.8%) [23], hydrotalcite (<27%) [24], Sn-β zeolite (<26%), etc. However, all of these non-enzymatic pathways have low d-tagatose yields [25]. Moreover, these methods require reactions under extreme conditions, which can lead to increased energy consumption and damage to substrates and products.
Biological methods use whole cells and isolated enzymes as catalysts. The advantages of such methods include mild reaction conditions, environmental friendliness, few by-products, and easy purification. Thus, the production of d-tagatose by biological methods has advantages over chemical methods in many aspects. In biological methods, the lzumoring strategy is often used to produce various rare sugars. This strategy uses different types of enzymes including epimerase, oxidoreductase, and aldose–ketose isomerases to achieve interconversion between monosaccharides and sugar alcohols [26]. d-tagatose can also be produced by these three types of enzymes, including tagatose 4-epimerase, galactitol dehydrogenase, and l-arabinose isomerase.
Tagatose 4-epimerase can epimerize d-fructose into d-tagatose. However, there are few reports of enzymes with d-fructose epimeric activity at the C-4 position in nature. Therefore, obtaining such enzymes by screening new ones or modifying old ones is the main work in this production pathway. Shin et al. [27] developed a new tagatose 4-epimerase through rational design and directed the evolution of the tagaturonate 3-epimerase from Thermotoga petrophila. The modified enzyme exhibited 184-fold-higher epimeric activity towards d-fructose compared to the original enzyme. Under optimal conditions of 80 °C, pH 8.5, and 1.5 mM Ni2+, the enzyme was able to produce 213 g/L of d-tagatose from 700 g/L of d-fructose within 2 h, with a conversion rate of 30%. Jeon et al. [28] expressed tagatose 4-epimerase from Thermotoga neapolitana in Corynebacterium glutamicum and improved the enzyme expression level by optimizing plasmid copy numbers. Under conditions of 60 °C and a specific amount of metal ions, the conversion rate reached 21.7%.
2. The Production of d-Tagatose by L-AIs
2.1. Molecular Structure and Catalytic Mechanism of L-AIs
The crystal structures of L-AIs from E. coli (PDB code: 2AJT) [29], Lactobacillus fermentum CGMCC2921 (PDB code: 4LQL) [30], Geobacillus kaustophilus (PDB code: 4R1O), and Thermotoga maritima MSB8 (PDB code: 7CWV) have been determined. Taking the L-AI from E. coli (ECAI) as an example [31], it is a hexamer with a total molecular weight of 336 kDa. Three asymmetric units of L-AI subunits form a trimer, as shown in Figure 4a. Two such trimers stack together to form the complete hexamer. Each subunit contains three structural domains: the N-terminal domain, the central domain, and the C-terminal domain. Within the complete hexamer of ECAI, there are six active sites located at the monomer–monomer interfaces (Figure 4a) and situated in the conjugate regions of adjacent subunits.
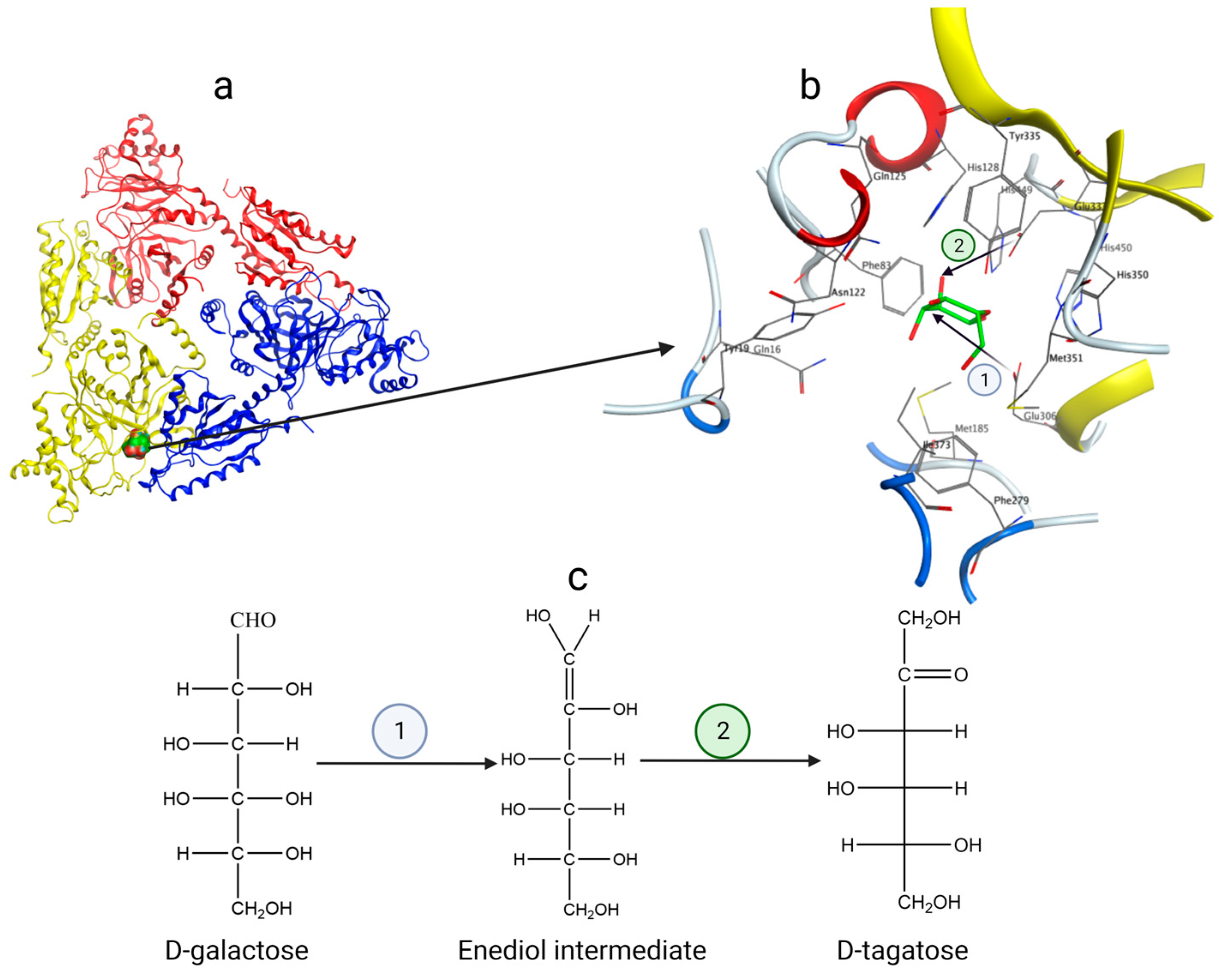
Figure 4. (a) The substrate and active site are located near the interface of two identical protein subunits; (b) ECAI catalytic residues Glu306 and Glu333 and amino acid residues in the region surrounding the active site; (c) Catalytic mechanism of L-AI—enediol intermediate.
The catalysis of d-galactose to d-tagatose by L-AIs follows the mechanism of enediol intermediate formation [32]. Taking ECAI as an example (Figure 4b), the residues E306 and E333 act as essential catalytic residues, while H350 and H450 stabilize the active site together with manganese ions. During isomerization (Figure 4), the Oε2 of Glu306 nucleophilically attacks the hydrogen atom of C-2 of d-galactose to deprotonate it and form a carbon–carbon double bond. The carbon–oxygen double bond is broken to form an oxygen anion, which combines with a proton to form an enediol intermediate; the Oε2 of Glu333 nucleophilically attacks the hydroxyl hydrogen of C-2 of d-galactose to generate a carbon–oxygen double bond at the C-2 position. This results in the cleavage of the carbon–carbon double bond between C-1 and C-2, producing a carbon anion that combines with a proton, ultimately yielding d-tagatose. This mechanism involves two proton transfers in d-galactose, one from O-2 to O-1 and the other from C-2 to C-1.
2.2. Properties of L-AIs
In order to efficiently produce d-tagatose, it is crucial to delve into the properties of L-AIs from various sources. The enzyme comes from a wide range of microbial sources, including Lactobacillus plantarum NC8 [33], Anoxybacillus flavithermus [34], Bacillus coagulans NL01 [35], Pediococcus pentosaceus PC-5 [36], Clostridium hylemonae [37], Lactobacillus sakei 23K [38], Lactobacillus fermentum CGMCC2921 [39], Bifidobacterium adolescentis [40], Thermo toga maritima [41], Thermotoga neapolitana [42], Lactococcus lactis [43], Bacillus thermoglucosidasius [44], Arthrobacter species 22c [45], Shewanella species ANA-3 [46], Bacillus licheniformis [47], Bacillus subtilis 168 [48], etc.
L-AIs from different microbial sources have different optimal temperature ranges. L-AIs sourced from mesophilic microorganisms exhibit optimal temperatures between 30 and 50 °C; those from thermophilic microorganisms have optimal temperatures ranging from 50 to 80 °C; and L-AIs from hyperthermophilic microorganisms have optimal temperatures exceeding 80 °C. Although L-AIs from hyperthermophiles have higher optimal temperatures and excellent thermal stability, their need for Co2+ to stabilize their structure at ultrahigh temperatures limits their application in the food industry [49][50]. Therefore, in actual industrial applications, L-AIs from thermophilic microorganisms are usually preferred.
L-AIs from different microbial sources exhibit variations in their optimal pH. Most reported L-AIs display their maximum activity under neutral or alkaline conditions. However, L-AIs with an optimal pH in the weakly acidic range offer advantages in industrial applications, including faster reaction rates and reduced by-product formation.
Metal ions play a crucial role in the activity and stability of L-AIs. Although not all L-AIs necessarily rely on the divalent metal ions to maintain their activity [51], for most L-AIs, divalent metal ions, especially Mn2+ and Co2+, are essential for exerting their activity and maintaining thermal stability. Since Co2+ is not permitted in the food industry, the addition of Mn2+ is more appropriate. Choi et al. [52] explored the structure of the L-AI from Geobacillus kaustophilus and found that the addition of Mn2+ transforms the L-AI structure from low oligomers to complete hexamers, indicating the significant role of metal ions in the oligomerization process. The authors also determined the melting temperatures of the hexamers by differential scanning calorimetry (DSC) and showed that the complete hexameric form is thermodynamically more stable.
Although most of the reported L-AIs were specific for l-arabinose and d-galactose, the specificity for l-arabinose was significantly higher than that for d-galactose. However, there are some exceptions. For example, the L-AIs from Bacillus subtilis 168 and Pseudoalteromonas haloplanktis [53] showed unique substrate specificity exclusively for l-arabinose. In addition, there are some enzymes classified as d-galactose isomerase because their optimal substrate is d-galactose instead of l-arabinose, such as the L-AI from Bifidobacterium adolescentis.
In addition to L-AIs, various sugar phosphate isomerases are also general aldose-ketose isomerases that can catalyze the biotransformation of non-phosphorylated monosaccharides. Patel et al. [54] characterized the phosphoglucose isomerase from Pseudomonas aeruginosa PAO1. This enzyme can also isomerize d-galactose to d-tagatose. The d-tagatose yield was 56% and the optimal activity was observed at 60 °C and pH 7.
A high temperature and slightly acidic conditions are considered ideal conditions for the efficient isomerization of d-galactose into d-tagatose. This is because the d-galactose isomerization process requires a high Gibbs free energy (4.96 kJ/mol) [55]. Therefore, the proper thermal conditions are necessary to overcome this energy barrier, which usually requires high temperature conditions [56]. High temperature conditions provide several advantages in this process. Firstly, within a specific range, higher temperatures lead to faster reaction rates, favoring the production of d-tagatose and improving the conversion; increasing the substrate solubility; reducing the viscosity of the reaction mixture, which improves the mass transfer efficiency and makes the reaction more efficient; and causing the risk of microbial contamination to be relatively low. However, it should be noted that extremely high temperature conditions above 80 °C may cause browning of the product and the generation of unnecessary by-products [57]. Therefore, the temperature range between 60 °C and 80 °C is usually chosen for industrial production. d-tagatose exhibits stability under acidic conditions and can remain relatively stable within the pH range of 3–7 [58], while side reactions will increase under alkaline conditions [59]. In addition, a high temperature and high d-galactose concentration may result in a decrease in the pH due to the Maillard reaction [60].
In conclusion, the L-AI from Lactobacillus sakei 23K showed optimal conditions at a low temperature and under acidic conditions, making it particularly suitable for the conversion of d-galactose to d-tagatose during the storage of milk and yogurt [61]. Xu et al. screened the L-AI from Lactobacillus fermentum CGMCC2921 and found its optimum temperature of 65 °C and the optimal pH of 6.5, which was within the range of suitability for industrial applications.
2.3. Production of d-Tagatose Using Lactose as Raw Material
d-galactose can usually be easily obtained from raw materials containing lactose. Therefore, it is necessary to combine lactose hydrolysis with the enzymatic isomerization of d-galactose to d-tagatose. This process can be accomplished under optimal conditions for hydrolysis and isomerization or through a one-pot method where hydrolysis and isomerization are carried out simultaneously [62]. The stepwise method allows hydrolysis and isomerization to be optimized separately under their respective optimal conditions, but high concentrations of d-glucose and d-galactose can lead to feedback inhibition of lactose hydrolysis, ultimately resulting in low conversion rates and yields. In such cases, the one-pot method is more advantageous as it simplifies the operations and minimizes the accumulation of d-galactose, thus improving the final yield of d-tagatose.
Furthermore, fully utilizing the residual d-glucose and d-galactose in the process is also an important consideration. These residues are typically wasted, and the similar physicochemical properties of d-galactose and d-tagatose introduce complex purification steps in downstream processes. These residual substances can serve multiple purposes. They can be used as energy sources to sustain cell viability or further converted into other products such as d-fructose and ethanol. Rhimi et al. [63] successfully co-expressed the L-AI from Bacillus stearothermophilus US100 and d-glucose isomerase from Streptomyces SK in E. coli to isomerize the remaining D-glucose into d-fructose. Zheng et al. [64] expressed the L-AI from Bacillus coagulans NL01 in E. coli and combined it with self-expressed β-galactosidase for the crude enzyme conversion of lactose at 50 °C, and then the residual d-glucose and d-galactose were further fermented to bioethanol using Saccharomyces cerevisiae NL22. This approach enhanced the ethanol production competitiveness and simplified the purification process of d-tagatose. Zhang et al. [65] utilized cheese whey for d-tagatose-production through whole-cell biotransformation in E. coli. Then, they fermented the residual d-glucose and d-galactose into d-arabitol and d-galactitol using Metschnikowia pulcherrima E1 yeast to maximize the conversion of lactose in the cheese whey into three high-value rare sugars (Figure 5).
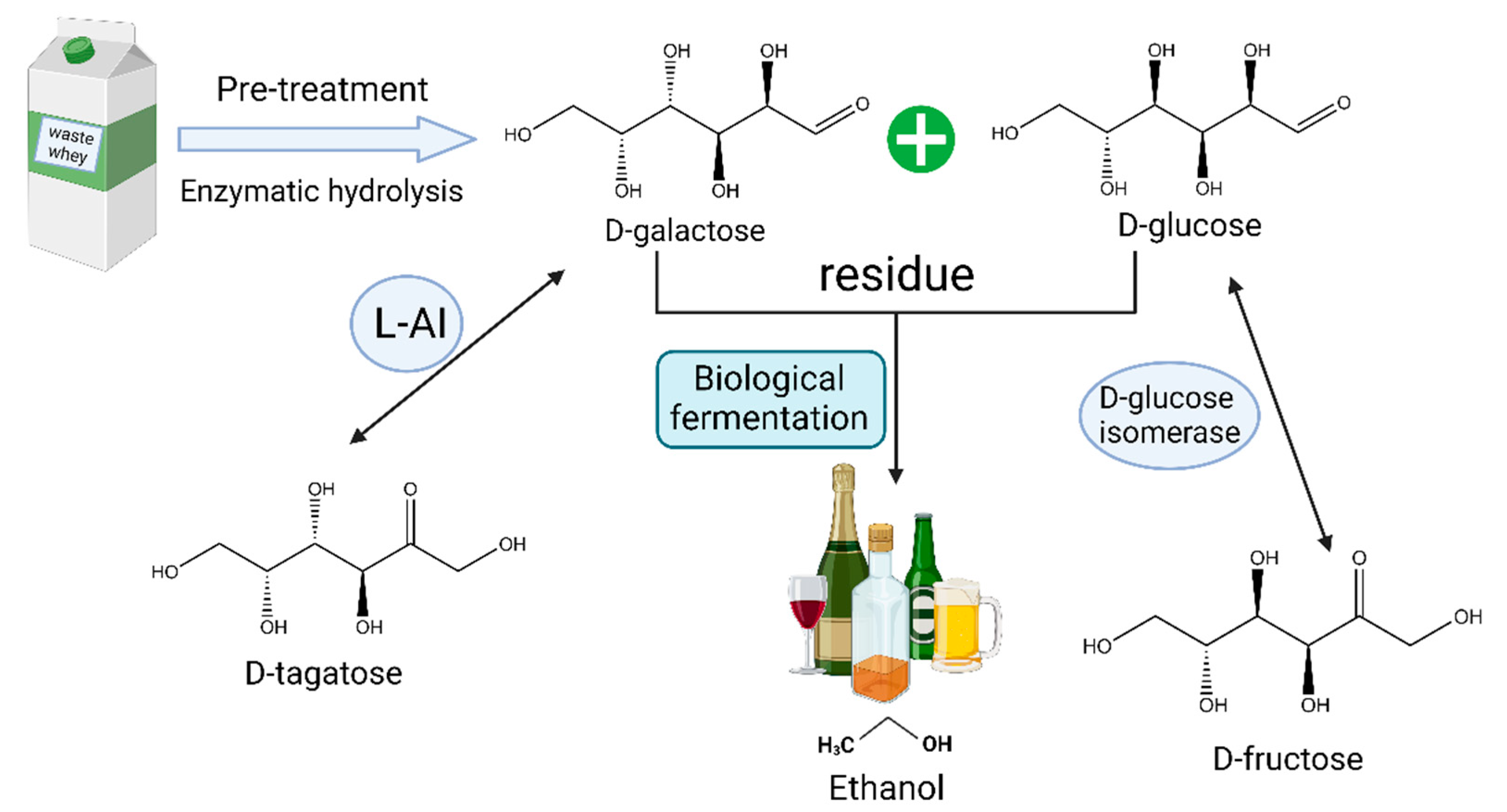
Figure 5. Utilization of lactose raw materials to produce d-tagatose, d-fructose, and bioethanol.
References
- Beerens, K.; Desmet, T.; Soetaert, W. Enzymes for the biocatalytic production of rare sugars. J. Ind. Microbiol. Biotechnol. 2012, 39, 823–834.
- Roy, S.; Chikkerur, J.; Roy, S.C.; Dhali, A.; Kolte, A.P.; Sridhar, M.; Samanta, A.K. Tagatose as a potential nutraceutical: Production, properties, biological roles, and applications. J. Food Sci. 2018, 83, 2699–2709.
- Donner, T.W.; Magder, L.S.; Zarbalian, K. Dietary supplementation with d-tagatose in subjects with type 2 diabetes leads to weight loss and raises high-density lipoprotein cholesterol. Nutr. Res. 2010, 30, 801–806.
- Buemann, B.; Toubro, S.; Raben, A.; Blundell, J.; Astrup, A. The acute effect of d-tagatose on food intake in human subjects. Br. J. Nutr. 2007, 84, 227–231.
- Ensor, M.; Banfield, A.B.; Smith, R.R.; Williams, J.; Lodder, R.A. Safety and efficacy of d-tagatose in glycemic control in subjects with type 2 diabetes. J. Endocrinol. Diabetes Obes. 2015, 3, 1065.
- Seoane, J.; Gomez-Foix, A.M.; O’Doherty, R.M.; Gomez-Ara, C.; Newgard, C.B.; Guinovart, J.J. Glucose 6-phosphate produced by glucokinase, but not hexokinase I, promotes the activation of hepatic glycogen synthase. J. Biol. Chem. 1996, 271, 23756–23760.
- Donner, T.; Wilber, J.; Ostrowski, D. d-tagatose, a novel hexose: Acute effects on carbohydrate tolerance in subjects with and without type 2 diabetes. Diabetes Obes. Metab. 1999, 1, 285–291.
- Espinosa, I.; Fogelfeld, L. Tagatose: From a sweetener to a new diabetic medication? Expert Opin. Investig. Drugs 2010, 19, 285–294.
- Levin, G.V. Tagatose, the new GRAS sweetener and health product. J. Med. Food 2002, 5, 23–36.
- Lu, Y.; Levin, G.V. Removal and prevention of dental plaque with d-tagatose. Int. J. Cosmet. Sci. 2002, 24, 225–234.
- Mayumi, S.; Kuboniwa, M.; Sakanaka, A.; Hashino, E.; Ishikawa, A.; Ijima, Y.; Amano, A. Potential of prebiotic d-tagatose for prevention of oral disease. Front. Cell Infect. Microbiol. 2021, 11, 767944.
- Hasibul, K.; Nakayama-Imaohji, H.; Hashimoto, M.; Yamasaki, H.; Ogawa, T.; Waki, J.; Tada, A.; Yoneda, S.; Tokuda, M.; Miyake, M.; et al. d-Tagatose inhibits the growth and biofilm formation of Streptococcus mutans. Mol. Med. Rep. 2018, 17, 843–851.
- Ensor, M.; Williams, J.; Smith, R.; Banfield, A.; Lodder, R.A. Effects of three low-doses of d-tagatose on glycemic control over six months in subjects with mild type 2 diabetes mellitus under control with diet and exercise. J. Endocrinol. Diabetes Obes. 2014, 2, 1057.
- Levin, G.V.; Zehner, L.R.; Saunders, J.P.; Beadle, J.R. Sugar substitutes: Their energy values, bulk characteristics, and potential health benefits. Am. J. Clin. Nutr. 1995, 62, 1161S–1168S.
- Normen, L.; Laerke, H.N.; Jensen, B.B.; Langkilde, A.M.; Andersson, H. Small-bowel absorption of d-tagatose and related effects on carbohydrate digestibility: An ileostomy study. Am. J. Clin. Nutr. 2001, 73, 105–110.
- Laerke, H.N.; Jensen, B.B.; Hojsgaard, S. In vitro fermentation pattern of d-tagatose is affected by adaptation of the microbiota from the gastrointestinal tract of pigs. J. Nutr. 2000, 130, 1772–1779.
- Fujimaru, T.; Park, J.-H.; Lim, J. Sensory characteristics and relative sweetness of tagatose and other sweeteners. J. Food Sci. 2012, 77, S323–S328.
- Armstrong, L.M.; Luecke, K.J.; Bell, L.N. Consumer evaluation of bakery product flavour as affected by incorporating the prebiotic tagatose. Int. J. Food Sci. Technol. 2009, 44, 815–819.
- Torrico, D.D.; Tam, J.; Fuentes, S.; Gonzalez Viejo, C.; Dunshea, F.R. d-tagatose as a sucrose substitute and its effect on the physico-chemical properties and acceptability of strawberry-flavored yogurt. Foods 2019, 8, 256.
- Hirst, E.; Hough, L.; Jones, J. Composition of the gum of Sterculia setigera: Occurrence of d-tagatose in nature. Nature 1949, 163, 177.
- Drabo, P.; Delidovich, I. Catalytic isomerization of galactose into tagatose in the presence of bases and Lewis acids. Catal. Commun. 2018, 107, 24–28.
- Gao, D.-M.; Kobayashi, T.; Adachi, S. Production of rare sugars from common sugars in subcritical aqueous ethanol. Food Chem. 2015, 175, 465–470.
- Milasing, N.; Khuwijitjaru, P.; Adachi, S. Isomerization of galactose to tagatose using arginine as a green catalyst. Food Chem. 2023, 398, 133858.
- Murzin, D.Y.; Murzina, E.V.; Aho, A.; Kazakova, M.A.; Selyutin, A.G.; Kubicka, D.; Kuznetsov, V.L.; Simakova, I.L. Aldose to ketose interconversion: Galactose and arabinose isomerization over heterogeneous catalysts. Catal. Sci. Technol. 2017, 7, 5321–5331.
- Zhao, J.; Wang, Z.; Jin, Q.; Feng, D.; Lee, J. Isomerization of galactose to tagatose: Recent advances in non-enzymatic isomerization. J. Agric. Food Chem. 2023, 71, 4228–4234.
- Granstrom, T.B.; Takata, G.; Tokuda, M.; Izumori, K. Izumoring: A novel and complete strategy for bioproduction of rare sugars. J. Biosci. Bioeng. 2004, 97, 89–94.
- Shin, K.-C.; Lee, T.-E.; Seo, M.-J.; Kim, D.W.; Kang, L.-W.; Oh, D.-K. Development of tagaturonate 3-epimerase into tagatose 4-epimerase with a biocatalytic route from fructose to tagatose. ACS Catal. 2020, 10, 12212–12222.
- Jeon, E.J.; Lee, Y.-M.; Choi, E.J.; Kim, S.-B.; Jeong, K.J. Production of tagatose by whole-cell bioconversion from fructose using Corynebacterium glutamicum. Biotechnol. Bioprocess. Eng. 2023, 28, 419–427.
- Manjasetty, B.A.; Chance, M.R. Crystal structure of Escherichia coli l-arabinose isomerase (ECAI), the putative target of biological tagatose production. J. Mol. Biol. 2006, 360, 297–309.
- Xu, Z.; Li, S.; Feng, X.; Zhan, Y.; Xu, H. Function of aspartic acid residues in optimum pH control of l-arabinose isomerase from Lactobacillus fermentum. Appl. Microbiol. Biotechnol. 2014, 98, 3987–3996.
- Shin, K.C.; Seo, M.J.; Kim, S.J.; Kim, Y.S.; Park, C.S. Characterization of l-Arabinose Isomerase from Klebsiella pneumoniae and Its Application in the Production of d-Tagatose from d-Galactose. Appl. Sci. 2022, 12, 4696.
- Prabhu, P.; Jeya, M.; Lee, J.-K. Probing the molecular determinant for the catalytic efficiency of l-arabinose isomerase from Bacillus licheniformis. Appl. Environ. Microbiol. 2010, 76, 1653–1660.
- Chouayekh, H.; Bejar, W.; Rhimi, M.; Jelleli, K.; Mseddi, M.; Bejar, S. Characterization of an l-arabinose isomerase from the Lactobacillus plantarum NC8 strain showing pronounced stability at acidic pH. FEMS Microbiol. Lett. 2007, 277, 260–267.
- Li, Y.; Zhu, Y.; Liu, A.; Sun, Y. Identification and characterization of a novel l-arabinose isomerase from Anoxybacillus flavithermus useful in d-tagatose production. Extremophiles 2011, 15, 441–450.
- Mei, W.; Wang, L.; Zang, Y.; Zheng, Z.; Ouyang, J. Characterization of an l-arabinose isomerase from Bacillus coagulans NL01 and its application for d-tagatose production. BMC Biotechnol. 2016, 16, 55.
- Men, Y.; Zhu, Y.; Zhang, L.; Kang, Z.; Izumori, K.; Sun, Y.; Ma, Y. Enzymatic conversion of d-galactose to d-tagatose: Cloning, overexpression and characterization of l-arabinose isomerase from Pediococcus pentosaceus PC-5. Microbiol. Res. 2014, 169, 171–178.
- Nguyen, T.K.; Hong, M.G.; Chang, P.S.; Lee, B.H.; Yoo, S.H. Biochemical properties of l-arabinose isomerase from Clostridium hylemonae to produce d-tagatose as a functional sweetener. PLoS ONE 2018, 13, e0196099.
- Rhimi, M.; Ilhammami, R.; Bajic, G.; Boudebbouze, S.; Maguin, E.; Haser, R.; Aghajari, N. The acid tolerant l-arabinose isomerase from the food grade Lactobacillus sakei 23K is an attractive d-tagatose producer. Bioresour. Technol. 2010, 101, 9171–9177.
- Xu, Z.; Qing, Y.; Li, S.; Feng, X.; Xu, H.; Ouyang, P. A novel l-arabinose isomerase from Lactobacillus fermentum CGMCC2921 for d-tagatose production: Gene cloning, purification and characterization. J. Mol. Catal. B Enzym. 2011, 70, 1–7.
- Zhang, G.; An, Y.; Parvez, A.; Zabed, H.M.; Yun, J.; Qi, X. Exploring a highly d-galactose specific l-arabinose isomerase from Bifidobacterium adolescentis for d-tagatose production. Front. Bioeng. Biotechnol. 2020, 8, 377.
- Lee, D.W.; Jang, H.J.; Choe, E.A.; Kim, B.C.; Lee, S.J.; Kim, S.B.; Hong, Y.H.; Pyun, Y.R. Characterization of a thermostable l-arabinose (d-galactose) isomerase from the hyperthermophilic eubacterium Thermotoga maritima. Appl. Environ. Microbiol. 2004, 70, 1397–1404.
- Kim, B.-C.; Lee, Y.-H.; Lee, H.-S.; Lee, D.-W.; Choe, E.-A.; Pyun, Y.-R. Cloning, expression and characterization of l-arabinose isomerase from Thermotoga neapolitana: Bioconversion of d-galactose to d-tagatose using the enzyme. FEMS Microbiol. Lett. 2002, 212, 121–126.
- Zhang, S.; Xu, Z.; Ma, M.; Zhao, G.; Chang, R.; Si, H.; Dai, M. A novel Lactococcus lactis l-arabinose isomerase for d-tagatose production from lactose. Food Biosci. 2022, 48, 101765.
- Seo, M.J. Characterization of an l-arabinose isomerase from Bacillus thermoglucosidasius for d-tagatose production. Biosci. Biotechnol. Biochem. 2013, 77, 385–388.
- Wanarska, M.; Kur, J. A method for the production of d-tagatose using a recombinant Pichia pastoris strain secreting β-d-galactosidase from Arthrobacter chlorophenolicus and a recombinant l-arabinose isomerase from Arthrobacter sp. 22c. Microb. Cell Factories 2012, 11, 113.
- Rhimi, M.; Bajic, G.; Ilhammami, R.; Boudebbouze, S.; Maguin, E.; Haser, R.; Aghajari, N. The acid-tolerant l-arabinose isomerase from the mesophilic Shewanella sp. ANA-3 is highly active at low temperatures. Microb. Cell Factories 2011, 10, 96.
- Prabhu, P.; Tiwari, M.K.; Jeya, M.; Gunasekaran, P.; Kim, I.W.; Lee, J.K. Cloning and characterization of a novel l-arabinose isomerase from Bacillus licheniformis. Appl. Microbiol. Biotechnol. 2008, 81, 283–290.
- Kim, J.H.; Prabhu, P.; Jeya, M.; Tiwari, M.K.; Moon, H.J.; Singh, R.K.; Lee, J.K. Characterization of an l-arabinose isomerase from Bacillus subtilis. Appl. Microbiol. Biotechnol. 2010, 85, 1839–1847.
- Jayamuthunagai, J.; Gautam, P.; Srisowmeya, G.; Chakravarthy, M. Biocatalytic production of d-tagatose: A potential rare sugar with versatile applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 3430–3437.
- Fan, C.; Liu, K.; Zhang, T.; Zhou, L.; Xue, D.; Jiang, B.; Mu, W. Biochemical characterization of a thermostable l-arabinose isomerase from a thermoacidophilic bacterium, Alicyclobacillus hesperidum URH17-3-68. J. Mol. Catal. B Enzym. 2014, 102, 120–126.
- Hong, Y.-H.; Lee, D.-W.; Pyun, Y.-R.; Lee, S.H. Creation of metal-independent hyperthermophilic l-arabinose isomerase by homologous recombination. J. Agric. Food Chem. 2011, 59, 12939–12947.
- Choi, J.M.; Lee, Y.-J.; Cao, T.-P.; Shin, S.-M.; Park, M.-K.; Lee, H.-S.; di Luccio, E.; Kim, S.-B.; Lee, S.-J.; Lee, S.J.; et al. Structure of the thermophilic l-Arabinose isomerase from Geobacillus kaustophilus reveals metal-mediated intersubunit interactions for activity and thermostability. Arch. Biochem. Biophys. 2016, 596, 51–62.
- Xu, W.; Fan, C.; Zhang, T.; Jiang, B.; Mu, W. Cloning, expression, and characterization of a novel l-arabinose isomerase from the psychrotolerant bacterium Pseudoalteromonas haloplanktis. Mol. Biotechnol. 2016, 58, 695–706.
- Patel, M.J.; Patel, A.T.; Akhani, R.; Dedania, S.; Patel, D.H. Bioproduction of d-tagatose from d-galactose using phosphoglucose isomerase from Pseudomonas aeruginosa PAO1. Appl. Biochem. Biotechnol. 2016, 179, 715–727.
- Hong, Y.H.; Lee, D.W.; Lee, S.J.; Choe, E.A.; Kim, S.B.; Lee, Y.H.; Cheigh, C.I.; Pyun, Y.R. Production of d-tagatose at high temperatures using immobilized Escherichia coli cells expressing l-arabinose isomerase from Thermotoga neapolitana. Biotechnol. Lett. 2007, 29, 569–574.
- Xu, Z.; Li, S.; Feng, X.; Liang, J.; Xu, H. l-Arabinose isomerase and its use for biotechnological production of rare sugars. Appl. Microbiol. Biotechnol. 2014, 98, 8869–8878.
- Kim, P. Current studies on biological tagatose production using l-arabinose isomerase: A review and future perspective. Appl. Microbiol. Biotechnol. 2004, 65, 243–249.
- Dobbs, C.M.; Bell, L.N. Storage stability of tagatose in buffer solutions of various compositions. Food Res. Int. 2010, 43, 382–386.
- Luecke, K.J.; Bell, L.N. Thermal stability of tagatose in solution. J. Food Sci. 2010, 75, C346–C351.
- Lim, B.-C.; Kim, H.-J.; Oh, D.-K. Tagatose production with pH control in a stirred tank reactor containing immobilized l-arabinose isomerase from Thermotoga neapolitana. Appl. Biochem. Biotechnol. 2007, 149, 245–253.
- Rhimi, M.; Chouayekh, H.; Gouillouard, I.; Maguin, E.; Bejar, S. Production of d-tagatose, a low caloric sweetener during milk fermentation using l-arabinose isomerase. Bioresour. Technol. 2011, 102, 3309–3315.
- Zhang, G.; Zabed, H.M.; Yun, J.; Yuan, J.; Zhang, Y.; Wang, Y.; Qi, X. Two-stage biosynthesis of d-tagatose from milk whey powder by an engineered Escherichia coli strain expressing l-arabinose isomerase from Lactobacillus plantarum. Bioresour. Technol. 2020, 305, 123010.
- Rhimi, M.; Messaoud, E.B.; Borgi, M.A.; Khadra, K.B.; Bejar, S. Co-expression of l-arabinose isomerase and d-glucose isomerase in E. coli and development of an efficient process producing simultaneously d-tagatose and d-fructose. Enzym. Microb. Technol. 2007, 40, 1531–1537.
- Zheng, Z.; Xie, J.; Liu, P.; Li, X.; Ouyang, J. Elegant and efficient biotransformation for dual production of d-tagatose and bioethanol from cheese whey powder. J. Agric. Food Chem. 2019, 67, 829–835.
- Zhang, G.; Zabed, H.M.; An, Y.; Yun, J.; Huang, J.; Zhang, Y.; Li, X.; Wang, J.; Ravikumar, Y.; Qi, X. Biocatalytic conversion of a lactose-rich dairy waste into d-tagatose, d-arabitol and galactitol using sequential whole cell and fermentation technologies. Bioresour. Technol. 2022, 358, 127422.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
29 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




