Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiangrong Chen | -- | 2485 | 2023-11-23 02:31:25 | | | |
| 2 | Rita Xu | Meta information modification | 2485 | 2023-11-23 02:43:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, X.; Abdallah, M.F.; Chen, X.; Rajkovic, A. Toxicities of AFB1 and FB1 In Vitro. Encyclopedia. Available online: https://encyclopedia.pub/entry/51958 (accessed on 08 February 2026).
Chen X, Abdallah MF, Chen X, Rajkovic A. Toxicities of AFB1 and FB1 In Vitro. Encyclopedia. Available at: https://encyclopedia.pub/entry/51958. Accessed February 08, 2026.
Chen, Xiangrong, Mohamed F. Abdallah, Xiangfeng Chen, Andreja Rajkovic. "Toxicities of AFB1 and FB1 In Vitro" Encyclopedia, https://encyclopedia.pub/entry/51958 (accessed February 08, 2026).
Chen, X., Abdallah, M.F., Chen, X., & Rajkovic, A. (2023, November 23). Toxicities of AFB1 and FB1 In Vitro. In Encyclopedia. https://encyclopedia.pub/entry/51958
Chen, Xiangrong, et al. "Toxicities of AFB1 and FB1 In Vitro." Encyclopedia. Web. 23 November, 2023.
Copy Citation
Mycotoxins stand out as some of the most threatening natural contaminants in food. Among these, aflatoxin B1 (AFB1) and fumonisin B1 (FB1) are prominent fungal metabolites, representing significant food safety risks due to their widespread co-occurrence in various food commodities and their profound toxic effects on humans.
mycotoxins
aflatoxin B1
fumonisin B1
combined toxicity
food safety
1. Introduction
Mycotoxin contamination in food represents serious threats toward public health [1]. Mycotoxins are known as toxic secondary metabolites, produced by several toxigenic fungal species, which invade agricultural/farm produce, under certain favorable environmental conditions [2]. Currently, more than 400 mycotoxins (including aflatoxins, citrinin, culmorin, ochratoxins, fumonisins, patulin, zearalenone, diacetoxyscirpenol, sterigmatocystin, nivalenol, T-2, HT-2, deoxynivalenol, enniatins, beauvericin, moniliformin, fusaproliferin, fusaric acid, mycophenolic acid, alternariol, alternariol monomethyl ether, tenuazonic acid, and ergot alkaloid) have been documented from a wide array of toxigenic fungal species, from Aspergillus, Fusarium, Penicillium, and Claviceps purpurea genera [3]. Among them, aflatoxin B1 (AFB1) and fumonisin B1 (FB1) are the most prominent compounds linked to a variety of serious human health disorders [4][5].
AFB1 is a difuranocoumarin derivative (Figure 1), produced mainly by toxigenic Aspergillus flavus and Aspergillus parasiticus species, and it contaminates different crops, such as nuts, dried fruits, oilseeds, and maize and other cereals. Since the discovery of AFB1 in 1960, after the famous incidence where it killed 100,000 young turkeys in the UK, which was called, at that time, Turkey X disease, several fatal outbreaks have been associated with the consumption of AFB1-contaminated food, as reported in India (the states of Gujrat and Rajasthan in 1974) and in Kenya (Eastern and Central Provinces, in 2004 [6]). The International Agency for Research on Cancer (IARC) classified AFB1 as a carcinogenic agent (group 1 carcinogens), due to its potent hepatocellular carcinoma (HCC) in human [7]. Other toxic effects of AFB1 include immunotoxic, mutagenic, and teratogenic properties in humans [8][9][10]. To protect the public against these effects, several national and international organizations have set regulatory limits for many mycotoxins in different food commodities, according to several factors, such as the toxic effect, contamination rate, and exposure. For instance, the European Union (EU) has set different regulatory limits for AFB1 in ready-to-eat dried figs (6 μg/kg), different types of nuts (5 μg/kg for hazelnuts and Brazil nuts; 8 μg/kg for almonds, pistachios, and apricot kernels; and 2 μg/kg for groundnuts), maize (2 μg/kg), and dried spices (5 μg/kg) [11].
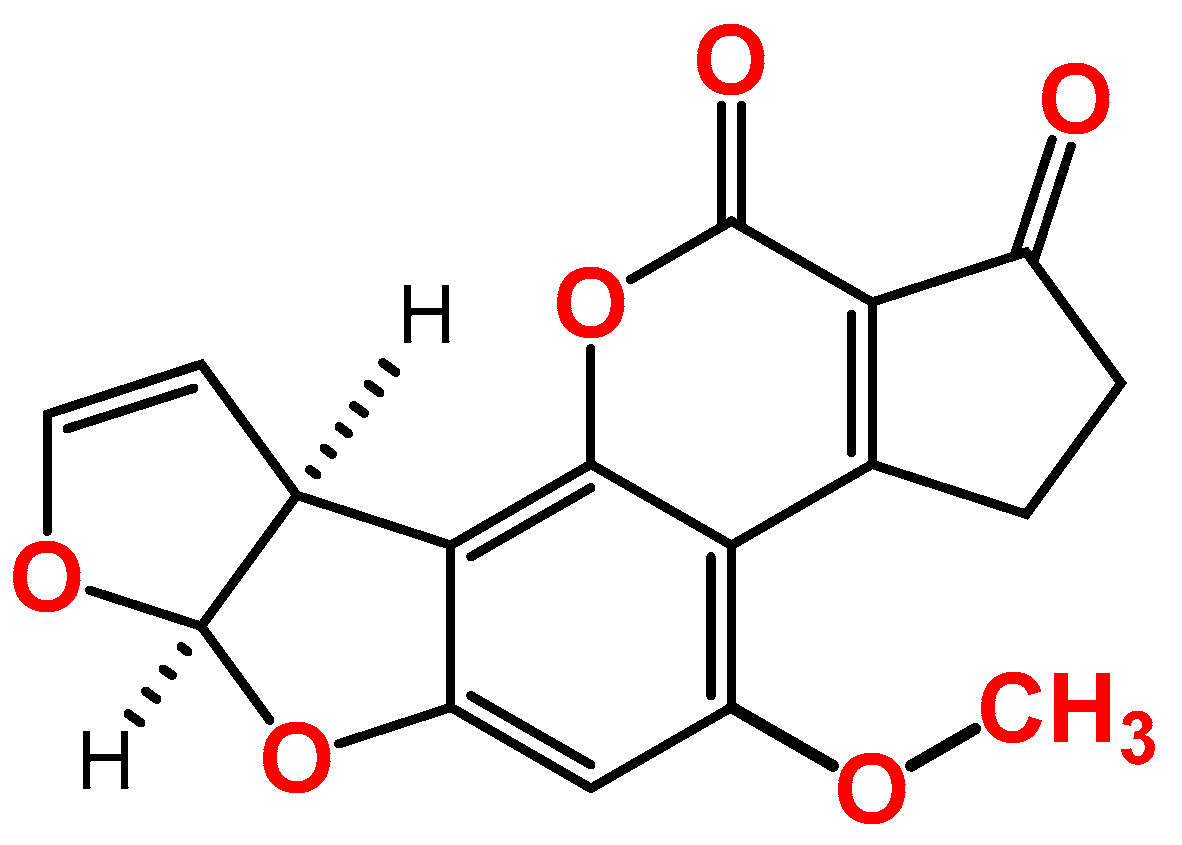
Figure 1. Chemical structure of aflatoxin B1.
FB1, a sphingosine analogue compound (Figure 2), was the first member of the fumonisin family to be described and characterized, in 1988, after isolation from the F. moniliforme MRC 826 fungus. The toxin is mainly produced by Fusarium verticillioides and Fusarium proliferatum species in cereals, including in corn (maize) and corn-based foods, but also in other cereals, such rice, oat, rye, barley, and wheat [12][13], and several foodborne outbreaks due to the consumption of FB1-contaminated food have been reported over the years in the world [14]. The IARC classified FB1 as a class 2B carcinogen (possible human carcinogen) [15]. It was suggested that FB1 could be associated with the incidence of esophageal cancer in humans in some areas of the world where FB1-contaminated maize is consumed daily, such as South Africa, Iran, and China [16][17]. The toxin poses other toxic effects, such as immunotoxicity, hepatotoxicity, and nephrotoxicity. The EU has set a maximum limit of 2000 μg/kg for the sum of FB1 and FB2 in processed maize for the final consumer [11]. Recently, the European Food Safety Authority (EFSA) has lowered the tolerated daily intake of FB1 to 1 μg/kg bw/day [11][18].

Figure 2. Chemical structure of fumonisin B1.
Both AFB1 and FB1 can co-occur in a variety of agricultural commodities, especially maize [19][20]. Therefore, humans are frequently co-exposed to both toxins on a daily basis. This co-exposure is likely to increase in the future when considering climate change as it is expected that the above mentioned mycotoxin-producing fungal species will be more toxigenic and, therefore, produce more AFB1 and FB1 at higher levels than those usually detected in the last decades [21]. In general, the co-exposure to two or more toxins may lead to additive, synergistic, or antagonist toxic effects [22][23][24]. EFSA has already developed some approaches for the exposure assessment of multiple pesticides and other contaminants in humans. Yet, the question regarding what the toxic outcome would be from the co-exposure to AFB1 and FB1, at different doses or scenarios of exposure, still remains unanswered.
2. Overview of the Toxic Effects of AFB1 In Vitro
Most of the available toxicological knowledge on aflatoxins is related to AFB1. AFB1 in different cell lines for human liver, kidney, intestines, bronchia, male genital system, bone, bone marrow, mammary gland, colon, and brain. Most studies focused on liver, intestine, and kidney as the main toxic effects of AFB1 include hepatotoxicity, enterotoxicity, and nephrotoxicity, respectively. The main selected models to investigate the toxicity of AFB1 in liver and intestine were HepG2 (human hepatocellular carcinoma) cells and Caco-2 (human colorectal adenocarcinoma) cells. HepG2 cells, originally derived from liver biopsies of a 15-year-old Caucasian male with a differentiated hepatocellular carcinoma, are frequently used as an in vitro alternative to primary human hepatocytes for studying the hepatotoxicity of xenobiotics. This is owing to their highly differentiation capability, and displaying many of the genotypic features of normal liver cells [25]. Also, these cells are able to synthesize plasma proteins, bile acid, and glycogen, as well as other functions, such as cholesterol and triglyceride metabolism, lipoprotein metabolism and transport, and insulin signaling. The Caco-2 cells have been applied in various intestinal studies with a high flexibility, high repeatability, and low cost [26]. In particular, as a model of intestinal epithelial barrier, it can spontaneously differentiate into a monolayer of cells with the characteristic of absorbing intestinal epithelial cells, with a brush border layer.
Once it is absorbed by the small intestine, AFB1 is metabolized in hepatic cells by cytochrome CYP450s enzymes, predominantly liver-localized enzymes, to the ultimate carcinogen AFB1-exo-8,9-epoxide [27]. This intermediate highly electrophilic metabolite reacts chemically with DNA and, therefore, causes mutations. However, AFB1 is also metabolized into many hydroxylation compounds through the P450 system, including aflatoxin Q1, aflatoxin P1, aflatoxin B2a, aflatoxin M1, aflatoxicol, and aflatoxicol H1 [27]. Apoptosis or programmed cell death is an evolutionarily conserved mechanism for the selective removal of aging, damaged, or other unwanted cells [28]. This mechanism plays a fundamental role in many physiological processes, and its deregulation can lead to a variety of pathological conditions, including carcinogenesis [28]. In Figure 3, AFB1 mainly activate apoptosis, by inducing several pathways: (1) oxidative stress, (2) mitochondrial pathway, (3) endoplasmic reticulum (ER) stress response, (4) Fas/FasL (Fas ligand) signaling pathway, (5) tumor necrosis factor-alpha (TNF-α) signal pathway (a key cytokine involved in inflammation, immunity, cellular homeostasis, and tumor progression) [29][30][31][32]. Oxidative stress is defined as an imbalance between the increased ROS and a low antioxidant mechanism activity. Increased oxidative stress can lead to damage to the cellular structure [33]. In oxidative stress, AFB1 can decrease antioxidant protein activities (glutathione, superoxide dismutase, and catalase), and increase the concentration of malondialdehyde, to trigger reactive oxygen species (ROS) production [34]. In addition, the oxidative stress caused by AFB1 disrupts mitochondrial function to induce apoptosis, and the manifestation is DNA damage [32][35]. DNA damage can disrupt mitochondrial homeostasis, and induce metabolic pathways resulting in mitochondrial dysfunction [36]. Studies showed that AFB1 increased the expression of anti-apoptotic proteins (Bcl-2 and Bcl-XL), significant mediators of apoptosis (caspase-9, caspase-3, and caspase-8), and decreased the expression of pro-apoptotic proteins (Bax, Bak, and Bid), to induce mitochondrial dysfunction and apoptosis [37]. Recent studies also showed that AFB1 exposure increased the ER stress via the activation of p53, AMP-activated protein kinase, the mammalian target of rapamycin (mTOR), and the c-Jun NH2-terminal kinases [38][39]. Among these activations under the ER stress, AFB1 activated p53 signaling, to disrupt mitochondrial function, to invoke cell apoptosis [37]. High concentrations of AFB1 (100 and 105 μM) suppressed p53 protein expression, and low doses of AFB1 exposure (10 and 16.9 μM) ameliorated this protein expression [40][41][42][43]. From the signaling pathways summarized above, mitochondria were essential mediators of these pathways. In addition to AFB1 impairing organ function by inducing apoptosis through these signaling pathways, the toxin can specifically disrupt cytochrome P450 activities, to trigger liver damage [44][45][46][47].

Figure 3. Mechanisms of aflatoxin B1 (AFB1) and fumonisin B1 (FB1) toxicity.
3. Overview of the Toxic Effects of FB1 In Vitro
FB1 is a water-soluble molecule, and typically has a low bioavailability (3–6%). It is rapidly distributed in liver and kidney, extensively biotransformed, and rapidly excreted, mostly in feces [48]. It is reported that the hydrolytic biotransformation metabolites, pHFB1 and HFB1, are present in limited amounts in body tissues [48]. FB1 toxicities in cell models of liver, intestine, bone, colon, brain, esophagus, and endothelia. As FB1 toxicities are associated with hepatotoxicity and enterotoxicity, most of these studies (n = 14) investigated the effect of FB1 in liver and intestine in which HepG2 and Caco-2 cells were the in vitro models of choice, accounting for 100% and 60%, respectively.
Around 57% of the presented 14 studies indicated that FB1 toxicity was related to the biosynthesis of sphingolipids, which are fundamental components of eukaryotic cells [49]. In addition to playing structural roles in cell membranes (including the synthesis of metabolites of ceramide, sphingosine, and sphingosine-1-phosphate), sphingolipids have attracted attention as bioactive signaling molecules involved in regulating cell growth, differentiation, aging, and apoptosis [50]. As the chemical structure of FB1 resembles sphingolipids, FB1 interferes with the metabolism of sphinganine and sphingosine in the synthesis of ceramide in mitochondria, complicating the sphingolipid biosynthesis pathway, and causing mitochondrial fragmentation [51][52]. Ceramide synthases are integral membrane proteins of the ER, and FB1 could inhibit ceramide synthases [53][54]. Based on the above studies [51][52][53][54], it indicates that FB1 could inhibit ceramide synthases, to affect all pathways and, consequently, invoking cell apoptosis. The mechanisms behind FB1-induced toxicity (Figure 3) include the induction of oxidative stress, the mitochondrial pathway, and ER stress (mTOR) [29][55][56]. In the oxidative stress pathway, FB1 has been shown to induces cytotoxicity, lipid peroxidation, ROS, and DNA damage in cell models of the liver, intestine, brain, and endothelia [57][58][59]. In the mitochondrial pathway, FB1 have the toxic effect to induce mitochondrial dysfunction [29][60]. Chen et al. reported, using Seahorse Respirometry Analysis, that FB1 induced mitochondrial membrane potential (MMP) damage and mitochondrial dysfunction, to disrupt the electron transport chain (ETC), and inhibit ATP production, after exposure for 24 h, in both HepG2 cells and Caco-2 cells [29]. Also, Khan et al. reported an alteration in MMP and ATP production following the exposure of oesophageal (SNO) cancer cells to FB1 for 48 h [60]. In the ER stress pathway, FB1 is attributed to the activation of the IRE1 α -JNK axis, the suppression of mTOR, and the activation of LC3I/II to reduce cellular apoptosis and autophagy in HepG2 cells [56]. In summary, FB1 could inhibit ceramide synthases, induce oxidative stress, disrupt mitochondrial pathway, and suppress the ER stress pathway to show the toxic effects to the human based on the in-vitro data.
4. Combined Toxicity of AFB1 and FB1 in Human Cells
The combined exposure to AFB1 and FB1 is of concern to public health. It has been reported that a synergistic interaction between AFB1 and FB1 is present via the induction of cell apoptosis [61][62]. Du et al. showed a synergistic interaction after HepG2 cell exposure to two sets of combinations: (1) 0.1 μM AFB1 and one μM FB1, (2) 5 μM AFB1 and 85 μM FB1 for 24 h. This synergistic interaction is related to the expression of apoptosis proteins (Bax, Caspase 3, and p53) via immunocytochemistry analysis [61]. Also, the authors reported that the synergetic proapoptotic activity of AFB1 and FB1 was likely caused by different mechanisms, due to the expression of the antagonistic caspase 8 [61]. In addition, the study by Mary et al., suggested a possible synergistic interaction toward genotoxicity in BRL-3A cells a mixture of AFB1 (20 μM) and FB1 (30 μM) after 48 h. including an increase in the arachidonic acid metabolism, cytochrome P450 activity, and p53 protein levels [62]. In this interaction, they argued that AFB1 had a major input into the mixture’s prooxidant activity, with cytochrome P450 and arachidonic acid being ROS contributors, but that FB1 was weak at invoking these pathways [62]. Chen et al. have also reported that the mixture of AFB1 (25.6 μM) and FB1 (224 μM) significantly increased the p53 protein, and downregulated the mitochondrial complexes in HepG2 cells [63]. Although the selected concentrations in the binary mixture of AFB1 and FB1 is different than the above mentioned studies, the ratio of both toxins is less than 20, and the synergistic interaction is still valid in hepatocytes. In addition, the same authors demonstrated that FB1 is contributing more than AFB1 to the mixture effects, based on RNA transcriptomic analysis [63], which is consistent with previous studies that showed that the binary mixture of AFB1 and FB1 would synergistically raise the hepatocarcinogenic properties. As shown in Figure 3, with AFB1 and FB1 having different mechanisms of action, there could be a potential of promoting each other via crossing pathways. In liver tumors, when AFB1 and FB1 were combined, the disruption of sphingolipid metabolism was promoted, which suggested that alterations in the associated sphingolipid signaling pathways were potentially responsible for the promotional activity of FB1 toward AFB1 [64]. Furthermore, FB1 could promote hepatocarcinogenesis when co-exposed to along with AFB1 [64]. Similarly, Torres et al. stated that FB1 has a potential to modulate AFB1 hepatoxicity, because FB1 could inhibit ceramide synthases, and the inhibition of sphingolipid signaling pathways could contribute to the tumorigenicity of AFB1 [65]. Therefore, within some ranges of combined AFB1 and FB1, they could cause synergistic toxicity in humans. At a lower ratio of combination (lower than 20) for both mycotoxins, the interaction is synergistic in the process of apoptosis in hepatic cells, such as the expression of the apoptosis-associate Bax and Bcl-2 proteins. However, when the combined ratio is slightly higher, the interaction of the two mycotoxins would no longer show an apparent synergistic effect but gradually tend toward an additive effect [61]. The combination of AFB1 (10 μM) and FB1 (300 μM) only increased the Bax, Caspase-8, Caspase-3, and p53, without a synergistic effect in HepG2 cells, and the combined ratio of AFB1 and FB1 is 30 (FB1/AFB1). On the other hand, an antagonistic interaction between AFB1 and FB1 may happen. McKean et al. mentioned a weak antagonistic effect in HepG2 cells of AFB1 and FB1 [66]. The combined AFB1 (1 μM) and FB1 (399 μM) did not reduce the cell viability of HepG2 cells after 24 h, and this combination ratio (FB1: 399 μM/AFB1: 1 μM = 399) is the highest applied in vitro concentrations found in the literature [66]. The summarized data showed that the combined ratio of AFB1 and FB1 could be the main parameter that affects the interaction of both toxins in hepatic cells. In their study, a strong additive interaction was found in BEAS-2B (human bronchial epithelial) cells after exposure to the combined AFB1 (100 μM) and FB1 (355.1 μM) over 24 h [66]. The interaction between these two toxins would vary, depending on the organs. These findings indicate that the interaction of AFB1 and FB1 is mainly manifested as a synergistic effect, and the additive/synergistic effect is primarily regulated by their ratio and organs. Therefore, the AFB1 and FB1 mixture may enhance toxic effects, and carry a more significant risk factor than their individual presence.
References
- Milicevic, D.; Nedeljkovic-Trailovic, J.; Masic, Z. Mycotoxins in food chain: Risk assessment and importance for public health. Tehnol. Mesa 2014, 55, 22–38.
- Gurikar, C.; Shivaprasad, D.P.; Sabillón, L.; Gowda, N.A.N.; Siliveru, K. Impact of mycotoxins and their metabolites associated with food grains. Grain Oil Sci. Technol. 2023, 6, 1–9.
- Palumbo, R.; Crisci, A.; Venâncio, A.; Abrahantes, J.C.; Dorne, J.L.; Battilani, P.; Toscano, P. Occurrence and co-occurrence of mycotoxins in cereal-based feed and food. Microorganisms 2020, 8, 74.
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. 2020, 60, 2773–2789.
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372.
- Kumar, P.; Mahato, D.K.; Mohanta, K.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170.
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767.
- Wouters, A.T.B.; Casagrande, R.A.; Wouters, F.; Watanabe, T.T.N.; Boabaid, F.M.; Cruz, C.E.F.; Driemeier, D. An outbreak of aflatoxin poisoning in dogs associated with aflatoxin B1-contaminated maize products. J. Vet. Diagn. Investig. 2013, 25, 282–287.
- IARC. Aflatoxin: Scientific Background, Control, and Implications; IARC (International Agency for Research on Cancer): Paris, France, 2012.
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161.
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006 (Text with EEA relevance). Off. J. Eur. Union 2023, 119, 103–157.
- Chen, J.; Wen, J.; Tang, Y.T.; Shi, J.C.; Mu, G.D.; Yan, R.; Cai, J.; Long, M. Research progress on fumonisin B1 contamination and toxicity: A review. Molecules 2021, 26, 5238.
- Gelderblom, W.C.A.; Jaskiewicz, K.; Marasas, W.F.O.; Thiel, P.G.; Horak, R.M.; Vleggaar, R.; Kriek, N.P.J. Fumonisins—Novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1998, 54, 1806–1811.
- Rosiles, M.R.; Bautista, J.; Fuentes, V.O.; Ross, F. An outbreak of Equine leukoencephalomalacia at Oaxaca, Mexico, sssociated with Fumonisin B1. J. Vet. Med. A Physiol. Pathol. Clin. Med. 1998, 45, 299–302.
- IARC. International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Paris, France, 2002; Volume 96, pp. 1–390.
- Shetty, P.H.; Bhat, R.V. Natural Occurrence of fumonisin B1 and its co-occurrence with aflatoxin B1 in Indian sorghum, maize, and Poultry Feeds. J. Agric. Food Chem. 1997, 45, 2170–2173.
- Alizadeh, A.M.; Roshandel, G.; Roudbarmohammadi, S.; Roudbary, M.; Sohanaki, H.; Ghiasian, S.A.; Taherkhani, A.; Semnani, S.; Aghasi, M. Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in Northeastern Iran. Asian Pac. J. Cancer Prev. 2012, 13, 2625–2628.
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B. Appropriateness to set a group health-based guidance value for fumonisins and their modified forms. EFSA J. 2018, 16, 5172.
- Sydenham, E.W.; Gelderblom, W.C.A.; Thiel, P.G.; Marasas, W.F.O. Evidence for the natural occurrence of fumonisin B1, a mycotoxin produced by Fusarium moniliforme, in corn. J. Agric. Food Chem. 1990, 38, 285–290.
- Massomo, S.M.S. Aspergillus flavus and aflatoxin contamination in the maize value chain and what needs to be done in Tanzania. Sci. Afr. 2020, 10, e00606.
- Fels-Klerx, H.J.V.D.; Liu, C.; Battilani, P. Modelling climate change impacts on mycotoxin contamination. World Mycotoxin J. 2016, 9, 717–726.
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328.
- Leggieri, M.C.; Toscano, P.; Battilani, P. Predicted aflatoxin b1 increase in europe due to climate change: Actions and reactions at global level. Toxins 2021, 13, 292.
- Akello, J.; Ortega-Beltran, A.; Katati, B.; Atehnkeng, J.; Augusto, J.; Mwila, C.M.; Mahuku, G.; Chikoye, D.; Bandyopadhyay, R. Prevalence of aflatoxin-and fumonisin-producing fungi associated with cereal crops grown in zimbabwe and their associated risks in a climate change scenario. Foods 2021, 10, 287.
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and functional characterization of human hepatoma HepG2 cells. In Protocols in In Vitro Hepatocyte Research; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015; Volume 1250, pp. 77–93.
- Ding, X.; Hu, X.; Chen, Y.; Xie, J.; Ying, M.; Wang, Y.; Yu, Q. Differentiated Caco-2 cell models in food-intestine interaction study: Current applications and future trends. Trends Food Sci. Technol. 2021, 107, 455–465.
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100.
- Renehan, A.G.; Booth, C.; Potteri, C.S. What is apoptosis, and why is it important? BMJ 2001, 322, 1536–1538.
- Chen, X.; Abdallah, M.F.; Grootaert, C.; Rajkovic, A. Bioenergetic status of the intestinal and hepatic cells after short term exposure to fumonisin B1 and aflatoxin B1. Int. J. Mol. Sci. 2022, 23, 6945.
- Xu, Q.; Shi, W.; Lv, P.; Meng, W.; Mao, G.; Gong, C.; Chen, Y.; Wei, Y.; He, X.; Zhao, J.; et al. Critical role of caveolin-1 in aflatoxin B1-induced hepatotoxicity via the regulation of oxidation and autophagy. Cell Death Dis. 2020, 11, 6.
- Yang, X.; Lv, Y.; Huang, K.; Luo, Y.; Xu, W. Zinc inhibits aflatoxin B1-induced cytotoxicity and genotoxicity in human hepatocytes (HepG2 cells). Food Chem. Toxicol. 2016, 92, 17–25.
- Zhu, L.; Huang, C.; Yang, X.; Zhang, B.; He, X.; Xu, W.; Huang, K. Proteomics reveals the alleviation of zinc towards aflatoxin B1-induced cytotoxicity in human hepatocyes (HepG2 cells). Ecotoxicol. Environ. Saf. 2020, 198, 110596.
- Inal, M.E.; Kanbak, G.; Sunal, E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin. Chim. Acta 2001, 305, 75–80.
- Costa, S.; Schwaiger, S.; Cervellati, R.; Stuppner, H.; Speroni, E.; Guerra, M.C. In vitro evaluation of the chemoprotective action mechanisms of leontopodic acid against aflatoxin B1 and deoxynivalenol-induced cell damage. J. Appl. Toxicol. 2009, 29, 7–14.
- Corcuera, L.A.; Arbillaga, L.; Vettorazzi, A.; Azqueta, A.; López de Cerain, A. Ochratoxin A reduces aflatoxin B1 induced DNA damage detected by the comet assay in Hep G2 cells. Food Chem. Toxicol. 2011, 49, 2883–2889.
- Fang, E.F.; Scheibye-Knudsen, M.; Chua, K.F.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. Nuclear DNA damage signalling to mitochondria in ageing. Nat. Rev. Mol. Cell Biol. 2016, 17, 308–321.
- Ji, J.; Wang, Q.; Wu, H.; Xia, S.; Guo, H.; Blaženović, I.; Zhang, Y.; Sun, X. Insights into cellular metabolic pathways of the combined toxicity responses of Caco-2 cells exposed to deoxynivalenol, zearalenone and Aflatoxin B1. Food Chem. Toxicol. 2019, 126, 106–112.
- Park, S.; Lee, J.Y.; You, S.; Song, G.; Lim, W. Neurotoxic effects of aflatoxin B1 on human astrocytes in vitro and on glial cell development in zebrafish in vivo. J. Hazard. Mater. 2020, 386, 121639.
- Zheng, N.; Zhang, H.; Li, S.; Wang, J.; Liu, J.; Ren, H.; Gao, Y. Lactoferrin inhibits aflatoxin B1- and aflatoxin M1-induced cytotoxicity and DNA damage in Caco-2, HEK, Hep-G2, and SK-N-SH cells. Toxicon 2018, 150, 77–85.
- Li, C.H.; Li, W.Y.; Hsu, I.N.; Liao, Y.Y.; Yang, C.Y.; Taylor, M.C.; Liu, Y.F.; Huang, W.H.; Chang, H.H.; Huang, H.; et al. Recombinant aflatoxin-degrading f420h2-dependent reductase from mycobacterium smegmatis protects mammalian cells from aflatoxin toxicity. Toxins 2019, 11, 259.
- Liu, R.; Jin, Q.; Huang, J.; Liu, Y.; Wang, X.; Zhou, X.; Mao, W.; Wang, S. In vitro toxicity of aflatoxin B 1 and its photodegradation products in HepG2 cells. J. Appl. Toxicol. 2012, 32, 276–281.
- Liu, Y.; Du, M.; Zhang, G. Proapoptotic activity of aflatoxin B1 and sterigmatocystin in HepG2 cells. Toxicol. Rep. 2014, 1, 1076–1086.
- Reddy, L.; Odhav, B.; Bhoola, K. Aflatoxin B1-induced toxicity in HepG2 cells inhibited by carotenoids: Morphology, apoptosis and DNA damage. J. Biol. Chem. 2006, 387, 87–93.
- Desaulniers, D.; Cummings-Lorbetskie, C.; Leingartner, K.; Xiao, G.H.; Zhou, G.; Parfett, C. Effects of vanadium (sodium metavanadate) and aflatoxin-B1 on cytochrome p450 activities, DNA damage and DNA methylation in human liver cell lines. Toxicol. Vitr. 2021, 70, 105036.
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Discovering the protective effects of resveratrol on aflatoxin b1-induced toxicity: A whole transcriptomic study in a bovine hepatocyte cell line. Antioxidants 2021, 10, 1225.
- Zhu, Q.; Ma, Y.; Liang, J.; Wei, Z.; Li, M.; Zhang, Y.; Liu, M.; He, H.; Qu, C.; Cai, J.; et al. AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct. Target. Ther. 2021, 6, 299.
- Nebert, D.W.; Dalton, T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer 2006, 6, 947–960.
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16, e05242.
- Yang, X.J.; Lu, H.Y.; Li, Z.Y.; Bian, Q.; Qiu, L.L.; Li, Z.; Liu, Q.; Li, J.; Wang, X.; Wang, S.L. Cytochrome P450 2A13 mediates aflatoxin B1-induced cytotoxicity and apoptosis in human bronchial epithelial cells. Toxicology 2012, 300, 138–148.
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96.
- Fugio, L.B.; Coeli-Lacchini, F.B.; Leopoldino, A.M. Sphingolipids and mitochondrial dynamic. Cells 2020, 9, 581.
- Lumsangkul, C.; Chiang, H.I.; Lo, N.W.; Fan, Y.K.; Ju, J.C. Developmental toxicity of mycotoxin fumonisin B1 in animal embryogenesis: An overview. Toxins 2019, 11, 114.
- Stiban, J.; Tidhar, R.; Futerman, A.H. Ceramide synthases: Roles in cell physiology and signaling. Adv. Exp. Med. Biol. 2010, 688, 60–71.
- Zitomer, N.C.; Mitchell, T.; Voss, K.A.; Bondy, G.S.; Pruett, S.T.; Garnier-Amblard, E.C.; Liebeskind, L.S.; Park, H.; Wang, E.; Sulllards, M.C.; et al. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine. A novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J. Biol. Chem. 2009, 284, 4786–4795.
- Minervini, F.; Garbetta, A.; D’Antuono, I.; Cardinali, A.; Martino, N.A.; Debellis, L.; Visconti, A. Toxic mechanisms induced by fumonisin B1 mycotoxin on human intestinal cell line. Arch. Environ. Contam. Toxicol. 2014, 67, 115–123.
- Singh, M.P.; Kang, S.C. Endoplasmic reticulum stress-mediated autophagy activation attenuates fumonisin B1 induced hepatotoxicity in vitro and in vivo. Food Chem. Toxicol. 2017, 110, 371–382.
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005, 213, 56–65.
- Stockmann-Juvala, H.; Mikkola, J.; Naarala, J.; Loikkanen, J.; Elovaara, E.; Savolainen, K. Fumonisin B1-induced toxicity and oxidative damage in U-118MG glioblastoma cells. Toxicology 2004, 202, 173–183.
- Zhao, X.; Wang, Y.; Liu, J.L.; Zhang, J.H.; Zhang, S.C.; Ouyang, Y.; Huang, J.T.; Peng, X.Y.; Zeng, Z.; Hu, Z.Q. Fumonisin B1 affects the biophysical properties, migration and cytoskeletal structure of human umbilical vein endothelial cells. Cell Biochem. Biophys. 2020, 78, 375–382.
- Khan, R.B.; Phulukdaree, A.; Chuturgoon, A.A. Fumonisin B1 induces oxidative stress in oesophageal (SNO) cancer cells. Toxicon 2018, 141, 104–111.
- Du, M.; Liu, Y.; Zhang, G. Interaction of aflatoxin B1 and fumonisin B1 in HepG2 cell apoptosis. Food Biosci. 2017, 20, 131–140.
- Mary, V.S.; Arias, S.L.; Otaiza, S.N.; Velez, P.A.; Rubinstein, H.R.; Theumer, M.G. The aflatoxin B1-fumonisin B1 toxicity in BRL-3A hepatocytes is associated to induction of cytochrome P450 activity and arachidonic acid metabolism. Environ. Toxicol. 2017, 32, 1711–1724.
- Chen, X.; Abdallah, M.F.; Grootaert, C.; Filip, V.N.; Rajkovic, A. New insights into the combined toxicity of aflatoxin B1 and fumonisin B1 in HepG2 cells using Seahorse respirometry analysis and RNA transcriptome sequencing. Environ. Int. 2023, 175, 107945.
- Carlson, D.B.; Williams, D.E.; Spitsbergen, J.M.; Ross, P.F.; Bacon, C.W.; Meredith, F.I.; Riley, R.T. Fumonisin B1 promotes aflatoxin B1 and N-methyl-N′-nitronitrosoguanidine-initiated liver tumors in rainbow trout. Toxicol. Appl. Pharmacol. 2001, 172, 29–36.
- Torres, O.; Matute, J.; Gelineau-Van Waes, J.; Maddox, J.R.; Gregory, S.G.; Ashley-Koch, A.E.; Showker, J.L.; Voss, K.A.; Riley, R.T. Human health implications from co-exposure to aflatoxins and fumonisins in maize-based foods in Latin America: Guatemala as a case study. World Mycotoxin J. 2015, 8, 143–159.
- McKean, C.; Tang, L.; Tang, M.; Billam, M.; Wang, Z.; Theodorakis, C.W.; Kendall, R.J.; Wang, J.S. Comparative acute and combinative toxicity of aflatoxin B1 and fumonisin B1 in animals and human cells. Food Chem. Toxicol. 2006, 44, 868–876.
More
Information
Subjects:
Toxicology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
23 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




