Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Monica Neagu | -- | 1840 | 2023-11-28 09:35:51 | | | |
| 2 | Catherine Yang | Meta information modification | 1840 | 2023-11-28 09:42:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Neagu, M.; Grisi, F.; Pulvirenti, A.; Simón-Vázquez, R.; García-González, C.A.; Boccia, A.C. Aerogel Compounds. Encyclopedia. Available online: https://encyclopedia.pub/entry/52125 (accessed on 12 January 2026).
Neagu M, Grisi F, Pulvirenti A, Simón-Vázquez R, García-González CA, Boccia AC. Aerogel Compounds. Encyclopedia. Available at: https://encyclopedia.pub/entry/52125. Accessed January 12, 2026.
Neagu, Monica, Fabia Grisi, Alfio Pulvirenti, Rosana Simón-Vázquez, Carlos A. García-González, Antonella Caterina Boccia. "Aerogel Compounds" Encyclopedia, https://encyclopedia.pub/entry/52125 (accessed January 12, 2026).
Neagu, M., Grisi, F., Pulvirenti, A., Simón-Vázquez, R., García-González, C.A., & Boccia, A.C. (2023, November 28). Aerogel Compounds. In Encyclopedia. https://encyclopedia.pub/entry/52125
Neagu, Monica, et al. "Aerogel Compounds." Encyclopedia. Web. 28 November, 2023.
Copy Citation
Aerogels have started to be considered as “advanced materials”; therefore, as a general consideration, aerogels’ toxicity testing should focus on their functionality which resides in their nanoscale open internal porosity.
aerogel
safety regulation
biomedical application
1. Introduction
Aerogels are uncommon materials that stand by themselves as nanosized structures that have a high specific surface area, low density and high porosity. In recent years, aerogels technology has been used to develop innovative tools in the food industry by the microencapsulation of additives or in the health system to incorporate drugs [1][2][3][4]. The raised question is whether aerogels are nanomaterials or not? Under the REACH Annexes, aerogels are explicitly excluded [5][6] as a “nanoform”. In several countries (e.g., Belgium, the USA or Canada), aerogels do not need to be reported to the national nanomaterials product inventories [7]. According to the new definition of nanomaterials, which was published in 2022, if the intended constitute material has at least 50% of nanomaterial, it can be assessed as such. Therefore, one can assume that, although there are no nanoform per se, aerogels can be analyzed using the nanomaterials guidelines [8]. Consequently, the majority of the materials that can be framed as an aerogel have low toxicity, but an enhanced bioactivity that results from their large inner surface area, high surface reactivity and/or an increased dissolution; all these particularities should be taken into account when assessing aerogels [9].
2. Aerogels’ Particularities as Innovative Materials
This type of material is solid, with a very light weight and displaying a coherent open porous matrix of lightly packed, bonded particles or nanoscale fibers. Their structure is obtained from a gel that is subjected to the removal of the pore fluid without damaging its inherent structure. Due to these particular characteristics, aerogels have a very high specific surface area [10]. Moreover, this special group of materials has distinct properties like high porosity, low bulk density, very good textural properties and, just as important, tunable surface chemistry that endows them with exquisite applicability in various domains [11]. Therefore, the combination of low density and super-nanoporosity with pores of 2–50 nm in diameter was explored for silica aerogels in industrial applications like thermal insulation of building materials and even in aerospace technologies. There are already commercialized products such as insulating pipes/boards/blankets/translucent panels in industrial applications. As biomedical application aerogel technology is rapidly expanding due to the fact that it can provide a material that is health-acquiescent, it can be molded according to specific needs; it can have high yield, reproducibility and low toxicity, thus satisfying the biomedical requirements. Therefore, choosing the best biocompatible basic material can be subjected to specific design platforms to obtain an advanced material suitable for a specific biomedical application [12].
The best technology to provide very light weight, high specific surface area and coherent open porous matrix of the aerogel material is the supercritical fluid-based drying of gels, a technology for obtaining this innovative material [13]. As the supercritical drying process is the best technical option for aerogels, the design optimization of the technology is a subject of intense collaborative research and efforts [14][15].
Currently, this technology is based on the gentle drying technique that allows for the best preservation of the fragile solid network structure from which the material is generated [16]. Moreover, this robust workflow can be developed for producing aerogel-biomolecule-loaded materials to be used in future wound healing applications. The obtained aerogels have exquisite properties, like a narrow size distribution, high surface area, good porosity that can harbor drug encapsulation and can further sustain a controlled release of the drug. Additionally, this aerogel has a robust capacity to absorb wound exudate, transforming itself into a soft elastic hydrogel, prolonging the incorporated drug release for up to 72 h. The report shows that alginate-based aerogel capsules can both control drug release and manage wound exudate, in acute and chronic wounds. These alginate aerogels can be tailored as specific doses and drug-release kinetics in personalized medicine, to meet individual patients’ needs [16].
Other techniques that remove the pore’s moisture like ambient drying or freeze-drying can be used to obtain xerogels and cryogels. These technologies can lead to materials with comparable properties to aerogels. Therefore, xerogels and cryogels can have similarities with aerogels and this depends on the initial gel source, the actual solid content and the stability of the obtained 3D structure, due to the fact that mechanical properties are doubled by the morphology and the physicochemical properties [17]. When the applied technology is evaporative drying, solvent exchanges and sialylation steps are needed to circumvent the shrinkage of the fine porous structure. An outline of the main steps in obtaining xerogels, cryogels and aerogels is presented in Figure 1.
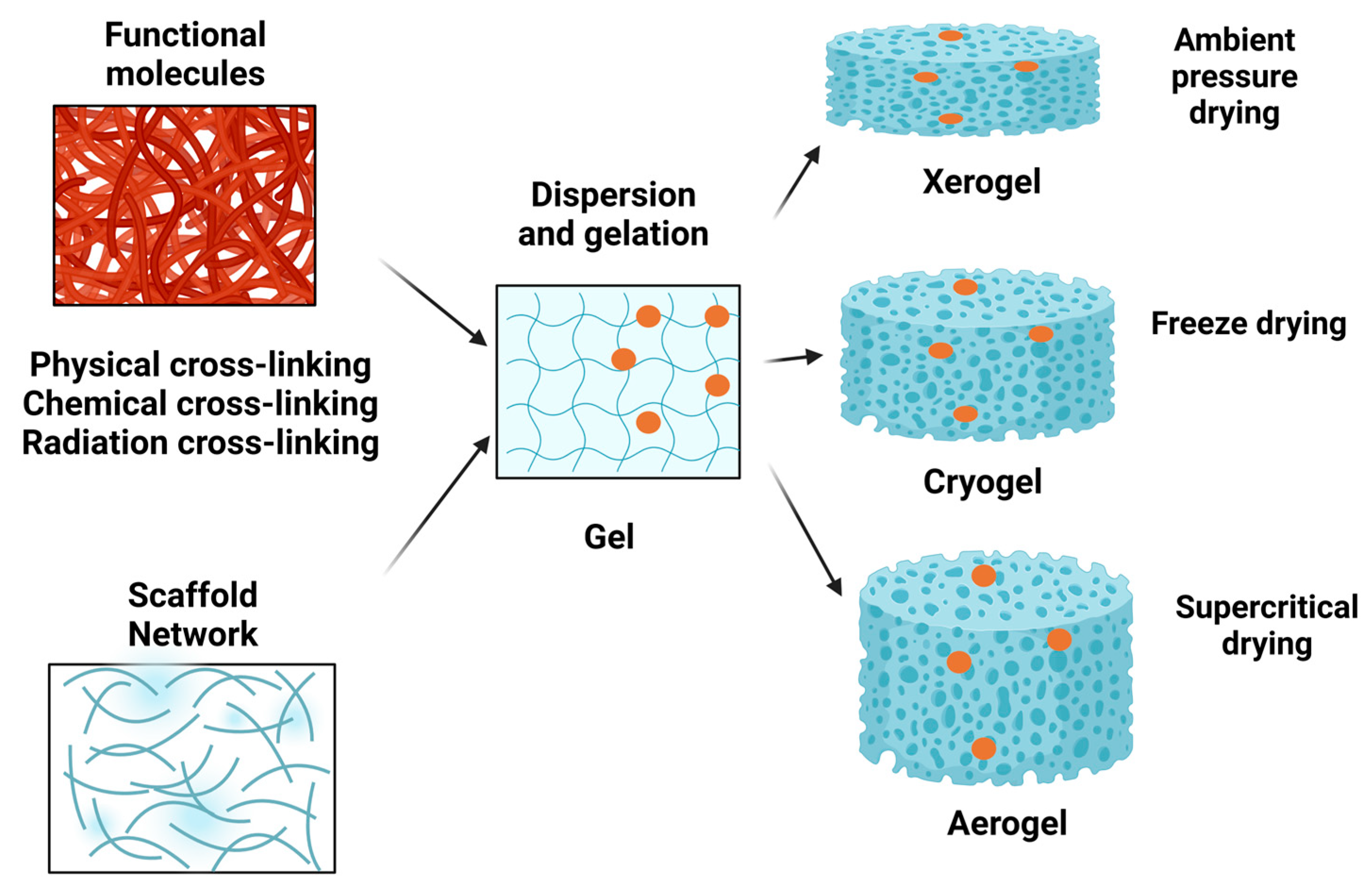
Figure 1. Main procedure steps for obtaining xerogels, cryogels and aerogels. Using several methods (physical, chemical, radiation) of cross-linking, various functional molecules can be embedded in a network structure upon dispersion and gelation. Afterwards, the gel can be subjected to ambient pressure drying for obtaining a xerogel, or to freeze-drying for obtaining a cryogel or to supercritical drying for obtaining an aerogel.
For biomedical applications, biopolymers are gaining constant interest due to several properties. Hence, they can originate from renewable resources that have a good economic impact, being highly bio-compatible and bio-degradable [3]. In this setting, the search has been focusing on abundant natural polymers, e.g., cellulose, starch or pectin. All these biopolymers can be transformed into gels and moreover into aerogels that can then become scaffolding materials harboring tissue-regenerating cells, they can be turned into artificial cartilage, they can be tailored as blood vessels, they can heal wounds and so many other biomedical applications. Moreover, in the environmental domain, aerogels can adsorb noble metals in the recycling process or can purify water from pollutants adsorbing heavy metals, oil, organic compounds and other pollutants [18][19].
From an ecological/toxicological point of view, the bio-polymer-based aerogels offer important advantages because no toxic compounds are involved in their preparation. Hence, bio-polymer aerogels are “biologically-friendly” and can be subjected to further testing for biomedical applications. Recently, new bio-based aerogels, like nanocellulose, proving specific self-assembling capabilities, can be the new players in the biomedicine applications domain [20]. Nanocellulose aerogels in fact use an abundant source as cellulose, and so it can represent the third-generation of innovative aerogel materials characterized by high porosity and large specific surface area, but also proving good bio-compatibility properties of the cellulose. Nanocellulose aerogels can be used in industrial applications (e.g., thermal insulation, electromagnetic interference shielding, purification, energy storage) but also in biomedical applications [21].
Apart from the nanocellulose-based aerogels, silica-based aerogels have high chemical versatility but lower mechanical properties so that their application does not comply with domains that need strength and stiffness. However, silica aerogels’ chemistry can be improved by carefully choosing silane precursors, by hybridization with poly-organo-siloxanes, organic polymers or fibers. With these improvements, their mechanical properties, namely strength and flexibility, silica aerogels can enlarge their applicability [22]. Natural fibers can reinforce the dried silica aerogel composites [23]. Another recent tendency is to mechanically reinforce silica-based aerogels with carbon nanotubes, which are carbon aerogels of graphene [24]. Silica-based aerogels and aerogels based on cellulose, polyurethane and so on can be endowed with additional useful properties, hierarchical porosity, super-flexibility and shape memory [25].
In the biomedical domain, aerogels can be drug-delivery matrices, they can be tailored to match various physiological administration routes like oral, pulmonary/nasal inhalation, topical administration and so on. Within these administration routes, aerogels should sustain drug-release kinetics, be both robust to resist the host’s defense mechanisms and flexible enough to modulate itself in a new biological environment. Therefore, aerogels can improve drug bioavailability and can be tailored to have a controlled drug delivery [26]. For these biomedical applications, aerogels can be designed to have pores from few microns to few millimeters in diameter [27]. As further elaborated in the other sections of this paper, aerogel particles, with their high porosity, can be carriers for pulmonary delivery having 20–25 µm diameters and penetrating in the lung’s alveoli [28].
Biopolymer-based aerogels have an as important advantage as carriers because they can have an improved dissolution rate of hydrophobic or poorly hydrophilic drugs. This is important as drugs can have good specificity to a target but low dissolution rate that overcomes their efficacy. Aerogels can be tailored to have a high drug pay load, can sustain the stability of drugs and any other properties specific for oral, topical, pulmonary or nasal administration routes [4][29][30]. Consequently, designing innovative aerogels increases the therapeutic efficiency because, for example, an oral inhalation administration drug will have an improved lung deposition, an enhanced drug permeation and an overall positive impact on the healthcare system and economics [31].
Another expanding domain of biomedical application of aerogels is the wound healing domain. The healing process is complex and it involves an acute inflammatory reaction that generates the final regeneration of the injured tissue. But when the processes of healing are dysfunctional, e.g., in diabetes, the healing process is stalled and the wound becomes chronic. Therefore, wound dressings are essential for the physical barrier that protects the wound milieu from environmental contaminants. Furthermore, aerogels can be designed to incorporate bio-active molecules for an increased rate of wound-healing events. Aerogels need to regulate the release of the bio-active compounds on site, in a controlled manner and providing targeted therapeutic effects. Moreover, the aerogel should prevent infections and may form a wet medium within the lesion, equilibrating the wound exudate and accelerating the healing process. For example, besides bio-active molecules, aerogels can be designed to incorporate sensors. In order to evaluate the protease activity within the lesion, the cellulose-based aerogel can be designed to have an incorporated sensor that senses the protease activity, this sensor evaluating wound pathology [32]. The healthcare market of wound dressing is expanding, so aerogels incorporated in advanced wound dressings [33] can impact the healthcare system in acute and chronic wounds from diabetic foot ulcer to pressure ulcer patients [34].
In the regenerative medicine domain, aerogel-based scaffolds can be tailored to mimic the extracellular matrix and specific bioactive molecules can be used to enhance regeneration and tissue integration [35][36]. Cellulose phosphate is a good cell scaffolding material because it enhances the growth of mesenchymal stem cells, it improves osteogenic differentiation, and does not display inflammatory responses [37]. An overview of aerogel applications in the biomedical field is presented in Figure 2 and Table 1.
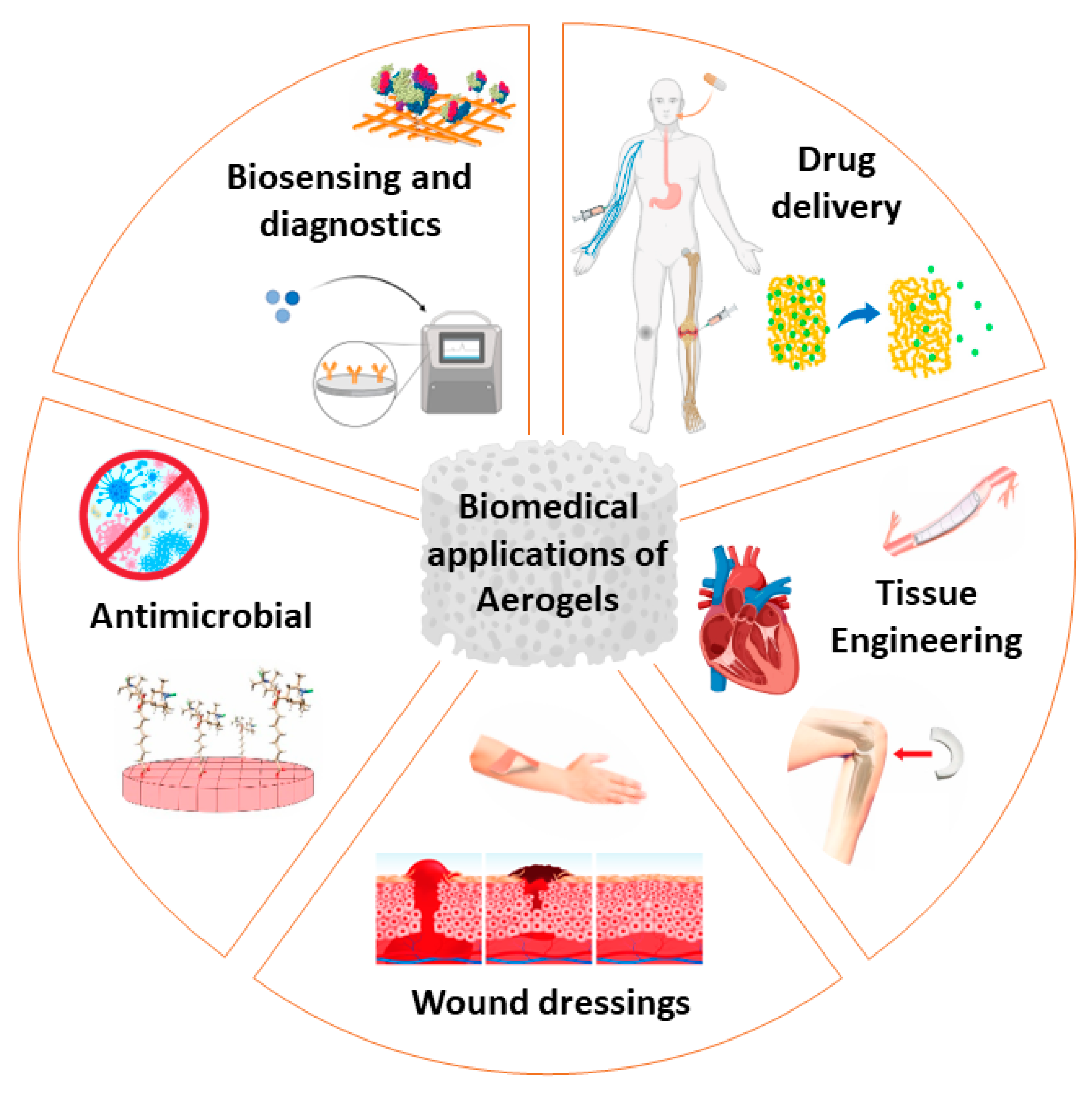
Figure 2. Aerogels’ biomedical applications as tissue-engineering scaffolds, wound-dressing materials, biosensing and diagnostics, drug-delivery matrices and antimicrobial materials.
Table 1. Aerogels in biomedical applications.
| Structure | Applications | References |
|---|---|---|
| Dialdehyde nanocellulose and collagen composite aerogels | Tissue engineering, wound dressing | [37] |
| Polyurea-nano-encapsulated macroporous silica aerogels, chitosan–silica hybrid aerogels | Tissue engineering, wound dressing | [38][39][40] |
| Alginate–starch aerogels | Tissue engineering, wound dressing | [16][41] |
| Alginate–lignin hybrid aerogels | Wound dressing | [35] |
| Ca-Zn-Ag alginate aerogels | Wound dressing | [42] |
| Macroporous silica aerogels | Biosensors | [43][44] |
| Cellulose nanofibrils | Biosensors and diagnostics | [4][43] |
| Silica aerogels | Biosensors and diagnostics | [3][4] |
| Polysaccharide aerogels | Food-related technologies | [4][44][45] |
| Alginate–chitosan aerogel | Drug delivery | [46] |
| Silica aerogels, surface-functionalized aerogels, composite aerogels | Drug delivery | [3][4][47] |
| Inorganic and organic aerogels | Drug delivery | [48] |
| Alginate-based aerogel | Drug delivery | [49] |
| Polysaccharide-based aerogels | Drug delivery | [3] |
| Alginate aerogel | Biocide | [50] |
Overall, aerogels’ particularities are to be used in the biomedical domain, bringing new medical solutions on one hand and reducing healthcare expenses on the other.
References
- Selmer, I.; Kleemann, C.; Kulozik, U.; Heinrich, S.; Smirnova, I. Development of egg white protein aerogels as new matrix material for microencapsulation in food. J. Supercrit. Fluids 2015, 106, 42–49.
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym 2015, 117, 797–806.
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438.
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel-based systems. J. Control. Release 2014, 177, 51–63.
- European Commission Recommendation on the Definition of Nanomaterial. Definition of Nanomaterial—European Observatory for Nanomaterials. 2011. Available online: europa.eu (accessed on 26 August 2023).
- Mech, A.; Wohlleben, W.; Ghanem, A.; Hodoroaba, V.D.; Weigel, S.; Babick, F.; Brüngel, R.; Friedrich, C.M.; Rasmussen, K.; Rauscher, H. Nano or Not Nano? A Structured Approach for Identifying Nanomaterials According to the European Commission’s Definition. Small 2020, 16, e2002228.
- Wigger, H.; Wohlleben, W.; Nowack, B. Redefining environmental nanomaterial lows: Consequences of the regulatory nanomaterial definition on the results of environmental exposure models. Environ. Sci. Nano 2018, 5, 1372–1385.
- Rauscher, H.; Kestens, V.; Rasmussen, K.; Linsinger, T.; Stefaniak, E.; European Commission; Joint Research Centre. Guidance on the Implementation of the Commission Recommendation 2022/C 229/01 on the Definition of Nanomaterial; Publications Office of the European Union: Luxembourg, 2023; ISSN 1831-9424. Available online: https://data.europa.eu/doi/10.2760/143118 (accessed on 23 August 2023).
- Steinhauser, K.G.; Sayre, P.G. Reliability of methods and data for regulatory assessment of nanomaterial risks. NanoImpact 2017, 7 (Suppl. C), 66–74.
- Liebner, F.; Pircher, N.; Schimper, C.; Haimer, E.; Rosenau, T. Aerogels: Cellulose-Based. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Mishra, M., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 2015.
- Durães, L.; Maleki, H.; Vareda, J.P.; Lamy-Mendes, A.; Portugal, A. Exploring the Versatile Surface Chemistry of Silica Aerogels for Multipurpose Application. MRS Adv. 2017, 2, 3511–3519.
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An opinion paper on aerogels for biomedical and environmental applications. Molecules 2019, 24, 1815–1830.
- Smirnova, I.; Gurikov, P. Aerogels in chemical engineering: Strategies toward tailor-made aerogels. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 307–334.
- Sahin, I.; Uzunlar, E.; Erkey, C. Investigation of kinetics of supercritical drying of alginate alcogel particles. J. Supercrit. Fluids 2019, 146, 78–88.
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A reconsideration on the definition of the term aerogel based on current drying trends. Microporous Mesoporous Mater. 2018, 258, 211–216.
- Sellitto, M.R.; Amante, C.; Aquino, R.P.; Russo, P.; Rodríguez-Dorado, R.; Neagu, M.; García-González, C.A.; Adami, R.; Del Gaudio, P. Hollow Particles Obtained by Prilling and Supercritical Drying as a Potential Conformable Dressing for Chronic Wounds. Gels 2023, 9, 492–505.
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306.
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121.
- Santos-Rosales, V.; Ardao, I.; Alvarez-Lorenzo, C.; Ribeiro, N.; Oliveira, L.A.; García-González, A.C. Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO2-Based Approach. Molecules 2019, 24, 871–888.
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631.
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569–2005603.
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74.
- Markevicius, G.; Ladj, R.; Niemeyer, P.; Budtova, T.; Rigacci, A. Ambient-dried thermal superinsulating monolithic silica-based aerogels with short cellulosic fibers. J. Mater. Sci. 2017, 52, 2210–2221.
- Lamy-Mendes, A.; Silva, R.F.; Durães, L. Advances in carbon nanostructure–silica aerogel composites: A review. J. Mater. Chem. A 2018, 6, 1340–1369.
- Ganesan, K.; Barowski, A.; Ratke, L.; Milow, B. Influence of hierarchical porous structures on the mechanical properties of cellulose aerogels. J. Sol-Gel Sci. Technol. 2019, 89, 156–165.
- García-González, C.A.; López-Iglesias, C.; Concheiro, A.; Alvarez-Lorenzo, C. Chapter 16, Biomedical Applications of Polysaccharide and Protein Based Aerogels. In Biobased Aerogels: Polysaccharide and Protein-Based Materials; The Royal Society of Chemistry: Cambridge, UK, 2018; pp. 295–323.
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144–2181.
- López-Iglesias, C.; Casielles, A.M.; Altay, A.; Bettini, R.; Alvarez-Lorenzo, C.; García-González, C.A. From the printer to the lungs: Inkjet-printed aerogel particles for pulmonary delivery. Chem. Eng. J. 2019, 357, 559–566.
- Sunargulov, T. Changes in the respiratory organs in experimental dust inhalation with silica aerogel. Arkhiv Patol. 1966, 28, 15–20.
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118.
- Tucker, G.; DeSilva, B.; Dressman, J.; Ito, M.; Kumamoto, T.; Mager, D.; Mahler, H.-C.; Maitland-van der Zee, A.H.; Pauletti, G.M.; Sasaki, H.; et al. Current Challenges and Potential Opportunities for the Pharmaceutical Sciences to Make Global Impact: An FIP Perspective. J. Pharm. Sci. 2016, 105, 2489–2497.
- Edwards, J.V.; Fontenot, K.R.; Liebner, F.; Condon, B.D. Peptide-Cellulose Conjugates on Cotton-Based Materials Have Protease Sensor/Sequestrant Activity. Sensors 2018, 18, 2334–2350.
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231.
- Ågren, M. Wound Healing Biomaterials–Volume 2: Functional Biomaterials; Elsevier Science: Cambridge, UK, 2016.
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8.
- García-González, C.A.; Concheiro, A.; Alvarez-Lorenzo, C. Processing of Materials for Regenerative Medicine Using Supercritical Fluid Technology. Bioconjug. Chem. 2015, 26, 1159–1171.
- Lu, T.H.; Li, Q.; Chen, W.S.; Yu, H.P. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138.
- Sabri, F.; Boughter, J.D., Jr.; Gerth, D.; Skalli, O.; Phung, T.C.; Tamula, G.R.; Leventis, N. Histological evaluation of the biocompatibility of polyurea crosslinked silica aerogel implants in a rat model: A pilot study. PLoS ONE 2012, 7, e50686.
- Yin, W.; Rubenstein, D. Biomedical applications of aerogels. In Aerogels Handbook; Advances in sol–gel derived materials and, technologies; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; pp. 683–694.
- Power, M.; Hosticka, B.; Black, E.; Daitch, C.; Norris, P. Aerogels as biosensors: Viral particle detection by bacteria immobilized on large pore aerogel. J. Non-Cryst. Solids 2001, 285, 303–308.
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159.
- Keil, C.; Hübner, C.; Richter, C.; Lier, S.; Barthel, L.; Meyer, V.; Subrahmanyam, R.; Gurikov, P.; Smirnova, I.; Haase, H. Ca-Zn-Ag alginate aerogels for wound healing applications: Swelling behavior in simulated human body fluids and effect on macrophages. Polymers 2020, 12, 2741–2758.
- Zhang, Y.; Nypelö, T.; Salas, C.; Arboleda, J.; Hoeger, I.C.; Rojas, O.J. Cellulose nanofibrils. J. Renew. Mater. 2013, 1, 195–211.
- Cumana, S.; Ardao, I.; Zeng, A.-P.; Smirnova, I. Glucose-6-phosphate dehydrogenase encapsulated in silica-based hydrogels for operation in a microreactor. Eng. Life Sci. 2014, 14, 170–179.
- Mikkonen, K.S.; Parikka, K.; Ghafar, A.; Tenkanen, M. Prospects of polysaccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013, 34, 124–136.
- Alnaief, M.; Obaidat, R.M.; Alsmadi, M.T.M. Preparation of Hybrid Alginate-Chitosan Aerogel as Potential Carriers for Pulmonary Drug Delivery. Polymers 2020, 12, 2223–2240.
- Smirnova, I.; García-González, C.A.; Gurikov, P. Pharmaceutical applications of aerogels. In Springer Handbook of Aerogels; Aegerter, M.A., Leventis, N., Koebel, M.M., Steiner III, S.A., Eds.; Springer: New York, NY, USA, 2023; pp. 1489–1504.
- Gaudio, P.D.; Auriemma, G.; Mencherini, T.; Porta, G.D.; Reverchon, E.; Aquino, R.P. Design of alginate-based aerogel for nonsteroidal anti-inflammatory drugs controlled delivery systems using prilling and supercritical-assisted drying. J. Pharm. Sci. 2013, 102, 185–194.
- Liebner, F.; Dunareanu, R.; Opietnik, M.; Haimer, E.; Wendland, M.; Werner, C.; Maitz, M.; Seib, P.; Neouze, M.-A.; Potthast, A.; et al. Shaped hemocompatible aerogels from cellulose phosphates: Preparation and properties. Holzforschung 2012, 66, 317–321.
- Wu, X.X.; Zhang, Y.; Hu, T.; Li, W.X.; Li, Z.L.; Hu, H.J.; Zhu, S.R.; Chen, W.Z.; Zhou, C.S.; Jiang, G.B. Long-term antibacterial composite via alginate aerogel sustained release of antibiotics and Cu used for bone tissue bacteria infection. Int. J. Biol. Macromol. 2021, 167, 1211–1220.
More
Information
Subjects:
Toxicology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
28 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




