Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biotechnology & Applied Microbiology
D-塔格糖是蔗糖天然单糖的低热量替代品,几乎同样甜。作为一种酮己糖,d-塔格糖具有缓解疾病和促进健康的特性。由于其本质上的稀缺性,d-塔格糖主要通过化学和生物方法生产。
- d-tagatose
- production
- l-arabinose isomerase
1. Introduction
近年来,传统的高吸收、高热量糖类逐渐被低热量、低吸收的稀有糖类所取代。作为这种稀有糖类的代表,d-塔格糖是一种天然存在的单糖,甜度只有蔗糖的92%,但热量只有三分之一。
d-塔格糖,分子式为C6H12O6,是 d-半乳糖的酮糖形式和 c-4 位 d-果糖的差向异构体(图 1)。D-塔格糖在自然界中非常罕见,少量D-塔格糖天然存在于奶酪、酸奶、热可可、灭菌奶粉和其他乳制品衍生产品中[1]。
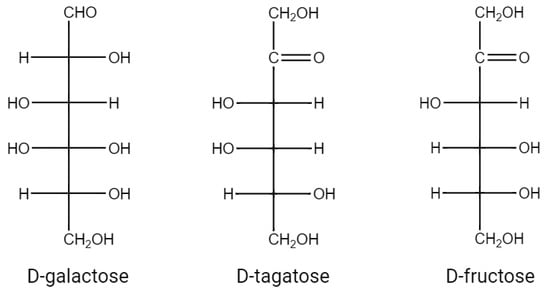
图 1.d-半乳糖、d-塔格糖、d-果糖的结构式。
d-塔格糖在改善疾病(控制肥胖、抗糖尿病和调节血液代谢物)和促进健康(抗衰老、抗氧化和益生元特性)方面的功能已被广泛评估(图2)[2]。研究显示,糖尿病患者通过长期使用d-tagatose治疗可逐渐减轻体重[3]。这是因为d-塔格糖不会导致脂肪沉积。Buemann等[4]的研究表明,未被吸收的d-塔格糖引起的渗透效应会导致肠膨胀。这可能会介导急性食欲抑制作用,并有助于减少能量摄入;d-塔格糖在血糖控制方面具有潜力,其血糖指数(GI)值为3[5]。当个体在饭前食用d-tagatose时,大约20%的d-tagatose被人体吸收,将血糖转化为糖原,并减缓糖原分解为葡萄糖的速度[6]。究其原因,可能是由于它的肝脏代谢过程,与d-果糖相似。D-塔格糖被果糖激酶磷酸化为塔格糖-1-磷酸。其较慢的分解速度导致塔格糖-1-磷酸的积累。塔格糖-1-磷酸刺激葡萄糖激酶,促进葡萄糖向葡萄糖-6-磷酸的转化。葡萄糖-6-磷酸刺激肝糖原合酶加速糖原形成。其余80%不被吸收,可能与小肠中的葡萄糖转运蛋白竞争或部分抑制,从而抑制和阻止葡萄糖吸收[7,8]。D-塔格糖通过增强关键血液因子(如红细胞计数、凝血酶原时间和活化部分凝血活酶时间)以及增加凝血因子来帮助改善血液健康[9]。因此,它是治疗贫血和血友病的候选者。D-塔格糖还具有预防口腔疾病的潜力[10]。Mayumi等[11]发现,它通过影响口腔病原体的糖酵解和下游代谢来选择性地抑制其生长。Hasibul等[12]发现,d-塔格糖可防止致龋物种变形链球菌生长和形成生物膜。通过d-塔格糖限制饮食可维持体内较低的血糖和胰岛素水平,从而延缓与年龄相关的疾病发展,并进一步延长实践者的寿命[13,14];此外,d-塔格糖是一种极好的益生元。研究表明[15],未被吸收的d-塔格糖会进入大肠,在那里它被微生物菌群选择性地发酵。这种发酵过程促进了有益菌群的增殖,同时抑制了有害菌群的生长,从而改善了肠道菌群。同时,d-塔格糖的发酵产生大量有益的短链脂肪酸,如丁酸[16],是结肠上皮细胞的良好能量来源。
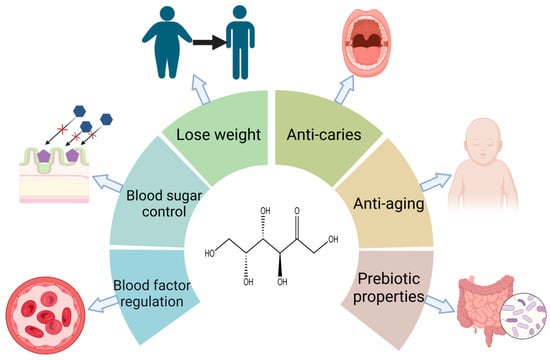
图2.d-塔格糖对人体的各种生理功能。
D-塔格糖是一种新兴的低热量甜味剂,具有替代蔗糖的潜力[17]。它可以提供各种浓度的甜味,而不会增加不良味道。在低糖产品中添加d-塔格糖可以为其提供理想的感官特性,这意味着它可以在不改变产品风味的情况下有益于健康[18,19]。由于 d-塔格糖已被批准为“公认安全”(GRAS),因此可用于糖果、饮料、营养保健品和膳食产品。D-塔格糖还可用作处方药的添加剂,以及牙膏、漱口水和化妆品中的甜味剂。
早在1949年,人们就在Sterculia setigera的牙龈中发现了d-tagatose[20]。然而,由于其稀缺性,很难直接从自然界中大规模提取d-塔格糖。为了使d-塔格糖的生产更加经济和高效,通常使用化学和生物方法(图3)。
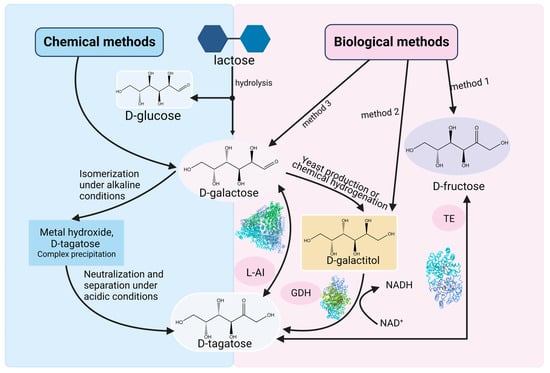
Figure 3. Current methods for producing d-tagatose via feasible chemical or biological routes. L-AI: l-arabinose isomerase, GDH: Galactitol dehydrogenase, TE: Tagatose 4-epimerase.
The typically used chemical method, also known as non-enzymatic isomerization [21], uses metal hydroxides as catalysts. This method consists of three steps: firstly, an insoluble d-tagatose complex is generated when d-galactose interacts with metal hydroxides, which are stable under alkaline conditions; in the second stage, the insoluble d-tagatose complex is neutralized with an acid to produce an insoluble salt; finally, filtering is used to separate d-tagatose from the insoluble salt. During the isomerization, the metal hydroxides perform two functions: isomerizing d-galactose to d-tagatose and degrading d-galactose into dicarbonyl compounds and acidic substances. This method has a high yield (>70%) but includes the following disadvantages: severe d-galactose degradation leads to a reduction in the yield of d-tagatose, while the decrease in the quality of the syrup makes the production of crystalline d-tagatose difficult; the removal of the degradation products requires complex extraction steps; and a large number of metal hydroxides and acids are consumed in the reaction, which ultimately has many negative impacts on the cost and the environment. There are also other methods such as supercritical fluid (<24%) [22], triethylamine (<34%), arginine (<16.8%) [23], hydrotalcite (<27%) [24], Sn-β zeolite (<26%), etc. However, all of these non-enzymatic pathways have low d-tagatose yields [25]. Moreover, these methods require reactions under extreme conditions, which can lead to increased energy consumption and damage to substrates and products.
Biological methods use whole cells and isolated enzymes as catalysts. The advantages of such methods include mild reaction conditions, environmental friendliness, few by-products, and easy purification. Thus, the production of d-tagatose by biological methods has advantages over chemical methods in many aspects. In biological methods, the lzumoring strategy is often used to produce various rare sugars. This strategy uses different types of enzymes including epimerase, oxidoreductase, and aldose–ketose isomerases to achieve interconversion between monosaccharides and sugar alcohols [26]. d-tagatose can also be produced by these three types of enzymes, including tagatose 4-epimerase, galactitol dehydrogenase, and l-arabinose isomerase.
Tagatose 4-epimerase can epimerize d-fructose into d-tagatose. However, there are few reports of enzymes with d-fructose epimeric activity at the C-4 position in nature. Therefore, obtaining such enzymes by screening new ones or modifying old ones is the main work in this production pathway. Shin et al. [27] developed a new tagatose 4-epimerase through rational design and directed the evolution of the tagaturonate 3-epimerase from Thermotoga petrophila. The modified enzyme exhibited 184-fold-higher epimeric activity towards d-fructose compared to the original enzyme. Under optimal conditions of 80 °C, pH 8.5, and 1.5 mM Ni2+, the enzyme was able to produce 213 g/L of d-tagatose from 700 g/L of d-fructose within 2 h, with a conversion rate of 30%. Jeon et al. [28] expressed tagatose 4-epimerase from Thermotoga neapolitana in Corynebacterium glutamicum and improved the enzyme expression level by optimizing plasmid copy numbers. Under conditions of 60 °C and a specific amount of metal ions, the conversion rate reached 21.7%.
2. The Production of d-Tagatose by L-AIs
2.1. Molecular Structure and Catalytic Mechanism of L-AIs
The crystal structures of L-AIs from E. coli (PDB code: 2AJT) [38], Lactobacillus fermentum CGMCC2921 (PDB code: 4LQL) [39], Geobacillus kaustophilus (PDB code: 4R1O), and Thermotoga maritima MSB8 (PDB code: 7CWV) have been determined. Taking the L-AI from E. coli (ECAI) as an example [40], it is a hexamer with a total molecular weight of 336 kDa. Three asymmetric units of L-AI subunits form a trimer, as shown in Figure 4a. Two such trimers stack together to form the complete hexamer. Each subunit contains three structural domains: the N-terminal domain, the central domain, and the C-terminal domain. Within the complete hexamer of ECAI, there are six active sites located at the monomer–monomer interfaces (Figure 4a) and situated in the conjugate regions of adjacent subunits.
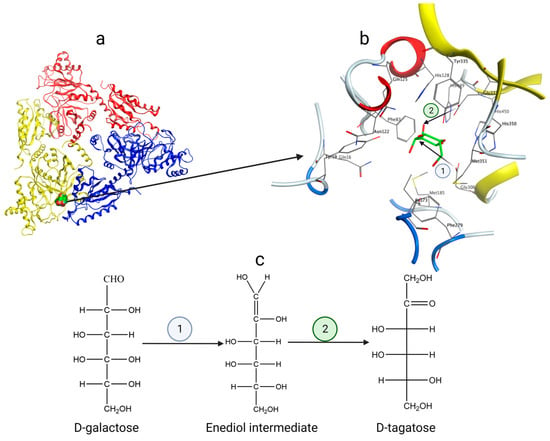
Figure 4. (a) The substrate and active site are located near the interface of two identical protein subunits; (b) ECAI catalytic residues Glu306 and Glu333 and amino acid residues in the region surrounding the active site; (c) Catalytic mechanism of L-AI—enediol intermediate.
The catalysis of d-galactose to d-tagatose by L-AIs follows the mechanism of enediol intermediate formation [41]. Taking ECAI as an example (Figure 4b), the residues E306 and E333 act as essential catalytic residues, while H350 and H450 stabilize the active site together with manganese ions. During isomerization (Figure 4), the Oε2 of Glu306 nucleophilically attacks the hydrogen atom of C-2 of d-galactose to deprotonate it and form a carbon–carbon double bond. The carbon–oxygen double bond is broken to form an oxygen anion, which combines with a proton to form an enediol intermediate; the Oε2 of Glu333 nucleophilically attacks the hydroxyl hydrogen of C-2 of d-galactose to generate a carbon–oxygen double bond at the C-2 position. This results in the cleavage of the carbon–carbon double bond between C-1 and C-2, producing a carbon anion that combines with a proton, ultimately yielding d-tagatose. This mechanism involves two proton transfers in d-galactose, one from O-2 to O-1 and the other from C-2 to C-1.
2.2. Properties of L-AIs
In order to efficiently produce d-tagatose, it is crucial to delve into the properties of L-AIs from various sources. The enzyme comes from a wide range of microbial sources, including Lactobacillus plantarum NC8 [42], Anoxybacillus flavithermus [43], Bacillus coagulans NL01 [44], Pediococcus pentosaceus PC-5 [45], Clostridium hylemonae [46], Lactobacillus sakei 23K [47], Lactobacillus fermentum CGMCC2921 [48], Bifidobacterium adolescentis [49], Thermo toga maritima [50], Thermotoga neapolitana [51], Lactococcus lactis [52], Bacillus thermoglucosidasius [53], Arthrobacter species 22c [54], Shewanella species ANA-3 [55], Bacillus licheniformis [56], Bacillus subtilis 168 [57], etc.
L-AIs from different microbial sources have different optimal temperature ranges. L-AIs sourced from mesophilic microorganisms exhibit optimal temperatures between 30 and 50 °C; those from thermophilic microorganisms have optimal temperatures ranging from 50 to 80 °C; and L-AIs from hyperthermophilic microorganisms have optimal temperatures exceeding 80 °C. Although L-AIs from hyperthermophiles have higher optimal temperatures and excellent thermal stability, their need for Co2+ to stabilize their structure at ultrahigh temperatures limits their application in the food industry [59,60]. Therefore, in actual industrial applications, L-AIs from thermophilic microorganisms are usually preferred.
L-AIs from different microbial sources exhibit variations in their optimal pH. Most reported L-AIs display their maximum activity under neutral or alkaline conditions. However, L-AIs with an optimal pH in the weakly acidic range offer advantages in industrial applications, including faster reaction rates and reduced by-product formation.
Metal ions play a crucial role in the activity and stability of L-AIs. Although not all L-AIs necessarily rely on the divalent metal ions to maintain their activity [61], for most L-AIs, divalent metal ions, especially Mn2+ and Co2+, are essential for exerting their activity and maintaining thermal stability. Since Co2+ is not permitted in the food industry, the addition of Mn2+ is more appropriate. Choi et al. [62] explored the structure of the L-AI from Geobacillus kaustophilus and found that the addition of Mn2+ transforms the L-AI structure from low oligomers to complete hexamers, indicating the significant role of metal ions in the oligomerization process. The authors also determined the melting temperatures of the hexamers by differential scanning calorimetry (DSC) and showed that the complete hexameric form is thermodynamically more stable.
Although most of the reported L-AIs were specific for l-arabinose and d-galactose, the specificity for l-arabinose was significantly higher than that for d-galactose. However, there are some exceptions. For example, the L-AIs from Bacillus subtilis 168 and Pseudoalteromonas haloplanktis [63] showed unique substrate specificity exclusively for l-arabinose. In addition, there are some enzymes classified as d-galactose isomerase because their optimal substrate is d-galactose instead of l-arabinose, such as the L-AI from Bifidobacterium adolescentis.
In addition to L-AIs, various sugar phosphate isomerases are also general aldose-ketose isomerases that can catalyze the biotransformation of non-phosphorylated monosaccharides. Patel et al. [64] characterized the phosphoglucose isomerase from Pseudomonas aeruginosa PAO1. This enzyme can also isomerize d-galactose to d-tagatose. The d-tagatose yield was 56% and the optimal activity was observed at 60 °C and pH 7.
A high temperature and slightly acidic conditions are considered ideal conditions for the efficient isomerization of d-galactose into d-tagatose. This is because the d-galactose isomerization process requires a high Gibbs free energy (4.96 kJ/mol) [65]. Therefore, the proper thermal conditions are necessary to overcome this energy barrier, which usually requires high temperature conditions [37]. High temperature conditions provide several advantages in this process. Firstly, within a specific range, higher temperatures lead to faster reaction rates, favoring the production of d-tagatose and improving the conversion; increasing the substrate solubility; reducing the viscosity of the reaction mixture, which improves the mass transfer efficiency and makes the reaction more efficient; and causing the risk of microbial contamination to be relatively low. However, it should be noted that extremely high temperature conditions above 80 °C may cause browning of the product and the generation of unnecessary by-products [66]. Therefore, the temperature range between 60 °C and 80 °C is usually chosen for industrial production. d-tagatose exhibits stability under acidic conditions and can remain relatively stable within the pH range of 3–7 [67], while side reactions will increase under alkaline conditions [68]. In addition, a high temperature and high d-galactose concentration may result in a decrease in the pH due to the Maillard reaction [69].
In conclusion, the L-AI from Lactobacillus sakei 23K showed optimal conditions at a low temperature and under acidic conditions, making it particularly suitable for the conversion of d-galactose to d-tagatose during the storage of milk and yogurt [70]. Xu et al. screened the L-AI from Lactobacillus fermentum CGMCC2921 and found its optimum temperature of 65 °C and the optimal pH of 6.5, which was within the range of suitability for industrial applications.
2.3. Production of d-Tagatose Using Lactose as Raw Material
d-galactose can usually be easily obtained from raw materials containing lactose. Therefore, it is necessary to combine lactose hydrolysis with the enzymatic isomerization of d-galactose to d-tagatose. This process can be accomplished under optimal conditions for hydrolysis and isomerization or through a one-pot method where hydrolysis and isomerization are carried out simultaneously [71]. The stepwise method allows hydrolysis and isomerization to be optimized separately under their respective optimal conditions, but high concentrations of d-glucose and d-galactose can lead to feedback inhibition of lactose hydrolysis, ultimately resulting in low conversion rates and yields. In such cases, the one-pot method is more advantageous as it simplifies the operations and minimizes the accumulation of d-galactose, thus improving the final yield of d-tagatose.
Furthermore, fully utilizing the residual d-glucose and d-galactose in the process is also an important consideration. These residues are typically wasted, and the similar physicochemical properties of d-galactose and d-tagatose introduce complex purification steps in downstream processes. These residual substances can serve multiple purposes. They can be used as energy sources to sustain cell viability or further converted into other products such as d-fructose and ethanol. Rhimi et al. [72] successfully co-expressed the L-AI from Bacillus stearothermophilus US100 and d-glucose isomerase from Streptomyces SK in E. coli to isomerize the remaining D-glucose into d-fructose. Zheng et al. [73] expressed the L-AI from Bacillus coagulans NL01 in E. coli and combined it with self-expressed β-galactosidase for the crude enzyme conversion of lactose at 50 °C, and then the residual d-glucose and d-galactose were further fermented to bioethanol using Saccharomyces cerevisiae NL22. This approach enhanced the ethanol production competitiveness and simplified the purification process of d-tagatose. Zhang et al. [74] utilized cheese whey for d-tagatose-production through whole-cell biotransformation in E. coli. Then, they fermented the residual d-glucose and d-galactose into d-arabitol and d-galactitol using Metschnikowia pulcherrima E1 yeast to maximize the conversion of lactose in the cheese whey into three high-value rare sugars (Figure 5).
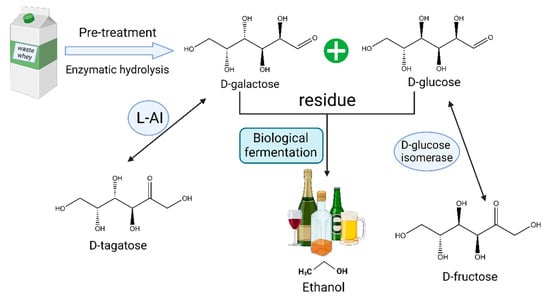
Figure 5. Utilization of lactose raw materials to produce d-tagatose, d-fructose, and bioethanol.
This entry is adapted from the peer-reviewed paper 10.3390/catal13111437
This entry is offline, you can click here to edit this entry!
