Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Geza Bujdosó | -- | 4038 | 2023-11-28 09:35:00 | | | |
| 2 | Lindsay Dong | Meta information modification | 4038 | 2023-11-28 09:59:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Martín-Valmaseda, M.; Devin, S.R.; Ortuño-Hernández, G.; Pérez-Caselles, C.; Mahdavi, S.M.E.; Bujdoso, G.; Salazar, J.A.; Martínez-Gómez, P.; Alburquerque, N. CRISPR/Cas as Genome-Editing Technique in Fruit Tree Breeding. Encyclopedia. Available online: https://encyclopedia.pub/entry/52124 (accessed on 07 February 2026).
Martín-Valmaseda M, Devin SR, Ortuño-Hernández G, Pérez-Caselles C, Mahdavi SME, Bujdoso G, et al. CRISPR/Cas as Genome-Editing Technique in Fruit Tree Breeding. Encyclopedia. Available at: https://encyclopedia.pub/entry/52124. Accessed February 07, 2026.
Martín-Valmaseda, Marina, Sama Rahimi Devin, Germán Ortuño-Hernández, Cristian Pérez-Caselles, Sayyed Mohammad Ehsan Mahdavi, Geza Bujdoso, Juan Alfonso Salazar, Pedro Martínez-Gómez, Nuria Alburquerque. "CRISPR/Cas as Genome-Editing Technique in Fruit Tree Breeding" Encyclopedia, https://encyclopedia.pub/entry/52124 (accessed February 07, 2026).
Martín-Valmaseda, M., Devin, S.R., Ortuño-Hernández, G., Pérez-Caselles, C., Mahdavi, S.M.E., Bujdoso, G., Salazar, J.A., Martínez-Gómez, P., & Alburquerque, N. (2023, November 28). CRISPR/Cas as Genome-Editing Technique in Fruit Tree Breeding. In Encyclopedia. https://encyclopedia.pub/entry/52124
Martín-Valmaseda, Marina, et al. "CRISPR/Cas as Genome-Editing Technique in Fruit Tree Breeding." Encyclopedia. Web. 28 November, 2023.
Copy Citation
CRISPR (short for “Clustered Regularly Interspaced Short Palindromic Repeats”) is a technology that research scientists use to selectively modify the DNA of living organisms. CRISPR was adapted for use in the laboratory from the naturally occurring genome-editing systems found in bacteria.
plants
molecular biology
genomic
transgenic transformation
1. Introduction
Fruit trees are crops of great economic importance worldwide as they are vital components of our food production systems. Abiotic stresses such as salinity or drought, the monoculture of disease-susceptible cultivars, excessive use of pesticides, and the appearance of new pathogens cause significant economic losses in the production of various fruit species and are important threats to the environment and to sustainable food production [1]. Fruit trees play an integral role in the food and nutrition industries due to their invaluable primary and secondary metabolites [2].
On the other hand, perennial fruit trees suffer different biological and environmental challenges throughout their life. Specifically, fruit trees are infected by a wide range of pathogenic agents, including fungi, bacteria, and viruses, which can lead to significant economic losses if not properly addressed or managed [1]. Furthermore, in the current climate change scenario that we face, it is increasingly common for fruit trees to not experience enough cold during winter due to increasing temperatures, and are also affected by drought in some areas.
Therefore, there is great interest in obtaining improved fruit varieties with high nutritional quality and resistant to different stresses. Additionally, it becomes imperative to grasp the roles of stress-tolerance-related genes and their regulatory mechanisms for the purpose of developing more resilient varieties. Breeding of fruit crops using conventional means has been effectual in terms of both quality and yield characteristics, although this is a slow breeding method with random consequences due to extrinsic and intrinsic factors such as a long juvenile period, self-incompatibility, heterozygosity, long times for selection of the seedlings, and a lack of correlation between seedlings and mature plants [2]. Traditional breeding methods have been enriched by the inclusion of transgenesis, a valuable tool for plant breeding that enables the introduction or modification of specific and important traits in a single step [3], also allowing functional genomic studies.
Despite their advantages, transgenesis has its own limitations, including the random integration of transgenes into the genome and the fact that many fruit trees species are recalcitrant or time-consuming in their transformation. Therefore, it is paramount to enhance transgenic research and dedicate additional efforts to enhance the efficiency of the regeneration and transformation procedures in fruit trees [4]. Equally, in higher plants, achieving the insertion of DNA sequences at a precise genomic location using homologous recombination, known as gene targeting (GT), has remained challenging due to the notably low efficiency of homologous recombination [5]. One approach to enhancing HR-dependent gene targeting involves inducing double-strand breaks (DSBs) in the genomic DNA at the desired target site [6].
Among the new technologies developed in recent years, various site-specific nucleases (SSNs) have emerged, enabling the precise creation of double-strand breaks (DSBs) at specific locations within the genome. These SSNs offer a highly innovative approach to genome engineering, facilitating targeted modifications such as gene silencing, gene correction, and gene addition [4]. SSNs have significant economic, time-saving, and streamlined advantages relative to conventional breeding methods, which may take up to approximately a decade in order to develop a variety [7]. This methodology can be used to study the genes involved in traits such as drought tolerance, disease resistance, and higher quality and yield [8]. SSNs can be used for different purposes to modify the structure and function of host genome in agricultural crops, such as the targeted mutation, modification, insertion, replacing, stacking, and translational modulation of the desired genes [7].
The classification of SSN-based genome-editing systems is according to the following categories: meganucleases, zinc finger nucleases (ZFNs), transcription-like effector nucleases (TALENs), and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), which is associated with the RNA-guided Cas double-stranded DNA-binding protein (CRISPR/Cas system) [4][9]. The main differences among them are their relative specificity and efficiency [10]. ZFNs and TALENs are engineered nucleases and their mode of action is based on the protein–DNA interaction. However, the CRISPR/Cas system depends on RNA–DNA coupling [9]. Even though the use of the synthetic nucleases ZFNs and TALES has allowed the targeting of many genomic sites, the application of these techniques for the edition of plant genomes has been limited [11]. CRISPR is, at this moment, the main technology that research scientists use to selectively modify the DNA of living organisms. CRISPR was adapted for use in the laboratory from naturally occurring genome-editing systems found in bacteria.
Since its discovery, the most used genome-editing tool used in plant research and breeding is CRISPR, associated with protein 9 (CRISPR/Cas9), using a designed RNA-guided Cas9 endonuclease [12]. Specifically, CRISPR/Cas9 from Streptococcus pyogenes (SpyCas9) has been successfully used for genome editing in many plant species [13]. However, CRISPR/Cas9 has some inconveniences, such as the limitations of target specificity, activity, efficiency, and targeting scope [14]. These limitations have been overcome by engineering the basic Crispr/Cas9 system and the discovery of other Cas enzymes from various species, extending the range of genome-editing tools [15].
The emergence of CRISPR/Cas technology initiated a new perspective on Genetically Modified Organism (GMO) regulations. The generation of GMOs using transgenesis involves the insertion of foreign DNA into the genome of the plant, which is not allowed in many countries around the world. However, the targeted modification of a gene using CRISPR/Cas technology, producing mutations, is in many cases similar to the application of mutagenic agents that are legally acceptable.
2. Mechanism of CRISPR/Cas System
In nature, CRISPR/Cas systems provide prokaryotes with an RNA-guided adaptive immunity against bacteriophages and plasmids [16]. These systems are encoded by the CRISPR array and the accompanying CRISPR-associated (Cas) genes. The CRISPR array contains two types of sequences, palindromic repeats and “spacer” sequences that are derived from a viral or plasmid genome. On the other hand, Cas genes codify different proteins involved in the process [17].
The adaptative immune response consists of three stages: adaptation, expression and maturation, and interference [18]. At the adaptation stage, Cas proteins recognize foreign genetic elements (protospacers) and insert them in between the repeats of the CRISPR array, forming new spacers. The expression and maturation stage consists of the transcription of the CRISPR array into pre-crRNA that is further processed, forming smaller mature crRNAs, one for each spacer. Then, the crRNA forms a complex with Cas proteins, and in some cases with the tracrRNA (transactivating crRNA) [19], which leads to the interference stage. The complex makes differentiations by base-pairing foreign nucleic acids that are complementary to crRNA sequences. In addition, a specific motif called a PAM (protospacer adjacent motif) is necessary for the stable binding to the target DNA, and it is crucial for the discrimination between self and non-self sequences [17].
There is a wide diversity of CRISPR/Cas systems that differ in the Cas protein sequences, gene compositions, and architecture of the genomic loci. According to Makarova et al. [20], CRISPR/Cas systems can be classified into 2 different classes, 6 types, and 33 subtypes, even though this classification is constantly evolving as new systems are being discovered. Class 1 and 2 differ in the number of Cas proteins involved in crRNA processing and interference, class 2 systems being simpler, as they only require one multidomain crRNA-binding protein.
Among class 2 systems, CRISPR/Cas9 and CRISPR/Cas12 are the most used for genome-engineering technologies because of their properties. Both are capable of cleaving dsDNA with just one Cas protein (Cas9/Cas12) being necessary for the recognition and cleavage of the DNA [21]; however, there are some differences in their mechanisms. In both systems, the Cas endonuclease assembles with the crRNA, which binds the target dsDNA by complementarity. For the CRISPR/Cas9 system, tracrRNA is also needed for the processing of crRNA and the interaction between Cas9 and the crRNA. This system has been engineered to create an RNA chimera (sgRNA) that acts as the crRNA and tracrRNA [22]. On the other hand, the CRISPR/Cas12 system does not need the tracrRNA, as it can process its own crRNA [21]. The PAM sequence is also different for each Cas protein. While for Cas9, the PAM sequence is NGG (being N any nucleobase), and it is located in the 3′ end, for Cas12, it is TTTV (V = A, C, and G), and it is located in the 5′ end [23]. Once they have recognized their corresponding PAM sequence, the nuclease activity of the Cas proteins is activated, leading to the dsDNA cleavage which produces a double-strand break (DSB) [24]. Cas9 generates blunt ends at the 3′ while Cas12 creates staggered ends at the 5′ [23]. In both cases, the DSB can trigger two endogenous DNA repair mechanisms: homology-directed repair (HDR) or non-homologous end joining (NHEJ) (Figure 1), both being interesting for genome-engineering applications [24]. HDR occurs if there is a homologous template, useful for changing or replacing sequences [25]. If there is no homologous template, NHEJ is triggered. This mechanism generates small insertions and deletions (indels) in order to ligate the broken ends as fast as possible, leading to the knockout of the gene [25] (Figure 1).
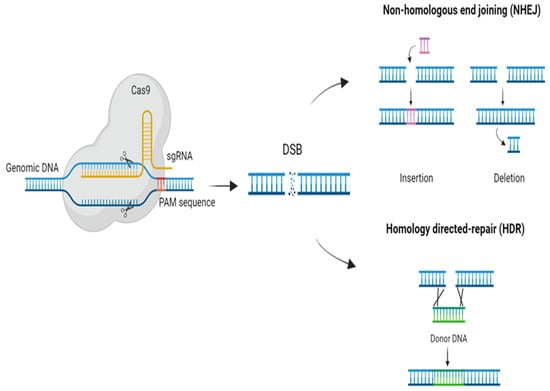
Figure 1. Gene-editing mechanism of CRISP/Cas9. A single RNA chimera (sgRNA) drives the complex CRISPR/Cas9 to the target DNA and the protospacer adjacent motif (PAM) enables the stable binding. Cas9 produces a double-strand break (DSB) that can trigger two endogenous DNA repair mechanisms: homology-directed repair (HDR) or non-homologous end joining (NHEJ).
3. Genetic Transformation Technology in Fruit Trees
Transformation of several fruit trees has been carried out for many traits and has been improved for a successful genetic transformation so far. Transgenic technology is increasingly used in fruit species to overcome the disadvantages of conventional traditional breeding methods and for gene function research [26]. However, there are several limitations in the transformation of fruit trees. Most of fruit trees are recalcitrant to regeneration and/or transformation, the processes are genotype-dependent, the process is time-consuming compared to for other species, and accurate selection with antibiotics or herbicides is necessary to avoid chimeric plants [4]. Furthermore, the lack of available and efficient explants for regeneration and transformation procedures (e.g., seedlings, leaves from micropropagated plants, or immature seeds) makes difficult the establishment of effective protocols [27].
Fruit quality improvement and biotic and abiotic tolerance/resistance have been achieved in fruit scion cultivars using direct transformation, but the use of genetically modified rootstocks to confer new characteristics to the non-transformed scion via transgrafting shows a potential improvement of fruit tree species, in particular those recalcitrant to transformation, and could mitigate public concerns about transgene dispersions or transgenic fruit consumption [27][28].
Although grapevine (Vitis vinifera) is considered a recalcitrant specie for transformation [29], over the past few years, numerous works have reported the successful transformation of various grape rootstocks and cultivars using Agrobacterium-mediated and or biolistic bombardment techniques [30]. These transformations have involved a range of target genes, such as genes involved in resistance and tolerance against diseases, pests, and abiotic stresses, as well as enhancing fruit quality [30][31].
In the same way as with grapevine, genetic transformation using Agrobacterium tumefaciens is the most used method to obtain transgenic apple (Malus domestica) plants [32]. The genetic modification of apples has been feasible since 1989 [33], and in the following years, most studies were focused on increasing the transformation efficiency. Agrobacterium-mediated transformation has become a conventional tool for functional genome studies on apples using overexpression or RNAi-based gene silencing [32].
Agrobacterium-mediated transformation of citrus was initially reported by Moore et al. [34] using internodal stem segments as explants, followed by the regeneration of shoots. Extensive research has resulted in the development of improved Agrobacterium protocols for the genetic modification of citrus plants [35][36]. Due to the difficulties of conventional citrus breeding (a complex reproductive biology, juvenility, a high heterozygosity level), genetic transformation has been considered as a possible alternative strategy for citrus improvement [37]. Modified plants from different citrus species have been generated with resistance to diseases such as huanglongbing and citrus canker caused by bacteria [38][39][40] and tristeza disease caused by Citrus tristeza virus [41], as have plants tolerant to different environmental stresses [40].
Although in most woody fruit species and especially in Prunus species, transformation and regeneration are frequently limited to a few genotypes [42], among Prunus, the European plum (Prunus domestica L.) is the species most frequently transformed [43]. However, Japanese plum transformation has been reported with low efficiency [44]. In the first works, several marker genes were introduced into the plum genome [45][46].
Sharka disease caused by the plum pox virus (PPV) is the most important disease of stone fruit, and the establishment of new cultivars resistant to sharka is one of the most focused topics in European plum breeding programs [47]. Among the different transgenic strategies used to achieve PPV resistance, successful results have been obtained via applications of RNA silencing techniques [48]. The first PPV-resistant transgenic Prunus was the plum C5 or “Honeysweet”, which was obtained via the Agrobacterium-mediated transformation of plum hypocotyl slices using a binary plasmid carrying the PPV-CP full-length gene [49]. The resistance of “Honeysweet” was due to the post-transcriptional gene silencing (PTGS) of the coat protein (CP) virus gene [50]. “HoneySweet” is freely available for fruit production in the United States and for use as a source of PPV resistance for developing new PPV-resistant plum cultivars worldwide, pending regulatory approval [51].
Engineered plum lines were produced via the RNA-interference-mediated silencing of the A. tumefaciens oncogenes ipt and iaaM to study the possibility of generating plum transgenic rootstocks resistant to crown gall disease. Several lines were infected with Agrobacterium strains in the greenhouse, showing a significant reduction in the development of the disease [52].
The use of transgenic Prunus rootstocks resistant to salinity and/or drought could improve productivity in arid and semi-arid regions affected by environmental stresses. Transgenic European plum lines tolerant to salt stress were obtained by overexpressing cytosolic superoxide dismutase (SOD) from spinach and/or cytosolic ascorbate peroxidase (APX) from peas [53][54]. Modulation of the enzymatic antioxidants and enhancement of non-enzymatic antioxidants like glutathione and ascorbate are responsible for the stress tolerance [54].
The European plum has also been transformed with the FLOWERING LOCUS T1 (FT1) gene from Populus trichocarpa, and transgenic plants that expressed high levels of FT1 flowered and produced fruits in the greenhouse within 1 to 10 months [55]. FT plums showed the ability to continuously produce flowers and fruit regardless of the day’s length or chilling time and survived winter temperatures. For these reasons, FT plums are used in crosses at the USDA ARS facility (Kearneysville, WV, USA) in what has been called “FasTrack” breeding [56]. The “FasTrack” system has allowed minimizing the generation cycle of plum plants from 3–7 years to one year round; it can be used under greenhouse conditions and the system allows the fast incorporation of important traits into plums.
Apricot (Prunus armeniaca L.) is a very recalcitrant species with important limitations in regeneration and transformation from explants of juvenile or mature origin. There are several works reporting the production of transgenic apricot plants expressing the marker genes gfp or uidA and nptII [57][58][59].
Although the main goal of transgenic research has been the generation of plants resistant to diseases [60], until now, there have been very few studies indicating the production of transgenic apricot lines with modified target genes for breeding objectives. To this end, Laimer da Câmara Machado et al. [61] produced some transgenic apricot lines with the CP of PPV that showed resistance to viral infection.
The polyethylene glycol (PEG)-mediated delivery method has also been employed in fruit tree genome-editing systems since it is especially useful for these species where the production of transgenic plants is very slow [62]. This CRISPR/Cas delivery method has been proposed as a good strategy to produce transgene-free edited plants by delivering ribonucleoprotein [63].
4. CRISPR/Cas-Mediated Gene Knock-In and Knock-Out in Fruit Trees
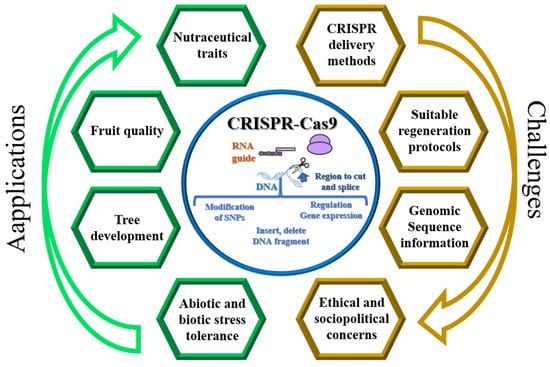
CRISPR/Cas has been applied to activate and knock out target genes in different fruit tree species, including related to tree development, yield, fruit quality, and tolerance to biotic and abiotic stresses, trying to answer to different challenges (Figure 2).

Figure 2. Overview of the applications and challenges of the CRISPR-Cas9 gene-editing technique in fruit trees.
4.1. Tree Growth and Development
The development and growth of the plant is a crucial factor that will determine the size, density, and ultimately the productivity of a plant. To enhance the planting density and subsequently increase productivity while improving nutrient and water use efficiency, the cultivation of dwarf fruit trees has become a prominent strategy [64][65]. Nevertheless, adjusting the plant height in these dwarf crops poses challenges that have led to investigations centered on phytohormones and genetic manipulation.
Phytohormones play pivotal roles in plant growth and architecture [66]. Among these, gibberellins (GAs) are recognized for their ability to stimulate plant elongation [2]. The disruption of genes involved in GA biosynthesis can result in dwarfed plant structures [67]. The MaGA20ox2 gene is involved in gibberellic acid biosynthesis and plant height in the Gros Michel banana cultivar, and CRISPR/Cas9 technology has been successfully used to modify the MaGA20ox2 gene and generate semi-dwarf mutants [68].
In the cytokinin context, Feng et al. [69] observed changes in gene expression associated with the cytokinin metabolic pathway and trans-zeatin concentration in apple rootstocks, distinguishing between vigorous and dwarf variants. They identified decreased expression of the IPT5b gene, characterized by high methylation levels in the promoter region, leading to impaired trans-zeatin synthesis and potentially causing dwarfism.
Estrigolactone (SL) is a recently identified plant hormone that plays a pivotal role in branching inhibition in plants. Two key genes involved in SL biosynthesis, CCD7 and CCD8, have been investigated in grapevine. CRISPR/Cas9 technology was employed, specifically for editing the VvCCD7 and VvCCD8 genes [70]. As a result of these genetic edits, it was observed that the ccd8 mutant exhibited a higher number of branches compared to wild-type plants, highlighting the significance of the VvCCD8 gene in grapevine branching regulation [71].
4.2. Early Flowering
The extended juvenile phase observed in many fruit trees leads to a prolonged non-flowering period, which can span from 3 to 15 years, depending on the specific fruit tree [72]. Elevated levels of terminal flowering protein (TFL) are typically associated with this youthful stage. TFL acts as a negative regulator of flowering by inhibiting the expression of several flowering-stimulating proteins, including FLOWERING LOCUS T (FT), LEAFY (LFY), and APETALA1 (AP1) [73].
To address this challenge, ref. [74] utilized CRISPR/Cas9 technology to edit the apple TFL1 gene. They employed two different sgRNAs to target the TFL1 gene, and they also used the same construct to edit the pear TFL1. It was observed that early flowering in transgenic pear lines (9%) and transgenic apple lines (93%) targeted the PcTFL1.1 and MdTFL1.1 genes, respectively.
4.3. Fruit Growth and Development
CRISPR/Cas holds significant potential for fine-tuning valuable quantitative traits in crop improvement, such as fruit size [75]. Nevertheless, it is crucial to emphasize that the manipulation of specific genes can lead to a wide range of effects. For instance, in the case of the SlKLUH gene, its copy number has been observed to positively correlate with fruit weight. Nonetheless, the deletion or reduction of SlKLUH often results in smaller fruits and can lead to other growth defects, such as smaller inflorescences and sterile flowers [76].
4.4. Shelf-Life and Fruit Ripening
One crucial aspect of post-harvest fruit quality revolves around its shelf-life, in which ethylene plays a pivotal role in both ripening and fruit softening. The fruit shelf-life can be extended by controlling ethylene biosynthesis and signal transduction, as highlighted in recent studies conducted on apricots and plums using the application of ethylene-related chemicals, either by inhibiting or increasing ethylene production [77].
Fruits with a prolonged shelf-life can be achieved by modifying the methylation patterns or by silencing key genes involved in ethylene biosynthesis, ripening processes, or their signaling pathways in fruit crops.
To illustrate, the CRISPR/Cas9 gene-editing technique was employed to eliminate MaACO1 (aminocyclopropane-1-carboxylate oxidase 1), the gene responsible for encoding the enzyme that converts ACC into ethylene. This genetic modification resulted in an extended fruit shelf-life period of up to 40 days when compared to wild-type bananas [78].
4.5. Fruit Color, Flavor, and Bioactive Compounds
Genomic editing of key genes offers the potential to design fruit crops with elevated levels of functional metabolites and pigments, which can have a significant impact on enhancing the quality and nutritional characteristics of fruits. Red fruits, rich in various bioactive components and nutrients such as antioxidants, minerals, vitamins, and dietary fiber, have been the subject of research [79]. In this context, CRISPR/Cas9 technology has demonstrated its ability to modulate specific traits in fruits.
Furthermore, plants can produce various secondary metabolites that play a significant role in growth control, component regeneration, and nutrient enhancement. Carotenoids, for instance, participate in processes like photosynthesis and have antioxidant functions, contributing to attractive colors in fruits and other plant organs [80]. Phytoene desaturase (PDS) has been identified as the rate-limiting enzyme in the carotenoid synthesis pathway [71].
4.6. Improving Stress Tolerance in Fruit Trees
Abiotic and biotic stresses, such as drought, extreme temperatures, pests, and diseases, pose substantial threats to global food production. These challenges can result in reduced crop yields, lower crop quality, and economic losses for farmers. To mitigate these issues, the advent of gene-editing systems has opened up promising avenues for agriculture. Gene-editing techniques like CRISPR/Cas9 enable precise modifications to an organism’s DNA, allowing researchers and breeders to target specific genes associated with stress resistance or desired traits [81].
Nowadays, CRISPR/Cas technology has been harnessed to confer tolerance to various environmental stresses, as demonstrated in recent studies [82]. Within this context, it has been observed that the Dehydration-Responsive Element-Binding (DREB) transcription factor (TF) plays a pivotal role in the regulation of several stress-inducible genes. It has been substantiated that DREB2-type proteins, a subtype of DREB proteins, play a significant role in enhancing drought, salinity, and heat tolerance in a variety of plants, including fruit-bearing trees [83].
CRISPR/Cas9 has evolved into an effective tool for introducing robust transcriptional regulatory elements into the promoter region of genes, governing the expression of stress-responsive genes. Consequently, this augmentation enhances their expression, thereby fortifying plant stress resilience.
The involvement of the Calcium-Dependent Protein Kinase (CDPK) in response to environmental stressors has been extensively documented by Wang and their team [84]. Overexpression of the apple CDPK gene, referred to as MdCIPK6L, has substantially heightened resistance to saline, osmotic, drought, and cold stresses without compromising root growth [85].
Editing genes that negatively regulate plant immunity is a strategy for obtaining disease-resistant crops. A notable example of this is the elimination of the NPR3 gene, a suppressor of defense responses, which has enhanced the resistance of cacao leaves to the Phytophthora tropicalis pathogen [86].
5. Off-Target Issues
One of the main advantages of CRISPR-Cas systems is their specificity; however, some off-target editing events can occur, leading to undesired modifications. Cas nuclease activity can be triggered even if there is an imperfect complementarity between sgRNA and the off-target genomic site [87], mainly when the mismatches are located far from the PAM sequence [88].
The presence of off-target mutation can be analyzed by sequencing potential off-target sites with a variable number of mismatches. Although it is important to explore off-target effects, there are few studies in fruit trees that address this aspect. In those where it has been carried out, it does not seem to be a critical factor. Thus, in sweet orange, no off-target mutations at potential sites were detected [23][89].
In order to decrease off-target modifications if they are a problem, some strategies can be followed. First, it is important to design highly specific gRNAs. For this, it is possible to use truncated sgRNAs (tru-gRNAs) that are formed of sequences shorter than 20 nucleotides, which reduces the likelihood of complementarity with mismatches while maintaining on-target efficiency [90]. In A. thaliana, tru-gRNAs have been used, resulting in no off-target modifications [91].
6. Conclusions
Successful genome-editing studies on fruit trees show that CRISPR/Cas can induce changes in target genes. In the future, the application of gene editing could allow the development of new generations of fruit crops with improved traits by targeting different genetic segments or even could facilitate the introduction of traits into elite cultivars without changing other traits. Although the use of genome-editing techniques promises a quick, easy, and inexpensive way to develop novel crop varieties with improved traits compared to before, in the case of fruit crops, the scarcity of efficient regeneration and transformation protocols in some species, the fact that many of these procedures are genotype-dependent, their polyploidy, and the convenience of segregating the transgenic parts of the CRISPR system are some of the problems that limit the potential of genetic editing techniques for fruit trees.
References
- Khan, A.; Korban, S.S. Breeding and genetics of disease resistance in temperate fruit trees: Challenges and new opportunities. Theor. Appl. Genet. 2022, 135, 3961–3985.
- Savadi, S.; Mangalassery, S.; Sandesh, M.S. Advances in genomics and genome editing for breeding next generation of fruit and nut crops. Genomics 2021, 113, 3718–3734.
- Scorza, R.; Callahan, A.; Dardick, C.; Ravelonandro, M.; Polak, J.; Malinowski, T.; Zagrai, I.; Cambra, M.; Kamenova, I. Genetic engineering of Plum pox virus resistance: ‘HoneySweet’plum—From concept to product. Plant Cell Tissue Organ Cult. 2013, 115, 1–12.
- Alburquerque, N.; Baldacci-Cresp, F.; Baucher, M.; Casacuberta, J.M.; Collonnier, C.; El Jaziri, M.; Nogué, F.; Burgos, L. New transformation technologies for trees. In Biosafety of Forest Transgenic Trees: Improving the Scientific Basis for Safe Tree Development and Implementation of EU Policy Directives; Vettori, C., Gallardo, F., Kazana, V., Häggman, H., Migliacci, F., Pilate, G., Fladung, M., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 31–66.
- Hanin, M.; Paszkowski, J. Plant genome modification by homologous recombination. Curr. Opin. Plant Biol. 2003, 6, 157–162.
- Wehrkamp-Richter, S.; Degroote, F.; Laffaire, J.B.; Paul, W.; Perez, P.; Picard, G. Characterisation of a new reporter system allowing high throughput in planta screening for recombination events before and after controlled DNA double strand break induction. Plant Physiol. Biochem. 2009, 47, 248–255.
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697.
- Puchta, H.; Fauser, F. Synthetic nucleases for genome engineering in plants: Prospects for a bright future. Plant J. 2014, 78, 727–741.
- Abdallah, N.A.; Prakash, C.S.; McHughen, A.G. Genome editing for crop improvement: Challenges and opportunities. GM Crops Food 2015, 6, 183–205.
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405.
- Puchta, H. Applying CRISPR/Cas for genome engineering in plants: The best is yet to come. Curr. Opin. Plant Biol. 2017, 36, 1–8.
- Wada, N.; Osakabe, K.; Osakabe, Y. Expanding the plant genome editing toolbox with recently developed CRISPR–Cas systems. Plant Physiol. 2022, 188, 1825–1837.
- Wada, N.; Ueta, R.; Osakabe, Y.; Osakabe, K. Precision genome editing in plants: State-of-the-art in CRISPR/Cas9-based genome engineering. BMC Plant Biol. 2020, 20, 234.
- Denes, C.E.; Cole, A.J.; Aksoy, Y.A.; Li, G.; Neely, G.G.; Hesselson, D. Approaches to enhance precise crispr/cas9-mediated genome editing. Int. J. Mol. Sci. 2021, 22, 8571.
- Zhang, D.; Zhang, Z.; Unver, T.; Zhang, B. CRISPR/Cas: A powerful tool for gene function study and crop improvement. J. Adv. Res. 2021, 29, 207–221.
- Wang, J.Y.; Pausch, P.; Doudna, J.A. Structural biology of CRISPR–Cas immunity and genome editing enzymes. Nat. Rev. Microbiol. 2022, 20, 641–656.
- Jiang, F.; Doudna, J.A. CRISPR-Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529.
- Amitai, G.; Sorek, R. CRISPR-Cas adaptation: Insights into the mechanism of action. Nat. Rev. Microbiol. 2016, 14, 67–76.
- Liao, C.; Beisel, C.L. The tracrRNA in CRISPR Biology and Technologies. Annu. Rev. Genet. 2021, 55, 161–181.
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83.
- Knott, G.J.; Doudna, J.A. CRISPR-Cas guides the future of genetic engineering. Science 2018, 361, 866–869.
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821.
- Jia, H.; Orbović, V.; Wang, N. CRISPR-LbCas12a-mediated modification of citrus. Plant Biotechnol. J. 2019, 17, 1928–1937.
- Hryhorowicz, M.; Lipiński, D.; Zeyland, J.; Słomski, R. CRISPR/Cas9 Immune System as a Tool for Genome Engineering. Arch. Immunol. Ther. Exp. 2017, 65, 233–240.
- Yin, K.; Gao, C.; Qiu, J.L. Progress and prospects in plant genome editing. Nat. Plants 2017, 3, 17107.
- Yin, Y.; Wang, C.; Xiao, D.; Liang, Y.; Wang, Y. Advances and Perspectives of Transgenic Technology and Biotechnological Application in Forest Trees. Front. Plant Sci. 2021, 12, 786328.
- Song, G.Q.; Prieto, H.; Orbovic, V. Agrobacterium-mediated transformation of tree fruit crops: Methods, progress, and challenges. Front. Plant Sci. 2019, 10, 226.
- Alburquerque, N.; Pérez-Caselles, C.; Faize, L.; Ilardi, V.; Burgos, L. Effective transfer of plum pox virus resistance from transgenic plum rootstocks to apricot scions. Authorea 2023, 14, 1216217.
- Orbovic, V.; Prieto, H. Editorial: New developments in Agrobacterium Mediated Transformation of tree fruit crops, volume II. Front. Plant Sci. 2023, 14, 1249563.
- Zhang, X.; Wu, Y.; Li, Z.; Song, C.; Wang, X. Advancements in plant regeneration and genetic transformation of grapevine (Vitis spp.). J. Integr. Agric. 2021, 20, 1407–1434.
- Campos, G.; Chialva, C.; Miras, S.; Lijavetzky, D. New Technologies and Strategies for Grapevine Breeding through Genetic Transformation. Front. Plant Sci. 2021, 12, 767522.
- Schröpfer, S.; Lempe, J.; Emeriewen, O.F.; Flachowsky, H. Recent Developments and Strategies for the Application of Agrobacterium-Mediated Transformation of Apple Malus × domestica Borkh. Front. Plant Sci. 2022, 13, 928292.
- James, D.J.; Passey, A.J.; Barbara, D.J.; Bevan, M. Genetic transformation of apple (Malus pumila Mill.) using a disarmed Ti-binary vector. Plant Cell Rep. 1989, 7, 658–661.
- Moore, G.A.; Jacono, C.C.; Neidigh, J.L.; Lawrence, S.D.; Cline, K. Agrobacterium-mediated transformation of citrus stem segments and Regeneration of Transgenic Plants. Plant Cell. Rep. 1992, 11, 238–242.
- Cervera, M.; Juárez, J.; Navarro, A.; Pina, J.A.; Durán-Vila, N.; Navarro, L.; Peña, L. Genetic transformation and regeneration of mature tissues of woody fruit plants bypassing the juvenile stage. Transgenic Res. 1998, 7, 51–59.
- Dutt, M.; Grosser, J.W. Evaluation of parameters affecting Agrobacterium-mediated transformation of citrus. Plant Cell Tissue Organ Cult. 2009, 98, 331–340.
- Peña, L.; Navarro, L., IV. Transgenic citrus. In Biotechnology in Agriculture and Forestry, Vol. 44. Transgenic Trees; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 39–54.
- Alquézar, B.; Carmona, L.; Bennici, S.; Peña, L. Engineering of citrus to obtain huanglongbing resistance. Curr. Opin. Biotechnol. 2021, 70, 196–203.
- Soares, J.M.; Tanwir, S.E.; Grosser, J.W.; Dutt, M. Development of genetically modified citrus plants for the control of citrus canker and huanglongbing. Trop. Plant Pathol. 2020, 45, 237–250.
- Nehela, Y.; Killiny, N. The unknown soldier in citrus plants: Polyamines-based defensive mechanisms against biotic and abiotic stresses and their relationship with other stress-associated metabolites. Plant Signal. Behav. 2020, 15, 1761080.
- Cervera, M.; Esteban, O.; Gil, M.; Gorris, M.T.; Martínez, M.C.; Peña, L.; Cambra, M. Transgenic expression in citrus of single-chain antibody fragments specific to Citrus tristeza virus confers virus resistance. Transgenic Res. 2010, 19, 1001–1015.
- Petri, C.; Burgos, L. Transformation of fruit trees. Useful breeding tool or continued future prospect? Transgenic Res. 2005, 14, 15–26.
- Petri, C.; Alburquerque, N.; Faize, M.; Scorza, R.; Dardick, C. Current achievements and future directions in genetic engineering of European plum (Prunus domestica L.). Transgenic Res. 2018, 27, 225–240.
- Urtubia, C.; Devia, J.; Castro, A.; Zamora, P.; Aguirre, C.; Tapia, E.; Barba, P.; Dell’Orto, P.; Moynihan, M.R.; Petri, C.; et al. Agrobacterium-mediated genetic transformation of Prunus salicina. Plant Cell Rep. 2008, 27, 1333–1340.
- Gonzalez-Padilla, I.M.; Webb, K.; Scorza, R. Early antibiotic selection and efficient rooting and acclimatization improve the production of transgenic plum plants (Prunus domestica L.). Plant Cell Rep. 2003, 22, 38–45.
- Tian, L.; Canli, F.A.; Wang, X.; Sibbald, S. Genetic transformation of Prunus domestica L. using the hpt gene coding for hygromycin resistance as the selectable marker. Sci. Hortic. 2009, 119, 339–343.
- Petri, C.; Ruiz, D.; Faize, M.; Burgos, L.; Alburquerque, N. Prunus domestica Plum. In Biotechnology of Fruit and Nut Crops; Litz, R.E., Pliego-Alfaro, F., Hormaza, J.I., Eds.; CABI: High Springs, FL, USA, 2020; pp. 512–531.
- Ilardi, V.; Tavazza, M. Biotechnological strategies and tools for Plum pox virus resistance: Trans-, intra-, cis-genesis, and beyond. Front. Plant Sci. 2015, 6, 379.
- Scorza, R.; Ravelonandro, M.; Callahan, A.M.; Cordts, J.M.; Fuchs, M.; Dunez, J.; Gonsalves, D. Transgenic plums (Prunus domestica L.) express the plum pox virus coat protein gene. Plant Cell Rep. 1994, 14, 18–22.
- Scorza, R.; Callahan, A.; Levy, L.; Damsteegt, V.; Webb, K.; Ravelonandro, M. Post-transcriptional gene silencing in plum pox virus resistant transgenic European plum containing the plum pox potyvirus coat protein gene. Transgenic Res. 2001, 10, 201–209.
- Scorza, R.; Ravelonandro, M.; Callahan, A.; Zagrai, L.; Polak, J.; Malinowski, T.; Cambra, M.; Levy, L.; Damsteegt, V.; Krška, B.; et al. Honeysweet (C5), the first genetically engineered plum pox vi’rus-resistant plum (Prunus domestica L.) cultivar. HortScience 2016, 51, 601–603.
- Alburquerque, N.; Faize, L.; Burgos, L. Silencing of Agrobacterium tumefaciens oncogenes ipt and iaaM induces resistance to crown gall disease in plum but not in apricot. Pest Manag. Sci. 2017, 73, 2163–2173.
- Faize, M.; Faize, L.; Petri, C.; Barba-Espin, G.; Diaz-Vivancos, P.; Clemente-Moreno, M.J.; Koussa, T.; Rifai, L.A.; Burgos, L.; Hernandez, J.A. Cu/Zn superoxide dismutase and ascorbate peroxidase enhance in vitro shoot multiplication in transgenic plum. J. Plant Physiol. 2013, 170, 625–632.
- Diaz-Vivancos, P.; Faize, M.; Barba-Espin, G.; Faize, L.; Petri, C.; Hernández, J.A.; Burgos, L. Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums. Plant Biotechnol. J. 2013, 11, 976–985.
- Srinivasan, C.; Dardick, C.; Callahan, A.; Scorza, R. Plum (Prunus domestica) trees transformed with poplar FT1 result in altered architecture, dormancy requirement, and continuous flowering. PLoS ONE 2012, 7, e40715.
- Scorza, R.; Dardick, C.D.; Callahan, A.M.; Srinivasan, C.; Delong, T.; Harper, J.; Raines, D.D.; Castro, S. FasTrack’-a revolutionary approach to long-generation cycle specialty crop breeding. In Proceedings of the Xth International Symposium Plum & Prune Genetics, Breeding & Pomology, University of California, Davis, CA, USA, 20–25 May 2012. Paper No. 101.
- Petri, C.; Wang, H.; Alburquerque, N.; Faize, M.; Burgos, L. Agrobacterium-mediated transformation of apricot (Prunus armeniaca L.) leaf explants. Plant Cell Rep. 2008, 27, 1317–1324.
- Petri, C.; López-Noguera, S.; Alburquerque, N.; Egea, J.; Burgos, L. An antibiotic-based selection strategy to regenerate transformed plants from apricot leaves with high efficiency. Plant Sci. 2008, 175, 777–783.
- Petri, C.; Wang, H.; Burgos, L.; Sánchez-Navarro, J.; Alburquerque, N. Production of transgenic apricot plants from hypocotyl segments of mature seeds. Sci. Hortic. 2015, 197, 144–149.
- Alburquerque, N.; Ruiz, D.; Burgos, L.; Petri, C. Prunus armeniaca Apricot. In Biotechnology of Fruit and Nut Crops; Litz, R.E., Pliego-Alfaro, F., Hormaza, J.I., Eds.; CABI: High Springs, FL, USA, 2020; pp. 496–511.
- Laimer da Câmara Machado, M.; da Câmara Machado, A.; Hanzer, V.; Weiss, H.; Regner, F.; Steinkeliner, H.; Mattanovich, D.; Plail, R.; Knapp, E.; Kalthoff, B.; et al. Regeneration of transgenic plants of Prunus armeniaca containing the coat protein gene of Plum Pox Virus. Plant Cell Rep. 1992, 11, 25–29.
- Pak, S.; Li, C. Progress and challenges in applying CRISPR/Cas techniques to the genome editing of trees. For. Res. 2022, 2, 6.
- Zhang, Y.; Cheng, Y.; Fang, H.; Roberts, N.; Zhang, L.; Vakulskas, C.A.; Niedz, R.P.; Culver, J.N.; Qi, Y. Highly Efficient Genome Editing in Plant Protoplasts by Ribonucleoprotein Delivery of CRISPR-Cas12a Nucleases. Front. Genome Ed. 2022, 4, 780238.
- Donadio, L.C.; Lederman, I.E.; Roberto, S.R.; Stucchi, E.S. Dwarfing-canopy and rootstock cultivars for fruit trees. Rev. Bras. Frutic. 2019, 41, 3.
- Dongariyal, A.; Chandra, A.K.; Dongriyal, A.; Kumar, A.; Sharma, P. Tending genome editing via CRISPR/Cas9-induced mutagenesis: Opportunity and challenges for yield, quality and nutritional improvement of fruit crops. Sci. Hortic. 2023, 311, 111790.
- Rudikovskii, A.V.; Stolbicova, A.V.; Rudikovskaya, E.G.; Dudareva, L.V. Role of phytohormones in the formation of dwarf and tall siberian crabapple (Malus baccata L. Borkh.). Zemdirbyste 2019, 106, 167–172.
- Cheng, J.; Zhang, M.; Tan, B.; Jiang, Y.; Zheng, X.; Ye, X.; Guo, Z.; Xiong, T.; Wang, W.; Li, J.; et al. A single nucleotide mutation in GID1c disrupts its interaction with DELLA1 and causes a GA-insensitive dwarf phenotype in peach. Plant Biotechnol. J. 2019, 17, 1723–1735.
- Shao, X.; Wu, S.; Dou, T.; Zhu, H.; Hu, C.; Huo, H.; He, W.; Deng, G.; Sheng, O.; Bi, F.; et al. Using CRISPR/Cas9 genome editing system to create MaGA20ox2 gene-modified semi-dwarf banana. Plant Biotechnol. J. 2020, 18, 17–19.
- Feng, Y.; Zhang, X.; Wu, T.; Xu, X.; Han, Z.; Wang, Y. Methylation effect on IPT5b gene expression determines cytokinin biosynthesis in apple rootstock. Biochem. Biophys. Res. Commun. 2017, 482, 604–609.
- Ren, C.; Guo, Y.; Kong, J.; Lecourieux, F.; Dai, Z.; Li, S.; Liang, Z. Knockout of VvCCD8 gene in grapevine affects shoot branching. BMC Plant Biol. 2020, 20, 47.
- Min, T.; Hwarari, D.; Li, D.; Movahedi, A.; Yang, L. CRISPR-Based Genome Editing and Its Applications in Woody Plants. Int. J. Mol. Sci. 2022, 23, 10175.
- Corte, L.E.D.; Mahmoud, L.M.; Moraes, T.S.; Mou, Z.; Grosser, J.W.; Dutt, M. Development of improved fruit, vegetable, and ornamental crops using the CRISPR/cas9 genome editing technique. Plants 2019, 8, 601.
- Pillitteri, L.J.; Lovatt, C.J.; Walling, L.L. Isolation and characterization of a terminal flower homolog and its correlation with juvenility in citrus. Plant Physiol. 2004, 135, 1540–1551.
- Charrier, A.; Vergne, E.; Dousset, N.; Richer, A.; Petiteau, A.; Chevreau, E. Efficient targeted mutagenesis in apple and first time edition of pear using the CRISPR-Cas9 system. Front. Plant Sci. 2019, 10, 40.
- Li, Q.; Sapkota, M.; van der Knaap, E. Perspectives of CRISPR/Cas-mediated cis-engineering in horticulture: Unlocking the neglected potential for crop improvement. Hortic. Res. 2020, 7, 36.
- Alonge, M.; Wang, X.; Benoit, M.; Soyk, S.; Pereira, L.; Zhang, L.; Suresh, H.; Ramakrishnan, S.; Maumus, F.; Ciren, D.; et al. Major Impacts of Widespread Structural Variation on Gene Expression and Crop Improvement in Tomato. Cell 2020, 182, 145–161.
- Salazar, J.A.; Ruiz, D.; Zapata, P.; Martínez-García, P.J.; Martínez-Gómez, P. Whole Transcriptome Analyses of Apricots and Japanese Plum Fruits after 1-MCP (Ethylene-Inhibitor) and Ethrel (Ethylene-Precursor) Treatments Reveal New Insights into the Physiology of the Ripening Process. Int. J. Mol. Sci. 2022, 23, 11045.
- Hu, H.; Scheben, A.; Verpaalen, B.; Tirnaz, S.; Bayer, P.E.; Hodel, R.G.J.; Batley, J.; Soltis, D.E.; Soltis, P.S.; Edwards, D. Amborella gene presence/absence variation is associated with abiotic stress responses that may contribute to environmental adaptation. New Phytol. 2022, 233, 1548–1555.
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706.
- Dall’Osto, L.; Holt, N.E.; Kaligotla, S.; Fuciman, M.; Cazzaniga, S.; Carbonera, D.; Frank, H.A.; Alric, J.; Bassi, R. Zeaxanthin protects plant photosynthesis by modulating chlorophyll triplet yield in specific light-harvesting antenna subunits. J. Biol. Chem. 2012, 287, 41820–41834.
- Zaidi, S.M.S.; Saud, A. Future of US-China Relations: Conflict, Competition or Cooperation? Asian Soc. Sci. 2020, 16, 1–14.
- Joshi, R.K.; Bharat, S.S.; Mishra, R. Engineering drought tolerance in plants through CRISPR/Cas genome editing. 3 Biotech 2020, 10, 400.
- Lian, X.; Zhao, X.; Zhao, Q.; Wang, G.; Li, Y.; Hao, Y. MdDREB2A in apple is involved in the regulation of multiple abiotic stress responses. Hortic. Plant J. 2021, 7, 197–208.
- Wang, Y.; Sun, T.; Li, T.; Wang, M.; Yang, G.; He, G. A CBL-interacting protein kinase TaCIPK2 confers drought tolerance in transgenic tobacco plants through regulating the stomatal movement. PLoS ONE 2016, 11, e0167962.
- Kaur, N.; Awasthi, P.; Tiwari, S. Fruit Crops Improvement Using CRISPR/Cas9 System; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128181409.
- Fister, A.S.; Landherr, L.; Maximova, S.N.; Guiltinan, M.J. Transient expression of CRISPR/Cas9 machinery targeting TcNPR3 enhances defense response in Theobroma cacao. Front. Plant Sci. 2018, 9, 268.
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell 2021, 184, 1621–1635.
- Slaymaker, I.M.; Gao, L.; Zetsche, B.; Scott, D.A.; Yan, W.X.; Zhang, F. Rationally engineered Cas9 nucleases with improved specificity. Science 2016, 351, 84–88.
- Huang, X.; Wang, Y.; Wang, N. Highly Efficient Generation of Canker-Resistant Sweet Orange Enabled by an Improved CRISPR/Cas9 System. Front. Plant Sci. 2022, 12, 769907.
- Fu, Y.; Sander, J.D.; Reyon, D.; Cascio, V.M.; Joung, J.K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 2014, 32, 279–284.
- Osakabe, Y.; Watanabe, T.; Sugano, S.S.; Ueta, R.; Ishihara, R.; Shinozaki, K.; Osakabe, K. Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Sci. Rep. 2016, 6, 26685.
More
Information
Subjects:
Horticulture
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
966
Revisions:
2 times
(View History)
Update Date:
28 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




