Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anastasia Stergioula | -- | 3198 | 2023-11-27 17:05:38 | | | |
| 2 | Mona Zou | Meta information modification | 3198 | 2023-11-28 10:24:08 | | | | |
| 3 | Mona Zou | -1 word(s) | 3197 | 2023-12-01 09:18:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Stergioula, A.; Pantelis, E.; Kontogeorgakos, V.; Lazaris, A.C.; Agrogiannis, G. Radio-Sensitizing Nanoparticles in Soft Tissue Sarcomas. Encyclopedia. Available online: https://encyclopedia.pub/entry/52103 (accessed on 07 February 2026).
Stergioula A, Pantelis E, Kontogeorgakos V, Lazaris AC, Agrogiannis G. Radio-Sensitizing Nanoparticles in Soft Tissue Sarcomas. Encyclopedia. Available at: https://encyclopedia.pub/entry/52103. Accessed February 07, 2026.
Stergioula, Anastasia, Evaggelos Pantelis, Vasileios Kontogeorgakos, Andreas C. Lazaris, Georgios Agrogiannis. "Radio-Sensitizing Nanoparticles in Soft Tissue Sarcomas" Encyclopedia, https://encyclopedia.pub/entry/52103 (accessed February 07, 2026).
Stergioula, A., Pantelis, E., Kontogeorgakos, V., Lazaris, A.C., & Agrogiannis, G. (2023, November 27). Radio-Sensitizing Nanoparticles in Soft Tissue Sarcomas. In Encyclopedia. https://encyclopedia.pub/entry/52103
Stergioula, Anastasia, et al. "Radio-Sensitizing Nanoparticles in Soft Tissue Sarcomas." Encyclopedia. Web. 27 November, 2023.
Copy Citation
High-atomic-number (Z) nanoparticles produce a cascade of low-energy secondary electrons and characteristic X-rays when ionized by X-ray irradiation. These secondary particles deposit their energy in the vicinity of the nanoparticles and, provided that the latter are selectively accumulated within tumor cells, this results in increased DNA damage and tumor cell deaths. The utilization of high-Z nanoparticles in the treatment of soft tissue sarcomas (STS) are reviewed. Both in vitro and in vivo experiments demonstrated that the dose is enhanced by approximately 1.2 when polyethelyne glycol (PEG)-modified gold nanoparticles, and from 1.4 to 1.8 when hafnium oxide nanoparticles (NBTXR3, Nanobiotix SA, France) are introduced into tumor cells and activated by X-ray beams. In a phase 2/3 clinical trial investigating the therapeutic benefit of using nanoparticles in preoperative external beam radiotherapy for locally advanced STS, the proportion of patients with a pathological complete response in their resected tumor was doubled when NBTXR3 nanoparticles were used. Additionally, a higher percentage of patients with complete tumor resection was observed in the NBTXR3 plus radiotherapy group. Similar toxicity profiles were found for both the NBTXR3 plus radiotherapy and the radiotherapy alone patient groups. The incorporation of radio-sensitizing nanoparticles in the preoperative radiotherapy of STS could enhance treatment outcomes.
nanoparticles
radiotherapy
pathologic response
soft tissue sarcoma
1. Physical and Radiobiological Basis of Using Nanoparticle in Radiotherapy
Nanoparticles consisting of atoms with a high atomic number (Z) in their chemical composition (e.g., gold (79Au), Hafnium (72Hf)) have been proposed as dose enhancement materials in radiotherapy treatments [1][2][3]. For a better understanding of the mechanism leading to this dose enhancement, one must distinguish between types of radiation. The strongest effect is presented for photons with energies (E) in the keV range (typically up to ≈300 keV for high-Z atoms). In this energy range, the vast majority of photon interactions occur via the photoelectric effect (PE), which is proportional to (Z/E)n, with n = 3–4 [4]. In the PE, the photon is absorbed by the atom, and a bound electron (called “photoelectron”) is ejected from the atom. The kinetic energy of the ejected photoelectron is equal to the energy of the photon minus the binding energy of the photoelectron. The ejection of the photoelectron leaves the atom in an excited state, which is promptly followed by a de-excitation phase involving the redistribution/rearrangement of the electronic states of the atom. This de-excitation process results in the emission of characteristic X-rays and low-energy Auger electrons. At photon energies greater than ≈300 keV, Compton scattering becomes the dominant interaction process. In this process, the photon is scattered by a weakly bound electron of the atom, leading to a transfer of an amount of energy from the incident photon to the electron which typically leaves the atom. The probability of Compton scattering is proportional to the electron density (𝜌𝑒) of the atom (𝜌𝑒=𝜌(𝑍/𝐴) where ρ and A are the mass density and mass number of the atom, respectively).
Provided that the scattered photons (if the primary photons interacted with the Compton effect), the produced characteristic X-rays, and the electrons are emitted in a dense medium, they can subsequently ionize surrounding biomolecules, as well as neighboring nanoparticles. This effect spreads at the nanometer level, since the range of the Auger electrons is limited to less than 100 nm and extends over micrometers away from the nanoparticle for the characteristic X-rays [2][5]. Scattered photons having higher energies have longer ranges due to the predominance of the Compton effect, resulting in very sparse distributions of ionizing events.
Figure 1a presents the interaction probability per unit mass (i.e., the mass attenuation coefficient) as a function of photon energy for soft tissue, soft tissue with 0.5% w/w Au, and soft tissue with 0.5% w/w Hf media. The selected mass concentrations of high-Z atoms fall within the typical range of concentrations (0.1% to 1% w/w) used in most studies involving nanoparticle radio-sensitizers [1][2][3]. Similar mass attenuation coefficient values can be seen for the presented energy range, except for photon energies near the K and L edges of the high-Z atoms, where the PE predominates. At these energies, an increase in the mass attenuation coefficient values can be observed for the soft tissue containing high-Z atoms. This increase depends on photon energy, atomic number, and the concentration of high-Z atoms. The ratio of the mass attenuation coefficients of the soft tissue containing high-Z atoms to the corresponding values of the soft tissue expresses the interaction probability enhancement and reaches up to 1.5 and 1.4 for the media containing Au or Hf atoms, respectively.
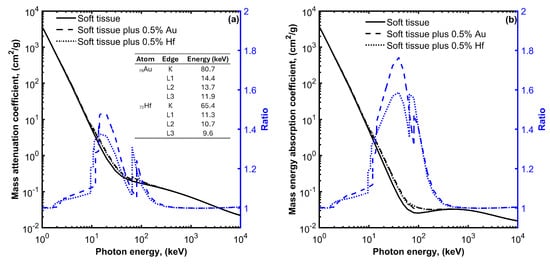
Figure 1. Mass attenuation coefficient (a) and mass energy absorption coefficient (b) for soft tissue, soft tissue with 0.5% gold (Au), and soft tissue with 0.5% hafnium (Hf) plot as a function of photon energy. On each plot, the ratio of the mass attenuation and mass energy absorption coefficient values divided by the corresponding values for the soft tissue without the high-Z materials are also plotted using blue color and refer to the y-axis on the right. The energies of the K and L absorption edges for Au and Hf are also shown in the table of Figure 1a.
The observed increase in the interaction probability per unit mass leads to a corresponding increase in the absorbed energy. This is evident in Figure 1b, where the mass energy absorption coefficients for soft tissue, soft tissue with 0.5% w/w Au, and soft tissue with 0.5% w/w Hf, media are plotted as a function of photon energy. An enhancement in the absorbed energy can be observed at photon energies ranging from 10 to 200 keV when Au or Hf high-Z atoms are introduced within the soft tissue. This enhancement reaches up to 1.8 and 1.6 for Au and Hf, respectively.
The absorbed energy leads to the generation of free radicals, particularly hydroxyl radicals (•OH), through the radiolysis of water. These •OH radicals readily react with biological molecules, including cellular DNA, initiating radiation-induced apoptosis through the generation of reactive oxygen species (ROS). ROS typically include the superoxide anion (O2−), the hydrogen peroxide (H2O2) and the hydroxyl radical (•OH), all of which contribute to cellular damage, including the oxidation of lipids, proteins, and DNA. This oxidative stress ultimately results in apoptotic and necrotic cell death also due to mitochondrial dysfunction [1][2][3]. It is worth noting that some nanoparticles have been found to induce the production of ROS and cell oxidative stress, even in the absence of ionizing radiation [1][2][3]. When these nanoparticles are activated by ionizing radiation, the oxidative stress is elevated (see Figure 2).
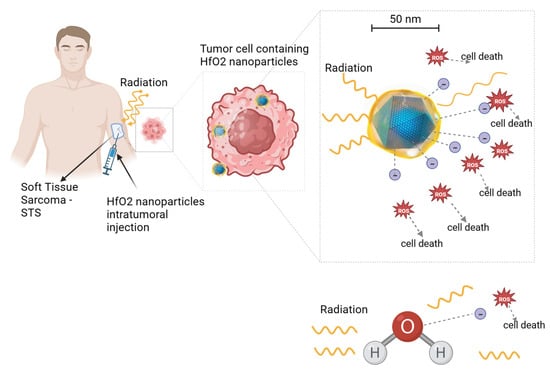
Figure 2. Illustration of hafnium oxide (HfO2) nanoparticle radio-enhancing function in soft tissue sarcoma radiotherapy. Upon ionizing radiation, HfO2 induces the generation of a cascade of secondary electrons that create more energy deposition in tumor cells than water molecules, hence promoting cancer cell death. A similar radio-enhancing function is also produced by other high-atomic-number nanoparticles. Besides intratumoral injection, radio-enhancing nanoparticles can be also administered intravenously and accumulate passively to the tumor cells through the enhanced permeability and retention (EPR) effect. Created with BioRender.com.
2. Characteristics of Nanoparticle Radio-Sensitizers
The main properties of nanoparticle radio-sensitizers include dose enhancement, biocompatibility, and targeting efficacy. These properties are contingent upon the specific characteristics of the nanoparticles. Table 1 provides a summary of the key characteristics of radio-sensitizing nanoparticles and their relevance. The material composition (i.e., the atomic number of the atoms comprising the nanoparticle), size, and intracellular nanoparticle concentration affect the probability of the interaction of radiation with matter and the deposited energy (i.e., the dose enhancement). The higher the deposited energy within the tumor, the higher the radio-sensitizing effect (i.e., the dose enhancement) and the pathologic response. It must be noted however, that while the number of interactions is proportional to the size of the nanoparticle, the energy absorbed in the surrounding biological matter is reduced for nanoparticles of relative increased dimensions [1][2] (see Figure 3). This is due to the absorption of an amount of energy carried by the secondary particles within the volume of the nanoparticle while its size increases (self-absorption). The material composition and chemical structure affect the nanoparticle toxicity. The nanoparticle biocompatibility depends also on their size, shape, and surface properties. Decorating nanoparticle surfaces with stealth coatings improves their biocompatibility. The size of the nanoparticles also affects their biodistribution, capture by the mononuclear phagocytic cells (reticulo-endothelial system), and clearance from the body. In the case of intravenous administration of nanoparticles, they accumulate passively to tumor cells through the enhanced permeability and retention (EPR) effect, which is affected by the nanoparticle size [1][2] (Figure 3). Besides the intravenous administration route, nanoparticles can be delivered to tumor cells through intratumoral injection. The shape (e.g., spherical) and surface properties (e.g., surface charge) of nanoparticles affect their circulation time and uptake by cells (i.e., target accumulation). Adding tumor targeting ligands on the nanoparticle surface improves their targeting efficacy.
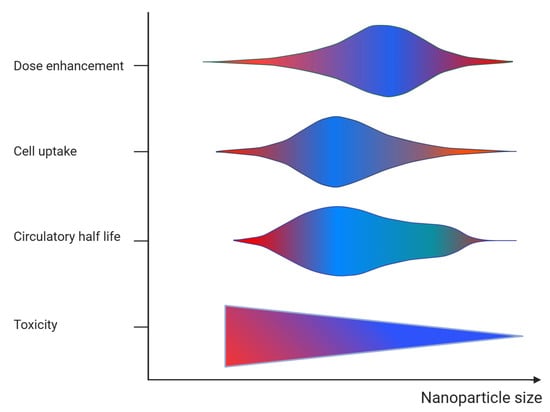
Figure 3. Graphical representation of the effect of nanoparticle size on the dose enhancement, target cell accumulation (cell uptake), blood circulation (circulatory half-life), and toxicity. The color refers to action of size on each parameter (blue = positive and red = negative).
Table 1. Key characteristics and corresponding relevance of the radio-sensitizing nanoparticles.
| Nanoparticle Characteristic | Relevance |
|---|---|
| Material composition/chemical structure |
|
| Size |
|
| Shape |
|
| Surface properties |
|
3. Soft Tissue Sarcoma and Nanoparticle Radio-Sensitizers
The literature was searched for articles that used nanoparticle radio-sensitizers in sarcoma tumors. The PubMed and Scopus electronic databases were searched using the terms: “soft tissue sarcoma” and “nanoparticles”, and “radiotherapy” or “radiation therapy”. A total of six studies were retrieved and analyzed [6][7][8][9][10][11]. Among these, two studies [6][7] reported findings from preclinical in vitro and in vivo experiments, while the remaining four studies [8][9][10][11] presented results from two clinical trials.
3.1. Preclinical Studies
Among the two preclinical studies, one used gold [6] and the other one hafnium oxide [7] nanoparticles. Joh et al. [6], investigated the usefulness of gold nanoparticle (GNP) radio-sensitizers in the treatment of two human sarcoma-derived cell lines, the HT1080 fibrosarcoma and the U2OS osteosarcoma. The gold nanoparticles used had a ~12 nm colloidal core decorated with polyethelyne glycol (PEG), enabling prolonged systemic circulation and enhanced accumulation by the tumor cells. The study involved both in vitro and in vivo experiments, with all irradiations being conducted using the Small Animal Radiation Research Platform (SARRP) low-energy X-ray radiotherapy system (Gulmay Medical, Inc., Camberley, UK). In the in vitro experiments, sarcoma cells were exposed to culture medium with PEG-modified nanoparticles (P-GNP) and then irradiated with different dose levels ranging from 0 to 6 Gy. The effect of the P-GNPs was quantified by comparing the density of DNA double-strand breaks (DSBs) in the irradiated and un-irradiated cells that had or had not been previously exposed to P-GNPs. In the in vivo experiments, the P-GNPs were administered intravenously in mice with engrafted HT1080 fibrosarcoma tumors. The mice were CT scanned prior and at specified time points post-P-GNP injection. A single dose of 20 Gy was delivered to the tumor, and the P-GNP sensitizing effect was quantified by measuring the change in the tumor volume in mice that were irradiated or not and that had received P-GNPs or not, as a function of time.
The combination of RT and P-GNP was found to increase the density of DSBs by approximately 1.6 times, compared to RT alone for both cell lines. Furthermore, irradiated cells with P-GNPs were found to exhibit decreased clonogenic survivability, with the required dose to achieve a surviving fraction of 0.1 being reduced by 1.16 and 1.07 for HT1080 and U2OS, respectively, compared to the irradiated cells without P-GNPs. In mice engrafted with fibrosarcoma tumor cells, the P-GNP selectively accumulated in the tumor and enabled durable imaging. Mice pretreated with P-GNP prior to RT exhibited a significantly improved tumor regression and overall survival. Long-term survival was observed in one third of the mice of this group compared to none with RT only.
Maggiorella et al. [7], investigated the utilization of NBTXR3 (Nanobiotix SA, France) nanoparticles for the treatment of mesenchymal and epithelial tumors. NBTXR3 is a nonpyrogen, sterile, white aqueous dispersion consisting of 50 nm hafnium oxide (HfO2) nanoparticles coated with a biocompatible agent that provides the nanoparticles with a negative surface charge and ensures their stability in aqueous solution at pH values between 6 and 8. The HT1080 fibrosarcoma and Ewing A673 family-type human sarcoma-derived cell lines were used. Several in vitro and in vivo experiments were performed using photon beam sources that included Ir-192 high-dose-rate brachytherapy (average energy = 380 keV), Co-60 (average energy = 1250 keV) and a 6 MV X-ray linear accelerator. In the in vivo studies, nanoparticles were administered through intratumoral injection.
A marked radiation enhancement was observed in the HT1080 fibrosarcoma cell line sensitized by NBTXR3. A significant decrease in the clonogenic surviving fraction was also found for both types of high-energy photon beams, presenting mean dose enhancement factors (DEF) of 1.8 and 1.4 for the Co-60 and 6 MV X-rays, respectively. NBTXR3 demonstrated high intratumoral localization, exhibiting extensive persistence and dispersion within the tumor tissue, while showing minimal diffusion into the extratumoral environment. The combination of NBTXR3 and RT resulted in a significant increase in the response of HT1080 tumor xenografts, with a DEF above 1.5 at doses of 4 and 8 Gy. Furthermore, the use of NBTXR3 and RT in Ewing sarcoma cells engrafted in mice demonstrated a delay in tumor regrowth compared to RT alone. An approximately twofold increase in tumor doubling time was observed, associated with a tumor growth inhibition of 82% for NBTXR3 activated by 15 Gy exposure, versus 72% for 15 Gy alone. Kaplan–Meier curves associated with the tumor regrowth delay revealed a statistically significant increase (p = 0.04) in the median survival time, with 31 days for NBTXR3 activated with 15 Gy compared to 25 days for 15 Gy alone.
3.2. Clinical Studies
The clinical studies found in the literature reported results from a first in human phase 1 [8] and a phase 2/3 clinical trial [9][10][11], both investigating the combination of NBTXR3 and RT in adult patients with locally advanced STSs. The first trial was a pilot study involving 22 patients and aimed to determine the recommended dose, assess the safety profile, and evaluate the feasibility of using NBTXR3 in combination with preoperative RT for adults with locally advanced STSs. Its main finding was that a single intratumoral injection of NBTXR3, equivalent to 10% of the initial tumor volume, was technically feasible and well-tolerated, with a manageable toxicity. The concentration of NBTXR3 in the injected solution was equal to 53.3 g/L. The NBTXR3 injections remained stable within the tumor volume and did not leak into the surrounding tissue or bloodstream after injection. Encouraging signs of antitumor activity were observed across various subtypes of sarcoma, with the authors reporting a median decrease in the maximal tumor diameter of 29% and a median change in volume of −40%.
Following the promising results of the phase 1 study, a randomized, multicenter, international phase 2/3 trial was conducted between 2015 and 2017. The trial compared preoperative RT alone with an investigational arm involving an intratumoral injection of NBTXR3 prior to RT [10]. The study enrolled a total of 180 patients with STSs of the extremity or trunk wall who required preoperative RT. The patients were randomly assigned in a 1:1 ratio. Four patients were excluded and a total of 176 patients were included in the analysis: 87 in the NBTXR3 and 89 in the RT alone group. In the NBTXR3 group, patients received a single intratumoral image-guided injection of NBTXR3, with the volume being equivalent to 10% of the baseline tumor volume. The injection points of NBTXR3 were defined based on the planned surgical incision line to ensure the removal of all NBTXR3 injection sites and tracts. Patients with a tumor volume at baseline larger than 3000 mL were excluded, because the required volume of NBTXR3 for injection would exceed 300 mL and was deemed infeasible. Both groups, NBTXR3 and control, received 3D conformal RT or IMRT, as determined by the discretion of the radio-oncologist. The total RT dose was 50 Gy, delivered in 25 fractions of 2 Gy over a period of 5 weeks, following the standard-of-care recommendations for preoperative RT in STSs of the extremity and trunk wall [12][13][14]. Premedication with steroids was introduced to reduce the risk of acute immune reaction. In the NBTXR3 group, RT began within 1–5 days after the NBTXR3 injection, while in the control group, RT commenced within 7 days after randomization. Following RT completion, all patients were scheduled for wide resection, adhering to the guidelines.
The primary endpoint of the trial was the assessment of pCR. In the intention-to-treat full analysis set, the proportion of patients achieving a pCR was 16% (14 out of 87) in the NBTXR3 group compared to 8% (7 out of 89) in the RT alone group (p = 0.044). Similarly, within the evaluable patient population for pathological response, the NBTXR3 group demonstrated a significantly higher proportion of patients with a pCR of 19% (14 patients out of 73) compared to the RT alone group, with a pCR of 9% (7 patients out of 81) (p = 0.047). In a planned exploratory examination of the proportion of patients achieving pCR, categorized by histological grade, it was observed that the difference between the treatment groups was more pronounced among patients with grade 2 and 3 tumors in comparison to those with grade 1 tumors. There was no significant disparity observed in the proportion of patients who achieved an objective response, as evaluated according to RECIST 1.1 criteria, between the treatment groups. In the NBTXR3 group, the objective response rate was 7% (6 out of 87), whereas in the RT alone group, it was 10% (9 out of 89) (p = 0.863). The secondary endpoint, evaluating the resection margin after neoadjuvant treatment, demonstrated that a larger proportion of patients in the NBTXR3 group achieved R0 margins compared to the RT alone group (p = 0.042). Furthermore, among the population eligible for resection margin assessment, the NBTXR3 group exhibited a higher percentage of patients with R0 margins (67 out of 83, or 81%) compared to the RT alone group (57 out of 86, or 66%; p = 0.030).
A long-term efficacy analysis was reported after a 2-year follow-up period [11]. The cumulative rate of local recurrence was found to be equal to 12.0% and 7.1% in the NBTXR3 plus RT and RT alone group, respectively. Moreover, the cumulative rate of distant recurrence was 33.3% in the NBTXR3 group and 26.2% in the RT alone group, based on the evaluable patient population. Throughout the entire study, a total of 46 patients died, with 24 patients of the NBTXR3 and 22 patients of the RT alone group. None of the deaths were related to the treatment, and the primary cause of death was progressive disease.
Overall similar toxicity profiles were found for both the NBTXR3 and the RT alone groups. In more detail, serious adverse events occurred in 39% of patients in the NBTXR3 group and in 30% of patients in the RT alone group. Serious treatment-emergent adverse events, which may not have been directly related to the treatment, were observed in 31% of patients in the NBTXR3 group and 16% of patients in the RT alone group. Within the NBTXR3 group, 11% of patients experienced treatment-emergent adverse events related to NBTXR3, with hypotension being the most frequent event. Serious adverse events related to radiotherapy were reported in both groups, with postoperative wound complication being the most common. No treatment-related deaths occurred. A long-term follow-up of 2 years showed that NBTXR3 did not have a negative impact on postsurgical wound complications or late radiation toxicities such as fibrosis and oedema. Additionally, NBTXR3 did not adversely affect patients’ health-related quality of life in terms of late-onset adverse effects or sequelae in patients with STS in the extremity.
References
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical Basis and Biological Mechanisms of Gold Nanoparticle Radiosensitization. Nanoscale 2012, 4, 4830–4838.
- Kuncic, Z.; Lacombe, S. Nanoparticle Radio-Enhancement: Principles, Progress and Application to Cancer Treatment. Phys. Med. Biol. 2018, 63, 02TR01.
- Schuemann, J.; Bagley, A.F.; Berbeco, R.; Bromma, K.; Butterworth, K.T.; Byrne, H.L.; Chithrani, B.D.; Cho, S.H.; Cook, J.R.; Favaudon, V.; et al. Roadmap for Metal Nanoparticles in Radiation Therapy: Current Status, Translational Challenges, and Future Directions. Phys. Med. Biol. 2020, 65, 21RM02.
- Podgorsak, E.B. (Ed.) Radiation Physics for Medical Physicists, 3rd ed.; Graduate Texts in Physics; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-25380-0.
- Vlastou, E.; Pantelis, E.; Efstathopoulos, E.P.; Karaiskos, P.; Kouloulias, V.; Platoni, K. Quantification of Nanoscale Dose Enhancement in Gold Nanoparticle-Aided External Photon Beam Radiotherapy. Cancers 2022, 14, 2167.
- Joh, D.Y.; Kao, G.D.; Murty, S.; Stangl, M.; Sun, L.; Zaki, A.A.; Xu, X.; Hahn, S.M.; Tsourkas, A.; Dorsey, J.F. Theranostic Gold Nanoparticles Modified for Durable Systemic Circulation Effectively and Safely Enhance the Radiation Therapy of Human Sarcoma Cells and Tumors. Transl. Oncol. 2013, 6, 722–731.
- Maggiorella, L.; Barouch, G.; Devaux, C.; Pottier, A.; Deutsch, E.; Bourhis, J.; Borghi, E.; Levy, L. Nanoscale Radiotherapy with Hafnium Oxide Nanoparticles. Futur. Oncol. 2012, 8, 1167–1181.
- Bonvalot, S.; Ecile Le Pechoux, C.; De Baere, T.; Kantor, G.; Buy, X.; Stoeckle, E.; Terrier, P.; Sargos, P.; Coindre, J.M.; Lassau, N.; et al. Cancer Therapy: Clinical First-in-Human Study Testing a New Radioenhancer Using Nanoparticles (NBTXR3) Activated by Radiation Therapy in Patients with Locally Advanced Soft Tissue Sarcomas. Clin. Cancer Res 2017, 23, 908–917.
- Bonvalot, S.; Rutkowski, P.; Thariat, J.O.; Carrere, S.; Sunyach, M.P.; Saada, E.; Ágoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M.; et al. Act.in.Sarc: An International Randomized Phase III Trial Evaluating Efficacy and Safety of First-in-Class NBTXR3 Hafnium Oxide Nanoparticles Activated By Preoperative Radiotherapy in Locally Advanced Soft Tissue Sarcoma. Int. J. Radiat. Oncol. 2018, 102, 1606.
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.-P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M.; et al. NBTXR3, a First-in-Class Radioenhancer Hafnium Oxide Nanoparticle, plus Radiotherapy versus Radiotherapy Alone in Patients with Locally Advanced Soft-Tissue Sarcoma (Act.In.Sarc): A Multicentre, Phase 2–3, Randomised, Controlled Trial. Lancet Oncol. 2019, 20, 1148–1159.
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.P.; Agoston, P.; Hong, A.M.; Mervoyer, A.; Rastrelli, M.; et al. Final Safety and HRQoL Results of the Phase 2/3 Act.In.Sarc Study With Preoperative NBTXR3 Plus Radiation Therapy Versus Radiation Therapy in Locally Advanced Soft-Tissue Sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 422–432.
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Soft Tissue Sarcoma (Version 2). 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf (accessed on 11 November 2023).
- Dangoor, A.; Seddon, B.; Gerrand, C.; Grimer, R.; Whelan, J.; Judson, I. UK Guidelines for the Management of Soft Tissue Sarcomas. Clin. Sarcoma Res. 2016, 6, 20.
- Salerno, K.E.; Alektiar, K.M.; Baldini, E.H.; Bedi, M.; Bishop, A.J.; Bradfield, L.; Chung, P.; DeLaney, T.F.; Folpe, A.; Kane, J.M.; et al. Radiation Therapy for Treatment of Soft Tissue Sarcoma in Adults: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2021, 11, 339–351.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
648
Revisions:
3 times
(View History)
Update Date:
01 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




