Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yuan, X.; Gao, X.; Liu, C.; Liang, W.; Xue, H.; Li, Z.; Jin, H. The Effects of Nanomaterials on Microalgae Growth. Encyclopedia. Available online: https://encyclopedia.pub/entry/52123 (accessed on 07 February 2026).
Yuan X, Gao X, Liu C, Liang W, Xue H, Li Z, et al. The Effects of Nanomaterials on Microalgae Growth. Encyclopedia. Available at: https://encyclopedia.pub/entry/52123. Accessed February 07, 2026.
Yuan, Xiaolong, Xiang Gao, Chang Liu, Wensheng Liang, Huidan Xue, Zhengke Li, Haojie Jin. "The Effects of Nanomaterials on Microalgae Growth" Encyclopedia, https://encyclopedia.pub/entry/52123 (accessed February 07, 2026).
Yuan, X., Gao, X., Liu, C., Liang, W., Xue, H., Li, Z., & Jin, H. (2023, November 28). The Effects of Nanomaterials on Microalgae Growth. In Encyclopedia. https://encyclopedia.pub/entry/52123
Yuan, Xiaolong, et al. "The Effects of Nanomaterials on Microalgae Growth." Encyclopedia. Web. 28 November, 2023.
Copy Citation
Microalgae are promising biological factories for the production of diverse natural products, such as proteins, lipids, exopolysaccharides (EPSs), carotenoids, and phenolic compounds. These biomolecules or metabolites can be used in industrial products ranging from biofuels, food additives, cosmetics, and pharmaceuticals to alternatives to chemically synthetic or animal-derived products. To improve the production efficiency of microalgae-derived metabolites, diverse microalgal cultivation methods have been developed, such as nitrogen deficiency, salinity stress, photoinduction, and carbon source addition. Nanotechnology or nanomaterial application has arisen as a new strategy in the production of valuable metabolites or bioproducts in microalgae. Nanomaterials (NMs) are materials that are manufactured with at least one dimension being less than 100 nanometers (nm). They have been found to influence the physiology and metabolism of algal cells by generating cell shading, physical damage, and oxidative stress.
nanomaterials
microalgae
metabolites
exopolysaccharides
bioactivity
1. The Interaction of Nanomaterials and Microalgal Cells
How nanomaterials (NMs) exert effects on cell growth is an important issue. They may be distributed around cells or penetrate the cell wall to function. Several reviews have summarized the interactions of NMs and microalgal cells, including adsorption, distribution, and ecotoxicity [1][2][3]. Several examples show their contact relationships. NMs can be distributed around cell walls when applied in microalgae cultivation, which limits cell exposure to nutrients and light [4]. Those NMs with a size smaller than that of the cell wall pore were supposed to enter the cells directly [5]. Larger NMs could penetrate the cell wall through embedding [6], membrane perforation, and endocytosis [3]. After entering the cell, NMs could physically contact various organelles and damage or alter their structures and functions [7][8]. Thus, it is critical to examine the mode of interaction (extracellular or intracellular) when discussing the effects of functional mechanisms of NMs applied in microalgal cultivation.
2. Improving Photosynthetic Utilization Efficiency
As the main form of energy used by algae, light is one of the most important factors affecting microalgal growth. Light energy is absorbed and transmitted through photosynthetic pigments, including chlorophyll, carotenoids, and phycobiliprotein, in photosynthetic systems. However, these pigments can only cover no more than 10% white light [9]. As the main photosynthetic pigments, chlorophyll a and b have only a dual-absorbance range of blue (450–480 nm) and red (605–700 nm) lights [9]. To maximize the use of solar energy, developing high-performance light conversion materials to improve the absorption efficiency of red and blue lights or utilizing the lights of other wavelengths for growth may be a feasible approach [10][11][12]. A mechanistic illustration of the application of NMs in improving light utilization efficiency and growth of microalgae is shown in Figure 1.
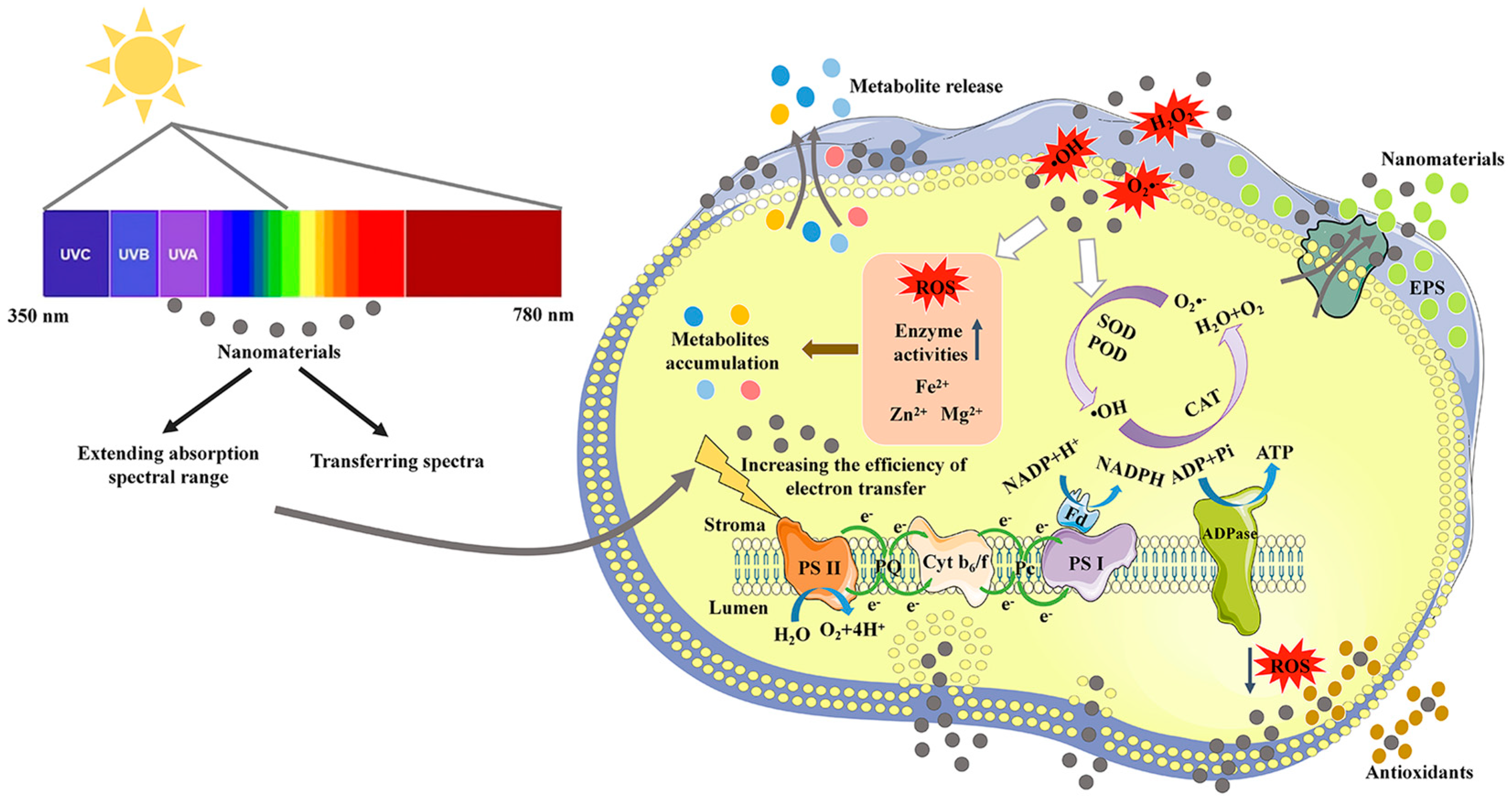
Figure 1. A mechanistic illustration of the application of nanomaterials in improving photosynthetic utilization efficiency and metabolite production of microalgae.
2.1. Increasing the Absorption of Red and Blue Lights
In liquid suspension cultivation, cell shading can cause insufficient absorption of blue and red lights by chlorophyll molecules [13]. Light utilization efficiency can be improved by light-harvesting NMs to maintain normal photosynthetic systems in microalgae [14]. One of the mechanisms is to enhance red or blue light absorption of microalgal cells. It was reported that some NPs selectively enhanced microalgal blue light absorption through plasma light scattering, increasing the growth of Chlamydomonas reinhardtii (Chlorophyta, Chlorophyceae) and Cyanothece (Cyanophyceae) 51142 by more than 30% when exposed to the full spectrum [15]. Additionally, the localized surface plasmon resonance (LSPR) of metal NPs was used to filter light at specific wavelengths within a bioreactor, and the LSPR wavelength could be tuned to the violet-blue or red regions [16]. The photosynthesis of Mung Bean was increased by more than two-fold, and the dry weight was increased by 15.39% [16].
2.2. Spectral Transformation of Infrared Light
Near-infrared (NIR) light, accounting for approximately 52% of the solar spectrum, is not effectively utilized by photosynthesis [17][18]. The photosynthetic efficiency will be improved if NIR light can be utilized by microalgae. A feasible route is to convert NIR light into visible light with photon-up conversion (UC) materials [19]. It was reported that NaYF4:Yb,Er, as a UC material, could efficiently transform NIR light to visible light (mainly green and red lights) via multiple-photon absorption [20][21]. Due to the potential of carbon dots (CDs) in light conversion, some studies explored the joint effect of NaYF4:Yb,Er, and CDs. For example, the construction of NaYF4:Yb,Er + CD nanocomposites further improved the conversion of NIR light to red light [22]. CDs modulated the UC emission of NaYF4:Yb,Er by efficient energy transfer [22].
2.3. Spectral Transformation of Yellow and Green Lights
In the photosynthetic system, the wavelengths at 500–600 nm (yellow and green lights) are not well absorbed by microalgae [9]. To improve the absorption efficiency of these lights, light-trapping NMs can be developed to convert poorly absorbed yellow and green lights into highly absorbed visible lights. Among light-trapping NMs, CDs were applied as good electron donors or acceptors in light energy conversion [23][24][25][26] due to their high quantum yield, chemical stability, and superior biocompatibility [27]. At present, these CDs can emit red light under yellow-green or green light. For example, the CDs with tunable emission could directionally shift unutilized yellow-green light (500–600 nm) to red light (580–700) to promote growth by 15% in Chlorella sp. [28]. They could also enhance microalgal photosynthetic activity by redshifting the incident light [29]. However, the synthesized CDs did not emit blue fluorescence under yellow or green light [29]. Under 1 mg/L CDs treatment, the photosystem II activity of Chlorella was significantly enhanced, and the growth rate was increased by 52.7% [29]. In addition, artificially synthesized polyolefin-based fluorescent dyes could convert green light (500–570 nm) to red light (580–650 nm) and contribute to microalgal biomass increase [30].
2.4. Spectral Transformation of Ultraviolet Light
Ultraviolet (UV) light (200–400 nm) usually has negative effects on microalgal growth and is also poorly adsorbed by microalgal photosynthetic systems [31]. In microalgae, it has rarely been reported that light-trapping NMs can convert UV light into highly absorbed visible light [32]. However, two aggregation-induced emission luminogens (AIEgens) were recently reported to absorb UV/blue light to emit green and yellow lights, which were efficiently used by Cyanobium bacillare (Cyanophyceae) [33]. Under AIEgens treatment, several photosynthetic parameters were significantly improved, and the growth (in terms of cell density) of C. bacillare was boosted five-fold [33]. More reports about UV light conversion are provided in the plant research area. For example, the synthesized CDs were reported to emit blue fluorescence under UV excitation [25][34][35]. Functionally modified CDs could enhance the spectral transformation of UV light, such as increasing graphitic-N and hydroxyl group contents [24], vinyl alcohol encapsulation [36], and amine functionalization [36]. A multifunctional CD was reported to match the chloroplast absorption spectrum (blue and red lights) by strong absorption of UV light [37]. In addition, aggregation-induced emission carbon dots (CD-AIEgens) had strong UV absorption in natural light [38], exhibiting application potential in mass cultivation.
2.5. The Quenching Effect and Stability Maintenance of Light Harvesting Nanomaterials
The aggregate state of NMs may cause the quenching effect of fluorophores in water, which could decrease the efficiency of spectral conversion. To solve this problem, sustainable aggregation-induced emission (AIE) materials were prepared from natural resources [39][40][41]. These resources include quercetin, lignin, and rosin, which can eliminate the quenching effect of the existing materials developed to enhance photosynthesis [40][41][42]. In the state of aggregation, AIE materials produced stronger excitation than traditional aggregation-caused quenching luminescent NMs [43][44]. In addition, the coupled application of AIEgens and CDs could obtain unique optical properties, such as high AIE-active fluorescence, efficient harvesting of UV light, and good photostability [39].
To avoid the aggregation-caused quenching in light-harvesting NMs, special scaffolds have also been developed, including macrocycles [45][46][47][48], DNA [49][50], and cyclic peptides [51][52]. The design and synthesis of ideal scaffolds are complex. To greatly simplify the fabrication steps of special scaffolds, the employment of AIE materials was presented [53][54]. In addition, supramolecular polymerization is an excellent strategy for constructing light-harvesting NMs, which can assemble the chromophores together to pack tightly and enhance supramolecular assembly-induced emission of the chromophores [54]. New polymerizations have been reported based on ureidopyrimidinone quadruple hydrogen bonding units [55] and tetraphenyl-ethylene [56]. Organic dyes are classical optical materials that can be used in AIE NPs. A hybrid dye system based on the tetraphenylene-encapsulated organic dye (Nile red) was synthesized, which had a considerable redshift distance (~126 nm), with a high energy-transfer efficiency of 99.37% and an antenna effect of 26.23% [57].
The NM solutions are easily dried or washed away when applied to plants and algae [58]. To gain a prolonged enhancement of photosynthesis by NMs, continuous leaf spraying or hydroponic conditions throughout the plant growth process are needed, but this results in high labor costs or large-scale facility construction [23][59][60]. In vitro spraying of adhesive fluorescent coatings on leaf surfaces with continuous fluorescence emission and good rain–erosion resistance provides a new tool for efficient photosynthesis enhancement [58]. CDs and some monomers could be used to synthesize covalently cross-linked polymers with prolonged fluorescent capacity, rain–erosion resistance, and stability [61]. In addition, a fluorescent polymer coating was developed, which consisted of UV-excited, blue light-emitting nitrogen-doped CDs as the fluorescent body to catalyze the covalent copolymerization of CDs and tannic acid [61]. This fluorescent polymer coating exhibited excellent fluorescence properties, stability, nontoxicity, and rain–erosion resistance [61]. Such light harvesting NMs used in plants should also have good potential to be applied in microalgae cultivation for enhancing photosynthetic efficiency.
3. Removing Reactive Oxygen Species
Various environmental stressors can induce reactive oxygen species (ROS) in microalgal cells, generating oxidative stress [31]. Intracellular antioxidants from algal cells can mitigate cellular damage. However, the effectiveness of these antioxidants is sometimes limited. Recently, antioxidant NPs were synthesized by amalgamation of material sciences with nanotechnology, including carbon nanotubes (CNTs), metal NPs, and metal oxide NPs [62][63]. In addition, the transport function of NMs was developed to transport antioxidants into cells to oxidative stress damage [64].
3.1. The Antioxidant Activities of Nanomaterials
Some NMs can scavenge ROS and mimic antioxidant molecules. Pristine CDs reduced the UV light damage by scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical in Chlorella vulgaris (Chlorophyta, Trebouxiophyceae) [65]. The antioxidant activity is related to the long-conjugated C=C chains of the CDs [66]. Similarly, cerium oxide NPs (CON) exhibited superoxide dismutase (SOD) activity, which reduces the levels of superoxide anions [67][68]. The antioxidant level of CON is related to nanocrystal diameter since smaller diameter nanocrystals are found to be more reactive towards H2O2 [69]. CON could be applied multiple times and used for several weeks since the particles remained colloidally stable [69]. To further improve the ROS scavenging ability under broad-scale environmental conditions (e.g., application in biological tissues and cells), CON could be wrapped with biocompatible polymers, such as PEGylated and dextran [70][71]. The dextran-coated CON was applied in the chloroplasts of photosynthetic organisms to improve the antioxidant capacity of their photosynthetic system [17][72].
Functionalized NMs derived from various biological extracts of living organisms, such as proteins, EPSs, and terpenes, show potential antioxidant activity. For example, extracellular protein (from Escherichia coli)-capped gold NPs showed excellent antioxidant ability [73]. The DPPH radical scavenging activity of gold NPs was found to be dose-dependent, with the maximum inhibition being greater than that of the extract alone [73]. The EPS-mediated silver (Ag) NPs also showed excellent antioxidant activities at a suitable concentration [74]. Terpene-rich extracts were used to synthesize the antioxidants of Ag NPs [61]. The obtained antioxidant ability was comparable to that of ascorbic acid [75]. In addition, the crude extracts of living organisms were also used to synthesize NM antioxidants, including leaf extracts [76][77][78], cell-free supernatants [78][79], and other extracts [80][81][82].
3.2. The Nanomaterials-Facilitated Transfer of Antioxidants
Many chemical compounds, either endogenous or exogenous, have been evaluated for their antioxidant properties, which have the potential to modulate oxidative stress [83]. Recently, NPs were found to efficiently enhance antioxidant activity and provide targeted delivery of certain antioxidants that show poor cell membrane permeation and cell internalization [64]. Encapsulation of vitamin E and catechol in Ag NPs facilitated the scavenging of DPPH radical, hydrogen peroxide, and nitric oxide [84]. The Ag NPs were also therapeutically applied for targeted delivery in breast cancer treatment [84]. Biodegradable polyanhydride NPs containing the mitochondrion-targeted antioxidant apocynin were explored for treating neuronal cells, and their pretreatment significantly protected cells against H2O2-induced toxicity [85]. The curcumin-encapsulated NPs with dual responses to oxidative stress and reduced pH could efficiently reduce the excess oxidants produced by lipopolysaccharide-stimulated macrophages [86]. These studies provide useful references for decreasing the damage of oxidative stress in microalgal cells generated from adverse environmental conditions. Moreover, precise delivery by NMs to specific locations, such as cell membrane, chloroplast, and nucleus in microalgae, needs to be explored with the aim of advancing the development of microalgal biotechnology.
References
- Wang, F.; Guan, W.; Xu, L.; Ding, Z.Y.; Ma, H.L.; Ma, A.Z.; Terry, N. Effects of nanoparticles on algae: Adsorption, distribution, ecotoxicity and fate. Appl. Sci. 2019, 9, 1534.
- Nguyen, M.K.; Moon, J.Y.; Lee, Y.C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotoxicol. Environ. Saf. 2021, 201, 110781.
- Chen, F.R.; Xiao, Z.G.; Yue, L.; Wang, J.; Feng, Y.; Zhu, X.S.; Wang, Z.Y.; Xing, B.S. Algae response to engineered nanoparticles: Current understanding, mechanisms and implications. Environ. Sci. Nano 2019, 6, 1026–1042.
- Li, F.; Liang, Z.; Zheng, X.; Zhao, W.; Wu, M.; Wang, Z. Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production. Aquat. Toxicol. 2015, 158, 1–13.
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386.
- Taylor, N.S.; Merrifield, R.; Williams, T.D.; Chipman, J.K.; Lead, J.R.; Viant, M.R. Molecular toxicity of cerium oxide nanoparticles to the freshwater alga Chlamydomonas reinhardtii is associated with supra-environmental exposure concentrations. Nanotoxicology 2016, 10, 32–41.
- Bhuvaneshwari, M.; Iswarya, V.; Archanaa, S.; Madhu, G.M.; Kumar, G.K.; Nagarajan, R.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of ZnO NPs towards fresh water algae Scenedesmus obliquus at low exposure concentrations in UV-C, visible and dark conditions. Aquat. Toxicol. 2015, 162, 29–38.
- Zhao, J.; Cao, X.S.; Liu, X.Y.; Wang, Z.Y.; Zhang, C.C.; White, J.C.; Xing, B.S. Interactions of CuO nanoparticles with the algae Chlorella pyrenoidosa: Adhesion, uptake, and toxicity. Nanotoxicology 2016, 10, 1297–1305.
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444.
- Wang, Y.H.; Xie, Z.M.; Wang, X.H.; Peng, X.; Zheng, J.P. Fluorescent carbon-dots enhance light harvesting and photosynthesis by overexpressing PsbP and PsiK genes. J. Nanobiotechnol. 2021, 19, 260.
- Ren, J.J.; Zhou, X.P.; Wang, Y.H. Dual-emitting CsPbX3@ ZJU-28 (X = Cl, Br, I) composites with enhanced stability and unique optical properties for multifunctional applications. Chem. Eng. J. 2020, 391, 123622.
- Liang, L.M.; Mei, L.F.; Liu, H.K.; Wang, C.C.; Liao, L.B. Intense broad-band absorption and blue-emitting Ca9La (PO4)5 (SiO4) Cl2: Eu2+ phosphor under near-ultraviolet excitation. J. Lumin. 2019, 206, 154–157.
- Katayama, T.; Nagao, N.; Goto, M.; Yusoff, F.M.; Sato, M.; Takahashi, K.; Furuya, K. Growth characteristics of shade-acclimated marine Chlorella vulgaris under high-cell-density conditions. J. Environ. Biol. 2018, 39, 747–753.
- Jiang, M.Y.; Yuan, Y.; Fang, Y.C.; Liu, S.X.; Li, J.; Chen, Z.J.; Pang, Q.Y.; Li, S.J. Intergrating photon up- and down-conversion to produce efficient light-harvesting materials for enhancing natural photosynthesis. J. Mater. Chem. A 2021, 9, 24308–24314.
- Torkamani, S.; Wani, S.N.; Tang, Y.J.; Sureshkumar, R. Plasmon-enhanced microalgal growth in miniphotobioreactors. Appl. Phys. Lett. 2010, 97, 043703.
- Xu, X.; Li, W.; Hu, C.; Lei, B.; Zhang, X.; Li, Y.; Zhan, Q.; Liu, Y.; Zhuang, J. Promoting the growth of mung bean plants through uptake and light conversion of NaYF4:Yb, Er@CDs nanocomposites. ACS Sustain. Chem. Eng. 2020, 8, 9751–9762.
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408.
- Ooms, M.D.; Dinh, C.T.; Sargent, E.H.; Sinton, D. Photon management for augmented photosynthesis. Nat. Commun. 2016, 7, 12699.
- Antal, T.; Harju, E.; Pihlgren, L.; Lastusaari, M.; Tyystjarvi, T.; Holsa, J.; Tyystjarvi, E. Use of near-infrared radiation for oxygenic photosynthesis via photon up-conversion. Int. J. Hydrogen Energy 2012, 37, 8859–8863.
- Sun, L.N.; Peng, H.; Stich, M.I.; Achatz, D.; Wolfbeis, O.S. pH sensor based on upconverting luminescent lanthanide nanorods. Chem. Commun. 2019, 33, 5000–5002.
- Dibaba, S.T.; Ge, X.Q.; Ren, W.L.; Sun, N. Recent progress of energy transfer and luminescence intensity boosting mechanism in Nd3+-sensitized upconversion nanoparticles. J. Rare Earth. 2019, 37, 791–805.
- Xu, X.K.; Zhang, X.J.; Hu, C.F.; Zheng, Y.H.; Lei, B.F.; Liu, Y.L.; Zhuang, J.L. Construction of NaYF4:Yb, Er(Tm)@CDs composites for enhancing red and NIR upconversion emission. J. Mater. Chem. C 2019, 7, 6231–6235.
- Li, Y.D.; Xu, X.K.; Wu, Y.; Zhuang, J.L.; Zhang, X.J.; Zhang, H.R.; Lei, B.F.; Hu, C.F.; Liu, Y.L. A review on the effects of carbon dots in plant systems. Mater. Chem. Front. 2020, 4, 437–448.
- Li, Y.; Pan, X.; Xu, X.; Wu, Y.; Zhuang, J.; Zhang, X.; Zhang, H.; Lei, B.; Hu, C.; Liu, Y. Carbon dots as light converter for plant photosynthesis: Augmenting light coverage and quantum yield effect. J. Hazard. Mater. 2021, 410, 124534.
- Milenković, I.; Borišev, M.; Zhou, Y. Photosynthesis enhancement in maize via nontoxic orange carbon dots. J. Agric. Food Chem. 2021, 69, 5446–5451.
- Bai, H.T.; Liu, H.X.; Chen, X.; Hu, R.; Li, M.; He, W.; Du, J.; Liu, Z.Y.; Qin, A.J.; Lam, J.W.Y.; et al. Augmenting photosynthesis through facile AIEgen-chloroplast conjugation and efficient solar energy utilization. Mater. Horiz. 2021, 8, 1433–1438.
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542.
- Xue, R.; Fu, L.; Dong, S.S.; Yang, H.W.; Zhou, D.D. Promoting Chlorella photosynthesis and bioresource production using directionally prepared carbon dots with tunable emission. J. Colloid Interface Sci. 2020, 569, 195–203.
- Zhao, Z.H.; Xue, R.; Fu, L.; Chen, C.L.; Ndayisenga, F.; Zhou, D.D. Carbon dots enhance the recovery of microalgae bioresources from wastewater containing amoxicillin. Bioresour. Technol. 2021, 335, 125258.
- Jang, H.; Namgoong, J.W.; Sung, M.; Chang, Y.; Kim, J.P. Synthesis and characterization of fluorescent dyes and their applications for the enhancement of growth rate of Chlorella vulgaris. Dye. Pigment. 2018, 158, 142–150.
- Makkick, N.; Mohn, F.H. Reactive oxygen species: Response of algal cells. J. Plant Physiol. 2000, 157, 183–193.
- Wang, Y.; Li, S.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles to augment photosynthesis of chloroplasts. Angew. Chem. Int. Ed. 2017, 56, 5308–5311.
- Liu, H.X.; Bai, H.; Yan, N.; Wong, T.Y.; Dang, D.; Ni, J.S.; Lam, J.W.; Lam, H.R.; Wok, T.K.; Wang, W.X.; et al. Boosting cyanobacteria growth by fivefold with aggregation-induced emission luminogens: Toward the development of a biofactory. ACS Sustain. Chem. Eng. 2021, 9, 15258–15266.
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Xiong, D.; Lee, Y.R. Facile hydrothermal synthesis of nitrogen rich blue fluorescent carbon dots for cell bio-imaging of Candida albicans. Process Biochem. 2020, 88, 113–119.
- Li, L.; Shi, L.; Jia, J.; Jiao, Y.; Gao, Y.; Liu, Y.; Dong, C.; Shuang, S. “On-off-on” detection of Fe3+ and F-, biological imaging, and its logic gate operation based on excitation-independent blue-fluorescent carbon dots. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 227, 117716.
- Xu, X.K.; Mao, X.P.; Zhuang, J.L.; Lei, B.F.; Li, Y.D.; Li, W.; Zhang, X.J.; Hu, C.F.; Fang, Y.T.; Liu, Y.L. PVA-coated fluorescent carbon dot nanocapsules as an optical amplifier for enhanced photosynthesis of lettuce. ACS Sustain. Chem. Eng. 2020, 8, 3938–3949.
- Li, W.; Wu, S.S.; Zhang, H.R.; Zhang, X.J.; Zhuang, J.L.; Hu, C.F.; Liu, Y.L. Enhanced biological photosynthetic efficiency using light-harvesting engineering with dual-emissive carbon dots. Adv. Funct. Mater. 2018, 28, 1804004.
- Xiao, D.M.; Jiang, M.Y.; Luo, X.F.; Liu, S.X.; Li, J.; Chen, Z.J.; Li, S.J. Sustainable carbon dot-based AIEgens: Promising light-harvesting materials for enhancing photosynthesis. ACS Sustain. Chem. Eng. 2021, 9, 4139–4145.
- Gu, Y.; Zhao, Z.; Su, H.F.; Zhang, P.F.; Liu, J.K.; Niu, G.L.; Li, S.W.; Wang, Z.Y.; Kwok, R.T.K.; Ni, X.L.; et al. Exploration of biocompatible AIEgens from natural resources. Chem. Sci. 2018, 9, 6497–6502.
- He, T.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Novel quercetin aggregation-induced emission luminogen (AIEgen) with excited-state intramolecular proton transfer for in vivo bioimaging. Adv. Funct. Mater. 2018, 28, 1706196.
- Ma, Z.; Liu, C.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Seeking brightness from nature: J-aggregation-induced emission in cellulolytic enzyme lignin nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 3169–3175.
- Ge, M.; Han, Y.; Ni, J.; Li, Y.; Han, S.; Li, S.; Yu, H.; Zhang, C.; Liu, S.; Li, J.; et al. Seeking brightness from nature: Sustainable carbon dots-based AIEgens with tunable emission wavelength from natural rosin. Chem. Eng. J. 2020, 413, 127457.
- Li, X.; Li, M.; Yang, M.; Xiao, H.; Wang, L.; Chen, Z.; Liu, S.; Li, J.; Li, S.; James, T.D. “Irregular” aggregation-induced emission luminogens. Coord. Chem. Rev. 2020, 418, 213358.
- Xu, S.D.; Duan, Y.K.; Liu, B. Precise molecular design for high-performance luminogens with aggregation-induced emission. Adv. Mater. 2019, 32, 1903530.
- Acharyya, K.; Bhattacharyya, S.; Sepehrpour, H.; Chakraborty, S.; Lu, S.; Shi, B.B.; Li, X.P.; Mukherjee, P.S.; Stang, P.J. Self-assembled fluorescent Pt (ii) metallacycles as artificial light-harvesting systems. J. Am. Chem. Soc. 2019, 141, 14565–14569.
- Sautter, A.; Kaletas, B.K.; Schmid, D.G.; Dobrawa, R.; Zimine, M.; Jung, G.; Stokkum, I.H.M.V.; Cola, L.D.; Williams, R.M.; Würthner, F. Ultrafast energy-electron transfer cascade in a multichromophoric light-harvesting molecular square. J. Am. Chem. Soc. 2005, 127, 6719–6729.
- Jullien, L.; Canceill, J.; Valeur, B.; Bardez, E.; Lehn, J.M. Antenna effect in multichromophoric cyclodextrins. Angew. Chem. Int. Ed. 1995, 33, 2438–2439.
- Pruchyathamkorn, J.; Kendrick, W.J.; Frawley, A.T.; Mattioni, A.; Caycedo-SolerHuelga, F.; Huelga, S.F.; Plenio, M.B.; Anderson, H.L. A cyanine dye rotaxane porphyrin nanoring complex as a model light harvesting system. Angew. Chem. Int. Ed. 2020, 59, 16455–16458.
- Ensslen, P.; Wagenknecht, H.A. One-dimensional multichromophor arrays based on DNA: From self-assembly to light-harvesting. Acc. Chem. Res. 2015, 48, 2724–2733.
- Dutta, P.K.; Varghese, R.; Nangreave, J.; Lin, S.; Yan, H.; Liu, Y.J. DNA-directed artificial light-harvesting antenna. Am. Chem. Soc. 2011, 133, 11985–11993.
- Song, Q.; Goia, S.; Yang, J.; Staniforth, H.; Stavros, V.G.; Perrier, S. Efficient artificial light-harvesting system based on supramolecular peptide nanotubes in water. J. Am. Chem. Soc. 2021, 143, 382–389.
- Wang, T.T.; Fan, X.T.; Li, J.Y.; Yan, X.; Liu, S.D.; Jiang, X.J.; Li, F.; Liu, J.Q. Giant proteinosomes as scaffolds for light harvesting. ACS Macro Lett. 2019, 8, 1128–1132.
- Yu, J.L.; Wu, M.X.; Xue, Z.Y.; Xia, Q.Q.; Liu, X.; Wang, X.H. Supramolecular assembly-induced emission enhancement vesicles regulated by pincer-like hosts containing pillararenes. Adv. Opt. Mater. 2022, 10, 2201496.
- Würthner, F. Aggregation-induced emission (AIE): A historical perspective. Angew. Chem. Int. Ed. 2020, 59, 14192–14196.
- Xiao, T.X.; Shen, Y.; Bao, C.; Ren, D.X.; Qian, H.W.; Zhang, L.L. Efficient artificial light-harvesting system constructed from supramolecumers with AIE prolar polyperty. ASC Adv. 2021, 11, 30041–30045.
- Zhang, L.; Qian, H.; Wu, Z.; Zhang, Q.; Li, S.; Cheng, M.; Xiao, T. Non-covalent dimer as donor chromophore for constructing artificial light-harvesting system in water. Molecules 2022, 27, 8876.
- Li, C.; Liu, Q.; Tao, S. Coemissive luminescent nanoparticles combining aggregation-induced emission and quenching dyes prepared in continuous flow. Nat. Commun. 2022, 13, 6034.
- Zhu, L.; Chen, L.; Gu, J.; Ma, H.; Wu, H. Carbon-based nanomaterials for sustainable agriculture: Their application as light converters, nanosensors, and delivery tools. Plants 2022, 11, 511.
- Hu, L.L.; Li, H.; Liu, C.A.; Song, Y.X.; Zhang, M.L.; Huang, H.; Liu, Y.; Kang, Z.H. Chiral evolution of carbon dots and the tuning on laccase activity. Nanoscale 2018, 10, 2333–2340.
- Wang, H.B.; Zhang, M.L.; Song, Y.X.; Li, H.; Huang, H.; Shao, M.W.; Liu, Y.; Kang, Z.H. Carbon dots promote the growth and photosynthesis of mung bean sprouts. Carbon 2018, 136, 94–102.
- Liu, Y.K.; Zhang, S.; Yang, F.; Wang, G.Z.; Jing, X.L.; Wang, X.F.; You, C.Y. New strategy of light quality regulation with leaf-spraying fluorescent coatings for enhancing photosynthesis efficiency. ASC Adv. 2021, 11, 26620–26628.
- Eftekhari, A.; Dizaj, S.M.; Chodari, L.; Sunar, S.; Hasanzadeh, A.; Ahmadian, E.; Hasanzadeh, M. The promising future of nano-antioxidant therapy against environmental pollutants induced-toxicities. Biomed. Pharmacother. 2018, 103, 1018–1027.
- Eftekhari, A.; Ahmadian, E.; Panahi-Azar, V.; Hosseini, H.; Tabibiazar, M.; Dizaj, S.M. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1-induced liver damage: In vitro/in vivo studies. Artif. Cells Nanomed. Biotechnol. 2017, 46, 411–420.
- Bhattacherjee, A.; Dhara, K.; Chakraborti, A.S. Argpyrimidine-tagged rutin-encapsulated biocompatible (ethylene glycol dimers) nanoparticles: Synthesis, characterization and evaluation for targeted drug delivery. Int. J. Pharm. 2016, 509, 507–517.
- Zhang, M.L.; Wang, H.G.; Song, Y.X.; Huang, H.; Shao, M.W.; Liu, Y.; Li, H.; Kang, Z.H. Pristine carbon dots boost the growth of Chlorella vulgaris by enhancing photosynthesis. ACS Appl. Bio Mater. 2018, 1, 894–902.
- Chong, Y.; Ge, C.; Fang, G.; Tian, X.; Ma, X.; Wen, T.; Wamer, W.G.; Chen, C.; Chai, Z.; Yin, J.J. Crossover between anti- and pro-oxidant activities of graphene quantum dots in the absence or presence of light. ACS Nano 2016, 10, 8690–8699.
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 14, 1056–1058.
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709.
- Lee, S.S.; Song, W.S.; Cho, M.J.; Puppala, H.L.; Nguyen, P.; Zhu, H.G.; Segator, L.; Colvin, Y.L. Antioxidant properties of cerium oxide nanocrystals as a function of nanocrystal diameter and surface coating. ACS Nano 2003, 7, 9693–9703.
- Perez, J.M.; Asati, A.; Nath, S.; Kaittanis, C. Synthesis of biocompatible dextran-coated nanoceria with pH-dependent antioxidant properties. Small 2008, 4, 552–556.
- Karakoti, A.S.; Singh, S.; Kumar, A.; Malinska, M.; Kuchibhatla, S.V.; Wozniak, K.; Self, W.T.; Seal, S. PEGylated nanoceria as radical scavenger with tunable redox chemistry. J. Am. Chem. Soc. 2009, 131, 14144–14145.
- Boghossian, A.A.; Sen, F.; Gibbons, B.M.; Sen, S.; Faltermeier, S.M.; Giraldo, J.P.; Zhang, C.T.; Zhang, J.Q.; Heller, D.A.; Strano, M.S.; et al. Application of nanoparticle antioxidants to enable hyperstable chloroplasts for solar energy harvesting. Adv. Energy Mater. 2013, 3, 881–893.
- Veeraapandian, S.; Sawant, S.N.; Doble, M. Antibacterial and antioxidant activity of protein capped silver and gold nanoparticles synthesized with Escherichia coli. J. Biomed. Nanotechnol. 2012, 8, 140–148.
- Sivasankar, P.; Seedevi, P.; Poongodi, S.; Sivakumar, M.; Murugan, T.; Sivakumar, L.; Sivakumar, K.; Balasubramanian, T. Characterization, antimicrobial and antioxidant property of exopolysaccharide mediated silver nanoparticles synthesized by Streptomyces violaceus MM72. Carbohydr. Polym. 2018, 181, 752–759.
- Patil, S.P.; Kumbhar, S.T. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 2017, 10, 76–81.
- Das, D.; Ghosh, R.; Mandal, P. Biogenic synthesis of silver nanoparticles using S1 genotype of Morus alba leaf extract: Characterization, antimicrobial and antioxidant potential assessment. SN Appl. Sci. 2019, 1, 498.
- Chandra, H.; Patel, D.; Kumari, P.; Jangwan, J.S.; Yadav, S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 212–220.
- Tuzun, B.S.; Fafal, T.; Tastan, P.; Kivcak, B.; Yelken, B.O.; Kayabasi, C.; Susluer, S.Y.; Gunduz, C. Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract. Green Process. Synth. 2020, 9, 153–163.
- Oladipo, I.C.; Lateef, A.; Elegbede, J.A.; Azeez, M.A.; Asafa, T.M.; Yekeen, T.A.; Akinboro, A.; Gueguim-Kana, E.B.; Beukes, L.S.; Oluyide, T.O.; et al. Enterococcus species for the one-pot biofabrication of gold nanoparticles: Characterization and nanobiotechnological applications. J. Photochem. Photobiol. B 2017, 173, 250–257.
- Manjunath, H.M.; Joshi, C.G. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus-Cladosporium cladosporioides. Process Biochem. 2019, 82, 199–204.
- Gao, Y.; Anand, M.A.V.; Ramachandran, V.; Karthikkumar, V.; Shalini, V.; Vijayalakshmi, S.; Ernest, D. Biofabrication of zinc oxide nanoparticles from Aspergillus niger, their antioxidant, antimicrobial and anticancer activity. J. Clust. Sci. 2019, 30, 937–946.
- Aygün, A.; Özdemir, S.; Gülcan, M.; Cellat, K.; Sen, F. Synthesis and characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J. Pharm. Bimed. Anal. 2020, 178, 112970.
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant. Antioxidants 2019, 9, 24.
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016, 470, 142–152.
- Brenza, T.M.; Ghaisas, S.; Ramirez, J.E.V.; Harischandra, D.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G.; Narasimhan, B. Neuronal protection against oxidative insult by polyanhydride nanoparticle-based mitochondria-targeted antioxidant therapy. Nanomedicine 2017, 13, 809–820.
- Pu, H.L.; Chiang, W.L.; Maiti, B.; Liao, Z.X.; Ho, Y.C.; Shim, M.S.; Chuang, E.Y.; Xia, Y.; Sung, H.W. Nanoparticles with dual responses to oxidative stress and reduced pH for drug release and anti-inflammatory applications. ACS Nano 2014, 8, 213–221.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
808
Revisions:
2 times
(View History)
Update Date:
29 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




