
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Axel Nierhaus | -- | 2704 | 2023-11-28 10:04:16 | | | |
| 2 | Lindsay Dong | Meta information modification | 2704 | 2023-12-04 02:18:49 | | |
Video Upload Options
There are no approved therapies to modulate the excessive immune response and limit hyperinflammation with the goal of preventing related organ failure and death. In this context, extracorporeal blood purification therapies aiming at the alteration of the host inflammatory response through broad-spectrum, non-selective removal of inflammatory mediators have come into focus. A novel hemoadsorption device (CytoSorb®, CytoSorbents Inc., Princeton, NJ, USA) has shown promising results in patients with hyperinflammation from various origins. Although a significant body of literature exists, there is ongoing research to address many important remaining questions, including the optimal selection of patient groups who might benefit the most, optimal timing for therapy initiation, optimal schedule for adsorber exchanges and therapy duration, as well as an investigation into the potential removal of concomitant antibiotics and other medications.
1. Background
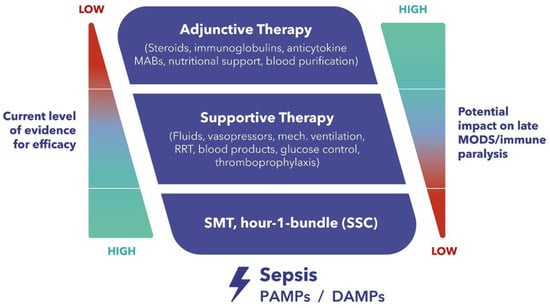
2. Potential Role of Extracorporeal Cytokine Adsorption
3. The CytoSorb Adsorber
3.1. Properties of the Device
3.2. Effects of CytoSorb Therapy on Circulating Cytokines
3.3. Effects of CytoSorb Therapy on Clinical Parameters
3.4. Patient Selection
3.5. Timing
3.6. Dosing
3.7. Therapeutic Goals
3.8. Safety
3.9. Procedural Details
3.10. Anti-Infectives
3.11. Anticoagulation
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810.
- van den Berg, M.; van Beuningen, F.E.; Ter Maaten, J.C.; Bouma, H.R. Hospital-related costs of sepsis around the world: A systematic review exploring the economic burden of sepsis. J. Crit. Care 2022, 71, 154096.
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273.
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268.
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302.
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045.
- Krenn, C.G.; Steltzer, H. Hemoadsorption for blood purification-incomparability of clinically available procedures. Med. Klin. Intensivmed. Notfmed 2021, 116, 449–453.
- Peng, Z.; Singbartl, K.; Simon, P.; Rimmele, T.; Bishop, J.; Clermont, G.; Kellum, J.A. Blood purification in sepsis: A new paradigm. Contrib. Nephrol. 2010, 165, 322–328.
- Rimmele, T.; Kellum, J.A. Clinical review: Blood purification for sepsis. Crit. Care 2011, 15, 205.
- Zhou, F.; Peng, Z.; Murugan, R.; Kellum, J.A. Blood purification and mortality in sepsis: A meta-analysis of randomized trials. Crit. Care Med. 2013, 41, 2209–2220.
- Rockx, B.; Baas, T.; Zornetzer, G.A.; Haagmans, B.; Sheahan, T.; Frieman, M.; Dyer, M.D.; Teal, T.H.; Proll, S.; van den Brand, J.; et al. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J. Virol. 2009, 83, 7062–7074.
- Van Reeth, K. Cytokines in the pathogenesis of influenza. Vet. Microbiol. 2000, 74, 109–116.
- Mavor, A.L.; Thewes, S.; Hube, B. Systemic fungal infections caused by Candida species: Epidemiology, infection process and virulence attributes. Curr. Drug Targets 2005, 6, 863–874.
- Brakhage, A.A. Systemic fungal infections caused by Aspergillus species: Epidemiology, infection process and virulence determinants. Curr. Drug Targets 2005, 6, 875–886.
- Clark, I.A.; Alleva, L.M.; Budd, A.C.; Cowden, W.B. Understanding the role of inflammatory cytokines in malaria and related diseases. Travel Med. Infect. Dis. 2008, 6, 67–81.
- Kellum, J.A.; Kong, L.; Fink, M.P.; Weissfeld, L.A.; Yealy, D.M.; Pinsky, M.R.; Fine, J.; Krichevsky, A.; Delude, R.L.; Angus, D.C.; et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch. Intern. Med. 2007, 167, 1655–1663.
- Nakae, H.; Endo, S.; Yamada, Y.; Inada, K. Bound and soluble adhesion molecule and cytokine levels in patients with severe burns. Burns 2000, 26, 139–144.
- Finnerty, C.C.; Jeschke, M.G.; Herndon, D.N.; Gamelli, R.; Gibran, N.; Klein, M.; Silver, G.; Arnoldo, B.; Remick, D.; Tompkins, R.G.; et al. Temporal cytokine profiles in severely burned patients: A comparison of adults and children. Mol. Med. 2008, 14, 553–560.
- Cuschieri, J.; Bulger, E.; Schaeffer, V.; Sakr, S.; Nathens, A.B.; Hennessy, L.; Minei, J.; Moore, E.E.; O’Keefe, G.; Sperry, J.; et al. Early elevation in random plasma IL-6 after severe injury is associated with development of organ failure. Shock 2010, 34, 346–351.
- Makhija, R.; Kingsnorth, A.N. Cytokine storm in acute pancreatitis. J. Hepatobiliary Pancreat. Surg. 2002, 9, 401–410.
- Laffey, J.G.; Boylan, J.F.; Cheng, D.C. The systemic inflammatory response to cardiac surgery: Implications for the anesthesiologist. Anesthesiology 2002, 97, 215–252.
- Lau, K.; Shah, H.; Kelleher, A.; Moat, N. Coronary artery surgery: Cardiotomy suction or cell salvage? J. Cardiothorac. Surg. 2007, 2, 46.
- Scharf, C.; Liebchen, U.; Paal, M.; Irlbeck, M.; Zoller, M.; Schroeder, I. Blood purification with a cytokine adsorber for the elimination of myoglobin in critically ill patients with severe rhabdomyolysis. Crit. Care 2021, 25, 41.
- Dilken, O.; Ince, C.; van der Hoven, B.; Thijsse, S.; Ormskerk, P.; de Geus, H.R.H. Successful Reduction of Creatine Kinase and Myoglobin Levels in Severe Rhabdomyolysis Using Extracorporeal Blood Purification (CytoSorb(R)). Blood Purif. 2020, 49, 743–747.
- Kimmel, J.D.; Gibson, G.A.; Watkins, S.C.; Kellum, J.A.; Federspiel, W.J. IL-6 adsorption dynamics in hemoadsorption beads studied using confocal laser scanning microscopy. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 390–396.
- Honore, P.M.; Hoste, E.; Molnar, Z.; Jacobs, R.; Joannes-Boyau, O.; Malbrain, M.; Forni, L.G. Cytokine removal in human septic shock: Where are we and where are we going? Ann. Intensive Care 2019, 9, 56.
- Poli, E.C.; Alberio, L.; Bauer-Doerries, A.; Marcucci, C.; Roumy, A.; Kirsch, M.; De Stefano, E.; Liaudet, L.; Schneider, A.G. Cytokine clearance with CytoSorb(R) during cardiac surgery: A pilot randomized controlled trial. Crit. Care 2019, 23, 108.
- Steiner, C. CytoSorbents Europe GmbH Müggelseedamm 131, 12587 Berlin. Available online: https://cytosorb-therapy.com (accessed on 25 September 2023).
- Kellum, J.A.; Song, M.; Venkataraman, R. Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-kappaB DNA binding, and improves short-term survival in lethal endotoxemia. Crit. Care Med. 2004, 32, 801–805.
- Peng, Z.Y.; Carter, M.J.; Kellum, J.A. Effects of hemoadsorption on cytokine removal and short-term survival in septic rats. Crit. Care Med. 2008, 36, 1573–1577.
- Peng, Z.Y.; Bishop, J.V.; Wen, X.Y.; Elder, M.M.; Zhou, F.; Chuasuwan, A.; Carter, M.J.; Devlin, J.E.; Kaynar, A.M.; Singbartl, K.; et al. Modulation of chemokine gradients by apheresis redirects leukocyte trafficking to different compartments during sepsis, studies in a rat model. Crit. Care 2014, 18, R141.
- Peng, Z.Y.; Wang, H.Z.; Carter, M.J.; Dileo, M.V.; Bishop, J.V.; Zhou, F.H.; Wen, X.Y.; Rimmele, T.; Singbartl, K.; Federspiel, W.J.; et al. Acute removal of common sepsis mediators does not explain the effects of extracorporeal blood purification in experimental sepsis. Kidney Int. 2012, 81, 363–369.
- Jansen, A.; Waalders, N.J.B.; van Lier, D.P.T.; Kox, M.; Pickkers, P. CytoSorb hemoperfusion markedly attenuates circulating cytokine concentrations during systemic inflammation in humans in vivo. Crit. Care 2023, 27, 117.
- Hawchar, F.; Rao, C.; Akil, A.; Mehta, Y.; Rugg, C.; Scheier, J.; Adamson, H.; Deliargyris, E.; Molnar, Z. The Potential Role of Extracorporeal Cytokine Removal in Hemodynamic Stabilization in Hyperinflammatory Shock. Biomedicines 2021, 9, 768.
- Rugg, C.; Klose, R.; Hornung, R.; Innerhofer, N.; Bachler, M.; Schmid, S.; Fries, D.; Strohle, M. Hemoadsorption with CytoSorb in Septic Shock Reduces Catecholamine Requirements and In-Hospital Mortality: A Single-Center Retrospective ‘Genetic’ Matched Analysis. Biomedicines 2020, 8, 539.
- Mehta, Y.; Singh, A.; Singh, A.; Gupta, A.; Bhan, A. Modulating the Inflammatory Response with Hemadsorption (CytoSorb) in Patients Undergoing Major Aortic Surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 673–675.
- Hawchar, F.; Laszlo, I.; Oveges, N.; Trasy, D.; Ondrik, Z.; Molnar, Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J. Crit. Care 2019, 49, 172–178.
- Akil, A.; Ziegeler, S.; Reichelt, J.; Rehers, S.; Abdalla, O.; Semik, M.; Fischer, S. Combined Use of CytoSorb and ECMO in Patients with Severe Pneumogenic Sepsis. Thorac. Cardiovasc. Surg. 2021, 69, 246–251.
- Friesecke, S.; Stecher, S.S.; Gross, S.; Felix, S.B.; Nierhaus, A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: A prospective single-center study. J. Artif. Organs 2017, 20, 252–259.
- Scharf, C.; Schroeder, I.; Paal, M.; Winkels, M.; Irlbeck, M.; Zoller, M.; Liebchen, U. Can the cytokine adsorber CytoSorb((R)) help to mitigate cytokine storm and reduce mortality in critically ill patients? A propensity score matching analysis. Ann. Intensive Care 2021, 11, 115.
- Hawchar, F.; Tomescu, D.; Trager, K.; Joskowiak, D.; Kogelmann, K.; Soukup, J.; Friesecke, S.; Jacob, D.; Gummert, J.; Faltlhauser, A.; et al. Hemoadsorption in the critically ill-Final results of the International CytoSorb Registry. PLoS ONE 2022, 17, e0274315.
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Melot, C.; Vincent, J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001, 286, 1754–1758.
- Sekino, M.; Murakami, Y.; Sato, S.; Shintani, R.; Kaneko, S.; Iwasaki, N.; Araki, H.; Ichinomiya, T.; Higashijima, U.; Hara, T. Modifications of peripheral perfusion in patients with vasopressor-dependent septic shock treated with polymyxin B-direct hemoperfusion. Sci. Rep. 2023, 13, 7295.
- Pan, J.; Peng, M.; Liao, C.; Hu, X.; Wang, A.; Li, X. Relative efficacy and safety of early lactate clearance-guided therapy resuscitation in patients with sepsis: A meta-analysis. Medicine 2019, 98, e14453.
- Schneider, A.G.; Andre, P.; Scheier, J.; Schmidt, M.; Ziervogel, H.; Buclin, T.; Kindgen-Milles, D. Pharmacokinetics of anti-infective agents during CytoSorb hemoadsorption. Sci. Rep. 2021, 11, 10493.




