The reasons initiating insulin resistance are not identified. Various metabolic derailments have been characterized. These are the outcome and not the initiation of insulin resistance. In animal models of type 2 diabetes and hypertension, a decreased hormonal stimulation of the synthesis of the cyclic AMP antagonist prostaglandylinositol cyclic phosphate (cyclic PIP) was determined. The resultant imbalance of the action of cyclic AMP and cyclic PIP shifts metabolic regulation to the dominance of catabolism and a decrease in imperative anabolism. This dominance develops gradually since the more cyclic AMP dominates, the more the synthesis of cyclic PIP will be inhibited. Vanishing actions of cyclic PIP are its 10-fold activation of glucose uptake in adipocytes, its inhibition of insulin release from pancreatic β-cells, its inhibition of PKA and its 7-fold activation of protein ser/thr phosphatase.
1. Introduction
Type 2 diabetes and hypertension are acute health problems of today’s world. The percentage of people with type 2 diabetes is continuously increasing, and there are at least 422 million people with diabetes and 1.28 billion adults with hypertension worldwide according to the WHO. Many scientists are engaged in identifying the primary causes that initiate the development of insulin resistance, a starting condition of type 2 diabetes. Using various inbred mouse strains, Nelson et al. determined the effects of diet on the development of insulin resistance primarily in muscle, liver and white adipose tissue
[1], and Mejhert and Ryden commented on this report
[2]. Petersen and Shulman published a comprehensive and detailed review on the “mechanism of insulin action and insulin resistance” and came to the conclusion that the “sheer complexity of biological systems means that any effort to understand insulin resistance with a unified, succinct and straightforward model may be a fool’s errand”
[3]. Galicia-Garcia et al.
[4] proposed that type 2 diabetes is caused by a combination of two primary factors, namely a defective insulin secretion of the pancreatic β-cells and the inability of insulin-sensitive tissues to respond appropriately to insulin, and they concluded that there is still a long way to go to fully understand insulin resistance.
The mechanism of insulin action, the understanding of which is a prerequisite for determining defects in insulin’s action, appears to be nearly solved: Insulin binds to its receptor, whose intrinsic tyrosine kinase is in this way activated; this kinase auto-phosphorylates itself and then phosphorylates primarily the insulin receptor substrates IRS1-4 and several Shc proteins
[5][6]. The IRS proteins are suggested to regulate metabolism, whereas Shc proteins appear to be involved in the regulation of proliferation by insulin. The IRS proteins are considered to be docking proteins to which various enzymes bind via Src-homology-2 (SH-2) domains and send signals further into a cell via phosphorylation pathways. Additionally, the IRS proteins are inactivated by serine/threonine phosphorylation by several different protein ser/thr kinases
[5].
Earl Sutherland articulated many years ago that a decrease in the strength of the insulin signal will increase the strength of the counterregulatory signal, leading to the dominance of regulations triggered by cyclic AMP
[7]. This view has not been adequately pursued. A characteristic of insulin resistance is that it progresses slowly and gradually for reasons not fully understood at the present time. Inhibition of single proteins/enzymes such as the IRS proteins cannot explain this slow progression, though they play a crucial role in signal transduction. Current thinking is that protein phosphorylation pathways are of primary importance
[5][6], but fails to consider that insulin antagonizes glucagon action. Insulin rapidly reverses cyclic AMP-triggered protein ser/thr phosphorylation. The key player in this scenario is the natural cyclic AMP antagonist, prostaglandylinositol cyclic phosphate (cyclic PIP)
[8][9]. The synthesis of cyclic PIP is stimulated for instance by insulin and also noradrenaline. Cyclic PIP synthesis is decreased in type 2 diabetic and also hypertensive rodents
[8].
2. Cyclic AMP
After its discovery by Earl Sutherland, cyclic AMP was for many years viewed as the unique intracellular regulator that activates the cyclic AMP-dependent protein kinase (PKA). By protein ser/thr phosphorylation, catabolic enzymes are predominantly activated and anabolic enzymes are predominantly inactivated. Further ways of action of cyclic AMP are (a) its activation of guanine exchange factors, (b) its binding to cyclic AMP binding proteins and (c) its regulation of cyclic nucleotide-gated ion channels
[10]. Cyclic AMP synthesis is stimulated for instance by glucagon and adrenaline via its β-receptors. Adenylate cyclase is activated by the Gαs subunit of the stimulatory, heterotrimeric Gs protein
[11]. The reversal of this activation is controlled by the GTPase activity of the Gαs protein which converts the bound GTP to GDP inactivating adenylate cyclase. Cyclic PIP inhibits adenylate cyclase dose-dependently up to 100%
[8]. In contrast, the Gi protein inhibits adenylate cyclase by approximately 30% at maximal hormone concentration
[12]. This effect is declared to be an adrenergic α
2-receptor action (see below).
3. The Natural Cyclic AMP Antagonist Cyclic Prostaglandylinositol Phosphate
The search for an antagonist to cyclic AMP started in the laboratory of the late Earl Sutherland. Cyclic PIP was primarily isolated from rat livers, which were extra-corporally perfused with buffer and stimulated with noradrenaline or insulin, homogenized within 1 to 3 min, and then put under denaturing conditions to minimize the enzymatic degradation of cyclic PIP. From 2 kg of rat livers, approximately 60 g of water-soluble, low-molecular-weight compounds were obtained, which also contained cyclic PIP. A rough calculation indicated that 500,000 to 1,000,000 molecules of this extract contain 1 molecule of cyclic PIP. But more challenging was the finding that cyclic PIP is one of the most labile molecules of this extract. It decays by 80% within 30 min held at an ionic strength comparable to a 0.5 molar sodium chloride solution, approximately a 3-fold concentrated physiological saline solution. The labilities of cyclic PIP are connected to the tension of the 5-ring phosphodiester, which is the most labile bond of cyclic PIP, as well as the allyl-ether bond combining two secondary alcohols and the beta-keto-hydroxy structure of the C5-ring of prostaglandin E (PGE). Chemically it is O-(prostaglandyl-E)-(15-4′)-(
myo-inositol 1′:2′-cyclic phosphate)
[8]. It is biosynthesized from PGE and activated inositol phosphate by cyclic PIP synthase
[8], which is active in a tyrosine-phosphorylated form
[13]. The synthesis of cyclic PIP is stimulated also by adrenergic α
1- and α
2-receptors
[14]. This result contradicts the present view of adrenergic α
1- and α
2-receptor action, as discussed in
[14][15]. The adrenergic receptors are transmembrane, G protein-coupled receptors; thus, cyclic PIP synthase should also be activated by a G protein
[13]. It is not known whether there are two different variants of cyclic PIP synthase, but it is known that the biosynthesis of the substrates for cyclic PIP synthesis involves phospholipase A2 and phospholipase C, both of which are activated by these two modes of activation as summarized in
[15]. The synthesis of cyclic AMP from ATP is a one-step reaction, and ATP is generally always present in a high enough amount in all cells to warrant maximal synthesis of cyclic AMP. In contrast, the biosynthesis of cyclic PIP needs at least five reaction steps (
Figure 1), and both substrates are obtained from membrane-bound lipids
[15].
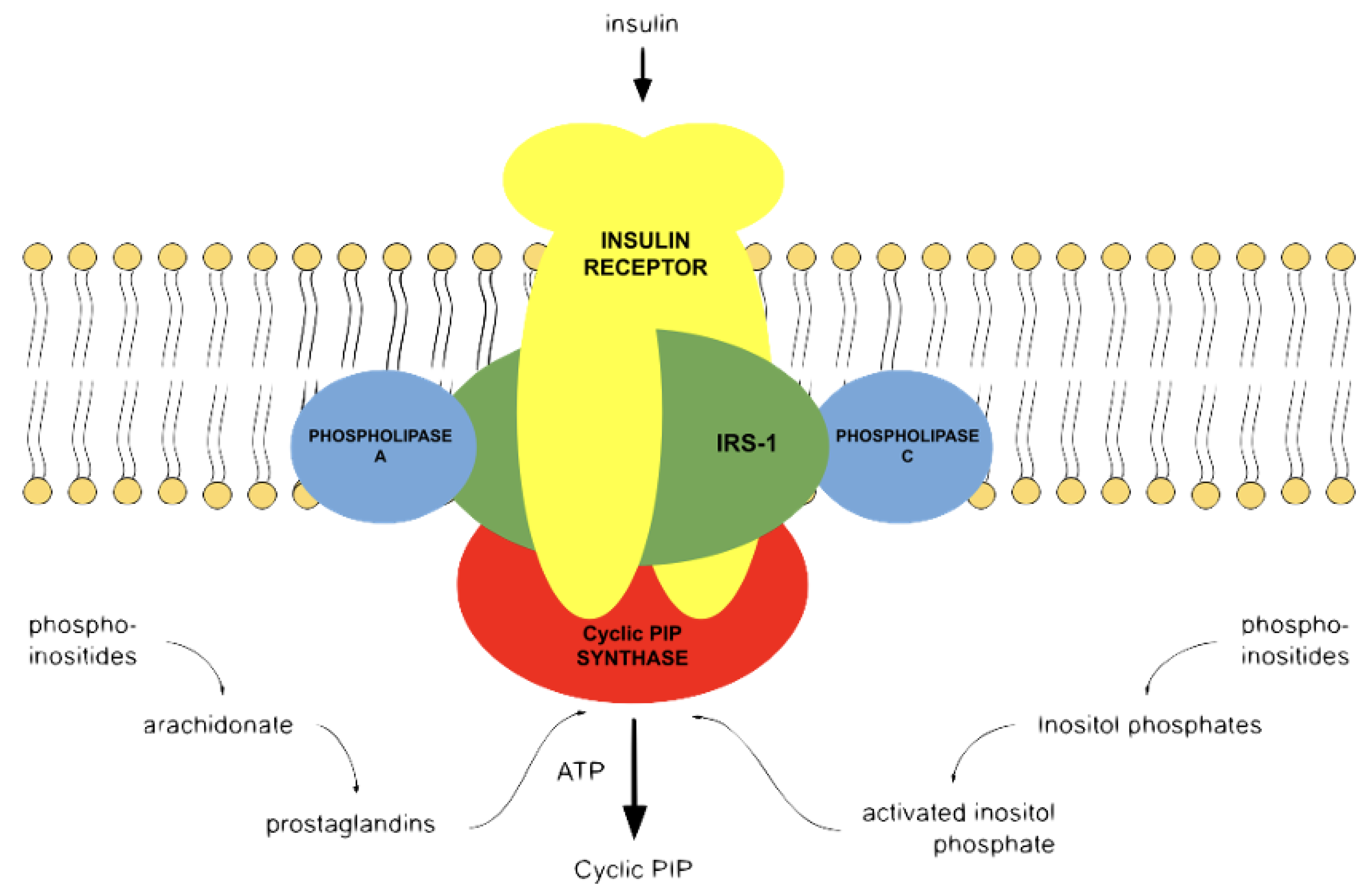
Figure 1. Cyclic PIP biosynthesis. After hormonal stimulation, phospholipase A2 liberates unsaturated fatty acids from membrane-bound phospholipids, and these unsaturated fatty acids are converted to prostaglandins; phospholipase C liberates various inositol phosphates, of which one is converted to activated inositol phosphate
[8]. Cyclic PIP synthase combines the inositol 1:2-cyclic phosphate part of activated inositol phosphate and PGE to cyclic PIP. The ATP is solely needed to activate cyclic PIP synthase by tyrosine phosphorylation
[13]. Apart from the insulin receptor tyrosine kinase, other protein kinases and protein phosphatases discussed in the text are not shown.
The primary action of cyclic AMP is to activate PKA, whereas cyclic PIP inhibits PKA. PKA is activated by increasing concentrations of cyclic AMP (10
−8 to 10
−6 molar) at least 10-fold. Increasing concentrations of cyclic PIP led to increasing, up to 100%, inhibition of basal and cyclic AMP-activated PKA. For an equal percentage of inhibition of basal and cyclic AMP-activated PKA, approximately 4 times more cyclic PIP is needed in the case of the cyclic AMP-activated kinase
[14][16]. It was assumed that protein ser/thr phosphatase, the counter-regulatory enzyme to PKA, would also be regulated by these two compounds. Phosphatase is a rather labile enzyme. In order to cope with this problem, scientists decided to isolate and characterize the catalytic subunits of this class of enzymes
[17]. For this reason, the holoenzyme of protein ser/thr phosphatase was partly purified
[9]. The obtained holoenzyme has a slightly higher molecular weight than PKA and is 7-fold activated by cyclic PIP and completely inhibited by cyclic AMP in the physiological action range
[8]. The effects of cyclic PIP in viable cells are as follows: (1) 10-fold activation of glucose uptake into adipocytes; (2) shut-off of insulin release from pancreatic β-cells; (3) inhibition of glucagon-stimulated autophagy and proteolysis; (4) 2,7-fold positive inotropic effect on the papillary muscle of the heart, which is, different from the action of cyclic AMP, connected with an elongation of the contraction time
[8]. As was the case with cyclic AMP
[10], further modes of action of cyclic PIP are likely to be found in the coming years as the chemical synthesis of cyclic PIP allows additional studies.