Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michael Wester | -- | 1748 | 2023-11-27 16:00:45 | | | |
| 2 | Camila Xu | Meta information modification | 1748 | 2023-11-28 02:22:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wester, M.; Arzt, M.; Sinha, F.; Maier, L.S.; Lebek, S. Phenotypes and Symptoms of HFpEF and Sleep-Disordered Breathing. Encyclopedia. Available online: https://encyclopedia.pub/entry/52100 (accessed on 13 January 2026).
Wester M, Arzt M, Sinha F, Maier LS, Lebek S. Phenotypes and Symptoms of HFpEF and Sleep-Disordered Breathing. Encyclopedia. Available at: https://encyclopedia.pub/entry/52100. Accessed January 13, 2026.
Wester, Michael, Michael Arzt, Frederick Sinha, Lars Siegfried Maier, Simon Lebek. "Phenotypes and Symptoms of HFpEF and Sleep-Disordered Breathing" Encyclopedia, https://encyclopedia.pub/entry/52100 (accessed January 13, 2026).
Wester, M., Arzt, M., Sinha, F., Maier, L.S., & Lebek, S. (2023, November 27). Phenotypes and Symptoms of HFpEF and Sleep-Disordered Breathing. In Encyclopedia. https://encyclopedia.pub/entry/52100
Wester, Michael, et al. "Phenotypes and Symptoms of HFpEF and Sleep-Disordered Breathing." Encyclopedia. Web. 27 November, 2023.
Copy Citation
Heart failure with preserved ejection fraction (HFpEF) is emerging as a widespread disease with global socioeconomic impact. Patients with HFpEF show a dramatically increased morbidity and mortality, and, unfortunately, specific treatment options are limited. One such comorbidity is sleep-disordered breathing (SDB), which affects up to 58% (or up to 80% in certain cohorts) of HFpEF patients. SDB presents in HFpEF patients either as predominantly obstructive sleep apnea (OSA) or as predominantly central sleep apnea (CSA).
heart failure
HFpEF
obstructive sleep apnea
central sleep apnea
1. Introduction
Heart failure with preserved ejection fraction (HFpEF) is a widespread disease with a prevalence of approximately 1% in developed countries and it is expected to rise to more than 5% among elderly populations (i.e., >70 years of age) [1]. The 5-year mortality rates of patients with HFpEF are estimated to be between 55% and 74% [2]. HFpEF symptoms such as dyspnea, fatigue, sleeping difficulties, depression, chest pain [1], and recurrent hospitalizations limit patients’ daily physical and social activities, as well as their capacity to work, thus leading to a poor quality of life [3]. HFpEF causes more than 0.5 million hospitalizations per year in Europe. Notably, hospitalizations contribute to 70–80% of the total health care costs for HFpEF patients, with an average yearly cost of ≈EUR 16,000 per patient [4][5].
These figures and trends are concerning because, in contrast to patients that have heart failure with reduced ejection fraction (HFrEF), currently only one class of pharmacological drugs has been shown to reduce morbidity in patients with HFpEF [1]. Therefore, attention has been redirected to lifestyle interventions such as exercise training [6][7] and the treatment of comorbidities [8] in order to prevent the progression of HFpEF and to reduce patient symptom burdens.
One such comorbidity is sleep-disordered breathing (SDB), which affects up to 58% (or up to 80% in certain cohorts) of HFpEF patients [9][10][11]. SDB presents in HFpEF patients either as predominantly obstructive sleep apnea (OSA) or as predominantly central sleep apnea (CSA) [9]. Treatment of OSA in patients with HFpEF provides an opportunity through which to improve quality of life [12] and exercise capacity [13], and it has the potential to prevent the progression of HFpEF via reductions in the arterial blood pressure and cardiac workload, as well prevention of cardiac remodeling [14][15]. Besides these, therapeutic strategies for patients with HFpEF and SDB are limited, especially with respect to pharmacological interventions. This highlights the need for a better understanding of the presence and effects, as well as its treatment, of SDB in patients with HFpEF.
2. Phenotypes and Symptoms of HFpEF and SDB
2.1. Epidemiology and Diagnosis of HFpEF
Left ventricular diastolic dysfunction, which is a precursor to HFpEF [16], is highly prevalent in asymptomatic community samples, and it affects almost one third of adults aged above 45 years [17]. As increasing age is a major risk factor [1] in the prevalence of diastolic dysfunction, and, as the prevalence of HFpEF is higher in the elderly, nearly half of all patients with heart failure (HF) have a preserved ejection fraction [1], which reaches to 65–77% in patients that are ≥67 years of age [18]. In absolute numbers, women outnumber men (ratio ≈ 2:1) [19]. However, this imbalance is, in part, caused by the higher life expectancy of women and the lower risk of death after the diagnosis of HFpEF; however, this discrepancy is alleviated after accounting for age and other risk factors [20].
Patients with HFpEF have a high rate of recurrent hospitalizations. After an episode of acute HF, the 30 day all-cause readmission rate is up to 21% and the HF-specific readmission rate up to 10%, thus reflecting the large burden of comorbidities in HFpEF patients [21]. The readmission rates for one year are up to 63% and 37% for all-cause and HF-specific readmissions, respectively [21]. The high rates for hospitalizations cause significant costs for the health care systems. Annual costs have been estimated to be up to USD 27,000 (in the USA) [22] or EUR 16,000 (in the EU) [5] per patient. The high prevalence and morbidity of HFpEF impose a significant burden on public health care.
Based on ejection fraction (EF), patients with chronic HF are classified as having HFpEF (EF ≥ 50%), heart failure with mildly reduced ejection fraction (HFmrEF, EF 40–49%), or HfrEF (EF < 40%) according to current European guidelines and position papers [1][23][24].
HFpEF is a clinical syndrome composed of many different etiologies, which complicates the aim of establishing a definition of clear diagnostic criteria [1]. The current guidelines of the European Society of Cardiology (ESC) define HFpEF as the combination of (1) the presence of symptoms and signs of HF (e.g., dyspnea, ankle swelling, and elevated jugular venous pressure), (2) a left ventricular ejection fraction of ≥50%, and (3) elevated levels of natriuretic peptides (BNP ≥ 35 pg/mL and/or NT-pro-BNP > 125 pg/mL) plus structural (left ventricular hypertrophy, left atrial enlargement, etc.) or functional (diastolic dysfunction) heart disease [1].
Also, there are empirically derived scoring systems for the diagnosis of HFpEF. Similar to their guideline criteria, the HFA-PEFF algorithm (Heart Failure Association, and PEFF stands for the steps of diagnostic work up, i.e., pretest assessment, echocardiographic and natriuretic peptide score, functional testing, and final etiology) [23] and the H2PEF score [25] both require normal left ventricular EF, signs or symptoms of HF, structural cardiac remodeling, signs of diastolic dysfunction, and biomarkers (e.g., abnormal brain natriuretic peptide). For patients with intermediate score values, echocardiographic stress testing (i.e., volume challenge or supine exercise) or invasive testing (right heart catheterization) can be added.
2.2. Epidemiology and Diagnosis of SDB
There are two main SDB sub-types that are diagnosed by poly(somno)graphy: central sleep apnea (CSA) and obstructive sleep apnea (OSA) [26]. Mechanistically, in CSA, the central respiratory signal pauses, thereby decreasing or ceasing airflow for ≥10 s [27][28][29]. In patients with OSA, a partial or complete collapse of the upper airway reduces the airflow (hypopnea), or it may even lead to a complete cessation of airflow (apnea) for ≥10 s [29]. An apnea-hypopnea index (AHI) of ≥5 events/h with characteristic symptoms (e.g., witnessed apneas, daytime sleepiness, and snoring) or an AHI of ≥15 events/h (regardless of symptoms) defines OSA [29][30]. The classification of SDB into CSA or OSA is based on the predominant (i.e., ≥50%) type of apneas/hypopneas [27][28][29]. CSA and OSA can occur separately, but could also occur concurrently within the same patient [27][28][29]. Patients with cardiovascular disease, particularly those with central sleep apnea, are less likely to exhibit classic SDB symptoms (which further complicates diagnoses [27][28][29][31][32]).
SDB is a widespread disease, and it currently affects about one billion individuals worldwide, as well as up to 40% in patients with cardiovascular disease [28][33]. In patients with HF (both HFpEF and HFrEF), the prevalence of SDB increases to 50% [9][10]. Notably, HF is associated with a high occurrence of CSA [9][27], whereas the severity of CSA is related to cardiac function.
2.3. Phenotypes of HFpEF—Comorbidities and Cluster Analyses
Ever since HFpEF was acknowledged as a distinct clinical syndrome, it has challenged cardiologists with respect to successfully finding definitive diagnostic criteria and offering effective treatment strategies. The difficulties and failures at this task most likely stem from the diversity of underlying etiologies, pathomechanisms, and relevant comorbidities, which defy a diagnostic or therapeutic “one-size-fits-all” approach [18].
Similar to SDB, HFpEF is strongly associated with several frequent diseases and pathologies such as metabolic syndrome (e.g., diabetes, obesity, and hypertension), pulmonary diseases (e.g., COPD and pulmonary hypertension), coronary artery disease, and chronic kidney disease [1][34]. A sedentary lifestyle and insufficient physical activity denominate a common risk factor for metabolic syndrome, arterial hypertension, and HFpEF [35]. Accordingly, increased physical activity has been shown to be among the few therapeutic strategies through which to realistically improve symptomatic outcomes in patients with HFpEF [6]. All of these comorbidities, specifically HFpEF and SDB, share many pathomechanisms that are closely intertwined and may often reciprocally reinforce their detrimental effects. Therefore, the treatment of those comorbidities may be crucial for avoiding the propagation of HFpEF.
Since the first attempt to phenotype HFpEF, many of the different approaches that utilize machine learning have identified distinct HFpEF phenotypes [36]. Based on clinical considerations, four phenotypes can be discerned: the “aging phenotype”, the “pulmonary hypertension phenotype”, the “coronary artery disease phenotype”, and the “obese phenotype” [37]. This clinical classification has been affirmed and specified by novel artificial intelligence-based deep-learning approaches that are based on clinical evaluation, echocardiographic, ECG, laboratory, and proteomic data [18][36][38] (the reviews of [39][40][41] provide an excellent overview).
The review by Peters et al. summarizes three main phenotypes: the “older, vascular aging phenotype”, the “metabolic, obese phenotype”, and the “relatively young, natriuretic peptide deficiency phenotype” (Figure 1) [41]. It is important to acknowledge that these phenotypes cannot be unequivocally discriminated as features of these phenotypes often overlap, and it is not yet clear if these phenotypes can develop into each other. The “older, vascular aging” phenotype accounts for approximately 30–50% of HFpEF patients. Its characteristics are higher age (>75 years), chronic kidney disease, arterial hypertension (together with arterial stiffness), and a high rate of adverse outcomes [41]. These patients often have elevated pulmonary arterial systolic pressure, as well as left atrial and/or right ventricular dysfunction. It seems that systemic inflammation is prevalent in these patients. The “metabolic, obese” phenotype accounts for approximately 25–30% of HFpEF patients. These patients are slightly younger than the “older” phenotype (60–70 years). These patients are obese and often have diabetes mellitus [41]. Epicardial adipose tissue may mechanistically favor HFpEF development in these patients. The comorbidities promote systemic inflammation, which is a contributing factor for HFpEF development. The last phenotype is the “relatively younger, natriuretic peptide deficiency” phenotype, which accounts for approximately 40–45% of HFpEF patients. These patients are relatively young (around 60 years), and they exhibit lower BNP/NT-pro-BNP levels due to increased adipose clearance. The absence of inflammation distinguishes this phenotype from the “metabolic, obese” phenotype. These patients have the lowest risk for adverse outcomes [41].
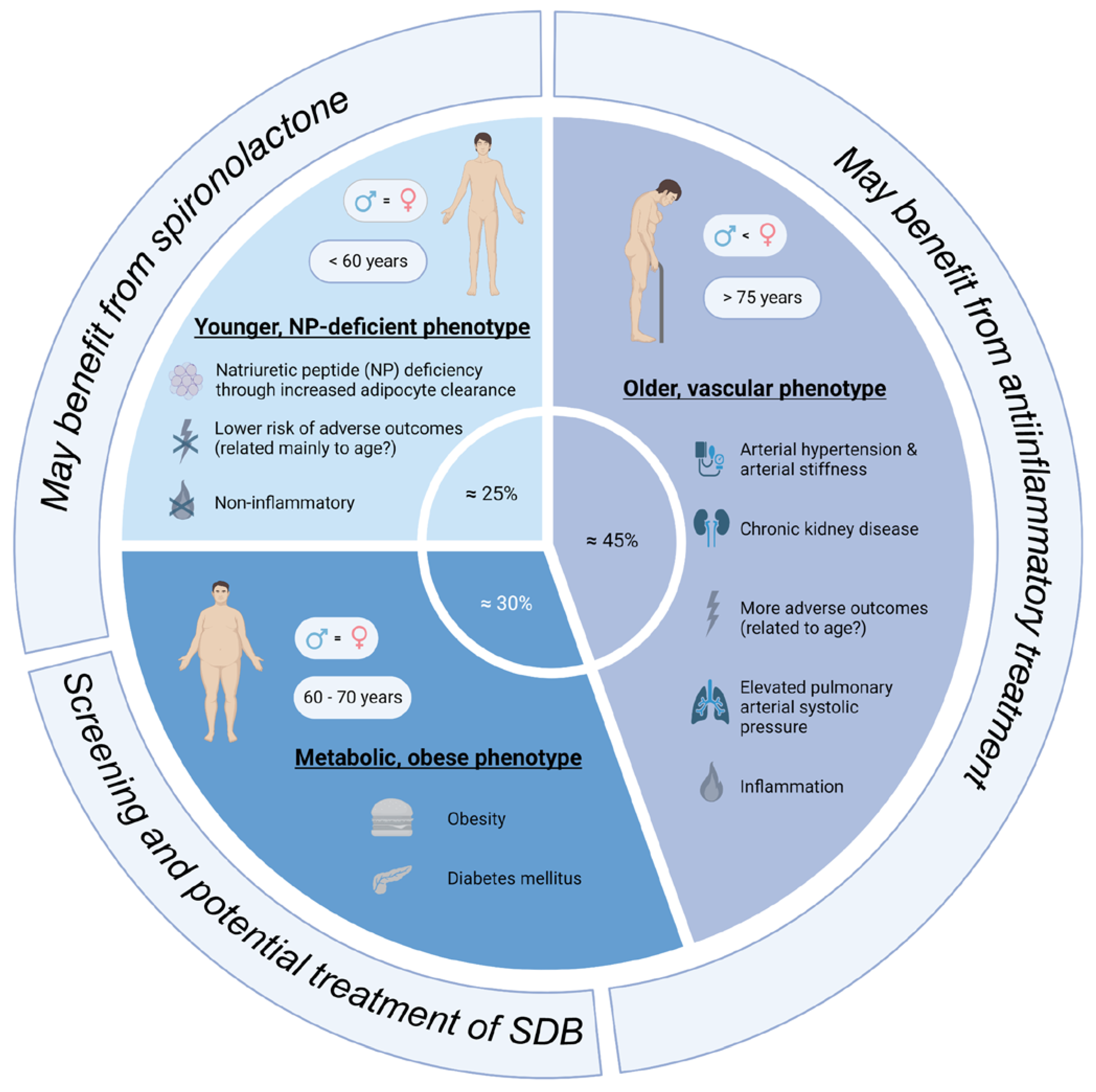
Figure 1. Overview of the main subtypes of HFpEF patients (created with biorender.com, accessed on 23 October 2023).
A cluster analysis of the TOPCAT study cohort also revealed three different phenotypes [42]. However, while the total HFpEF study cohort of TOPCAT did not benefit from treatment with spironolactone, a machine learning approach identified one HFpEF phenotype that, in actuality, showed an improved survival rate [42]. This phenotype was very similar to the “obese phenotype” [37] and the “natriuretic peptide deficiency syndrome” [18], which is highly reminiscent of typical SDB patients (even though SDB was not explicitly mentioned). This “obese phenotype” is characterized by the metabolic syndrome, which increases arterial stiffness, promotes systemic inflammation, and activates the sympathetic nervous system [37]. The most common comorbidities of this phenotype are obstructive sleep apnea, diabetes mellitus, and chronic kidney disease [37]. The prevalence of the “obese phenotype” in the TOPCAT study cohort was 31% [42]. SDB is highly prevalent in obese (40–60%) compared to non-obese (10–20%) HFpEF patients [37][43], which highlights the importance of SDB as a potentially treatable and modifiable comorbidity in HFpEF.
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Lam, C.S.P.; Donal, E.; Kraigher-Krainer, E.; Vasan, R.S. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2011, 13, 18–28.
- Heo, S.; Lennie, T.A.; Okoli, C.; Moser, D.K. Quality of life in patients with heart failure: Ask the patients. Heart Lung 2009, 38, 100–108.
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.P.; Sato, N.; Shah, A.N.; et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133.
- Lesyuk, W.; Kriza, C.; Kolominsky-Rabas, P. Cost-of-illness studies in heart failure: A systematic review 2004-2016. BMC Cardiovasc. Disord. 2018, 18, 74.
- Edelmann, F.; Gelbrich, G.; Düngen, H.-D.; Fröhling, S.; Wachter, R.; Stahrenberg, R.; Binder, L.; Töpper, A.; Lashki, D.J.; Schwarz, S.; et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J. Am. Coll. Cardiol. 2011, 58, 1780–1791.
- Crisci, G.; De Luca, M.; D’Assante, R.; Ranieri, B.; D’Agostino, A.; Valente, V.; Giardino, F.; Capone, V.; Chianese, S.; Rega, S.; et al. Effects of Exercise on Heart Failure with Preserved Ejection Fraction: An Updated Review of Literature. J. Cardiovasc. Dev. Dis. 2022, 9, 241.
- von Haehling, S.; Arzt, M.; Doehner, W.; Edelmann, F.; Evertz, R.; Ebner, N.; Herrmann-Lingen, C.; Garfias Macedo, T.; Koziolek, M.; Noutsias, M.; et al. Improving exercise capacity and quality of life using non-invasive heart failure treatments: Evidence from clinical trials. Eur. J. Heart Fail. 2021, 23, 92–113.
- Arzt, M.; Oldenburg, O.; Graml, A.; Schnepf, J.; Erdmann, E.; Teschler, H.; Schoebel, C.; Woehrle, H. Prevalence and predictors of sleep-disordered breathing in chronic heart failure: The SchlaHF-XT registry. ESC Heart Fail. 2022, 9, 4100–4111.
- Borrelli, C.; Gentile, F.; Sciarrone, P.; Mirizzi, G.; Vergaro, G.; Ghionzoli, N.; Bramanti, F.; Iudice, G.; Passino, C.; Emdin, M.; et al. Central and Obstructive Apneas in Heart Failure With Reduced, Mid-Range and Preserved Ejection Fraction. Front. Cardiovasc. Med. 2019, 6, 125.
- Herrscher, T.E.; Akre, H.; Øverland, B.; Sandvik, L.; Westheim, A.S. High prevalence of sleep apnea in heart failure outpatients: Even in patients with preserved systolic function. J. Card. Fail. 2011, 17, 420–425.
- Gaisl, T.; Rejmer, P.; Thiel, S.; Haile, S.R.; Osswald, M.; Roos, M.; Bloch, K.E.; Stradling, J.R.; Kohler, M. Effects of suboptimal adherence of CPAP therapy on symptoms of obstructive sleep apnoea: A randomised, double-blind, controlled trial. Eur. Respir. J. 2020, 55, 1901526.
- Fox, H.; Bitter, T.; Sauzet, O.; Rudolph, V.; Oldenburg, O. Automatic positive airway pressure for obstructive sleep apnea in heart failure with reduced ejection fraction. Clin. Res. Cardiol. 2021, 110, 983–992.
- Fisser, C.; Götz, K.; Hetzenecker, A.; Debl, K.; Zeman, F.; Hamer, O.W.; Poschenrieder, F.; Fellner, C.; Stadler, S.; Maier, L.S.; et al. Obstructive sleep apnoea but not central sleep apnoea is associated with left ventricular remodelling after acute myocardial infarction. Clin. Res. Cardiol. 2021, 110, 971–982.
- Pengo, M.F.; Soranna, D.; Giontella, A.; Perger, E.; Mattaliano, P.; Schwarz, E.I.; Lombardi, C.; Bilo, G.; Zambon, A.; Steier, J.; et al. Obstructive sleep apnoea treatment and blood pressure: Which phenotypes predict a response? A systematic review and meta-analysis. Eur. Respir. J. 2020, 55, 1901945.
- Kane, G.C.; Karon, B.L.; Mahoney, D.W.; Redfield, M.M.; Roger, V.L.; Burnett, J.C.; Jacobsen, S.J.; Rodeheffer, R.J. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011, 306, 856–863.
- Redfield, M.M.; Jacobsen, S.J.; Burnett, J.C.; Mahoney, D.W.; Bailey, K.R.; Rodeheffer, R.J. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA 2003, 289, 194–202.
- Shah, S.J. 20th Annual Feigenbaum Lecture: Echocardiography for Precision Medicine-Digital Biopsy to Deconstruct Biology. J. Am. Soc. Echocardiogr. 2019, 32, 1379–1395.e2.
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602.
- Dewan, P.; Rørth, R.; Raparelli, V.; Campbell, R.T.; Shen, L.; Jhund, P.S.; Petrie, M.C.; Anand, I.S.; Carson, P.E.; Desai, A.S.; et al. Sex-Related Differences in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2019, 12, e006539.
- Cheng, R.K.; Cox, M.; Neely, M.L.; Heidenreich, P.A.; Bhatt, D.L.; Eapen, Z.J.; Hernandez, A.F.; Butler, J.; Yancy, C.W.; Fonarow, G.C. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am. Heart J. 2014, 168, 721–730.
- Clark, H.; Rana, R.; Gow, J.; Pearson, M.; van der Touw, T.; Smart, N. Hospitalisation costs associated with heart failure with preserved ejection fraction (HFpEF): A systematic review. Heart Fail. Rev. 2022, 27, 559–572.
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639.
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 861–870.
- Arzt, M.; Oldenburg, O.; Graml, A.; Erdmann, E.; Teschler, H.; Wegscheider, K.; Suling, A.; Woehrle, H. Phenotyping of Sleep-Disordered Breathing in Patients With Chronic Heart Failure With Reduced Ejection Fraction-the SchlaHF Registry. J. Am. Heart Assoc. 2017, 6, e005899.
- Cowie, M.R.; Linz, D.; Redline, S.; Somers, V.K.; Simonds, A.K. Sleep Disordered Breathing and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 608–624.
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858.
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394.
- Mehra, R.; Chung, M.K.; Olshansky, B.; Dobrev, D.; Jackson, C.L.; Kundel, V.; Linz, D.; Redeker, N.S.; Redline, S.; Sanders, P.; et al. Sleep-Disordered Breathing and Cardiac Arrhythmias in Adults: Mechanistic Insights and Clinical Implications: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e119–e136.
- Kadhim, K.; Middeldorp, M.E.; Elliott, A.D.; Jones, D.; Hendriks, J.M.L.; Gallagher, C.; Arzt, M.; McEvoy, R.D.; Antic, N.A.; Mahajan, R.; et al. Self-Reported Daytime Sleepiness and Sleep-Disordered Breathing in Patients With Atrial Fibrillation: SNOozE-AF. Can. J. Cardiol. 2019, 35, 1457–1464.
- Arzt, M.; Young, T.; Finn, L.; Skatrud, J.B.; Ryan, C.M.; Newton, G.E.; Mak, S.; Parker, J.D.; Floras, J.S.; Bradley, T.D. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch. Intern. Med. 2006, 166, 1716–1722.
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698.
- Adamczak, D.M.; Oduah, M.T.; Kiebalo, T.; Nartowicz, S.; Bęben, M.; Pochylski, M.; Ciepłucha, A.; Gwizdała, A.; Lesiak, M.; Straburzyńska-Migaj, E. Heart Failure with Preserved Ejection Fraction-a Concise Review. Curr. Cardiol. Rep. 2020, 22, 82.
- Elagizi, A.; Kachur, S.; Carbone, S.; Lavie, C.J.; Blair, S.N. A Review of Obesity, Physical Activity, and Cardiovascular Disease. Curr. Obes. Rep. 2020, 9, 571–581.
- Shah, S.J.; Katz, D.H.; Selvaraj, S.; Burke, M.A.; Yancy, C.W.; Gheorghiade, M.; Bonow, R.O.; Huang, C.-C.; Deo, R.C. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015, 131, 269–279.
- Samson, R.; Jaiswal, A.; Ennezat, P.V.; Cassidy, M.; Le Jemtel, T.H. Clinical Phenotypes in Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2016, 5, e002477.
- Hedman, Å.K.; Hage, C.; Sharma, A.; Brosnan, M.J.; Buckbinder, L.; Gan, L.-M.; Shah, S.J.; Linde, C.M.; Donal, E.; Daubert, J.-C.; et al. Identification of novel pheno-groups in heart failure with preserved ejection fraction using machine learning. Heart 2020, 106, 342–349.
- Galli, E.; Bourg, C.; Kosmala, W.; Oger, E.; Donal, E. Phenomapping Heart Failure with Preserved Ejection Fraction Using Machine Learning Cluster Analysis: Prognostic and Therapeutic Implications. Heart Fail. Clin. 2021, 17, 499–518.
- Heinzel, F.R.; Shah, S.J. The future of heart failure with preserved ejection fraction: Deep phenotyping for targeted therapeutics. Herz 2022, 47, 308–323.
- Peters, A.E.; Tromp, J.; Shah, S.J.; Lam, C.S.P.; Lewis, G.D.; Borlaug, B.A.; Sharma, K.; Pandey, A.; Sweitzer, N.K.; Kitzman, D.W.; et al. Phenomapping in heart failure with preserved ejection fraction: Insights, limitations, and future directions. Cardiovasc. Res. 2023, 118, 3403–3415.
- Cohen, J.B.; Schrauben, S.J.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Yarde, M.; Wang, Z.; Bhattacharya, P.T.; Chirinos, D.A.; et al. Clinical Phenogroups in Heart Failure With Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail. 2020, 8, 172–184.
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation 2017, 136, 6–19.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
438
Revisions:
2 times
(View History)
Update Date:
28 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




