Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Erika Segato | -- | 3119 | 2023-11-22 10:31:35 | | | |
| 2 | Sirius Huang | Meta information modification | 3119 | 2023-11-23 01:57:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Monzani, D.; Liberale, C.; Segato, E.; De Cecco, F.; Arietti, V.; Palma, S.; Sacchetto, L.; Nocini, R. Role of Fibrinogen, Homocysteine and MetS in SSHL. Encyclopedia. Available online: https://encyclopedia.pub/entry/51908 (accessed on 07 February 2026).
Monzani D, Liberale C, Segato E, De Cecco F, Arietti V, Palma S, et al. Role of Fibrinogen, Homocysteine and MetS in SSHL. Encyclopedia. Available at: https://encyclopedia.pub/entry/51908. Accessed February 07, 2026.
Monzani, Daniele, Carlotta Liberale, Erika Segato, Francesca De Cecco, Valerio Arietti, Silvia Palma, Luca Sacchetto, Riccardo Nocini. "Role of Fibrinogen, Homocysteine and MetS in SSHL" Encyclopedia, https://encyclopedia.pub/entry/51908 (accessed February 07, 2026).
Monzani, D., Liberale, C., Segato, E., De Cecco, F., Arietti, V., Palma, S., Sacchetto, L., & Nocini, R. (2023, November 22). Role of Fibrinogen, Homocysteine and MetS in SSHL. In Encyclopedia. https://encyclopedia.pub/entry/51908
Monzani, Daniele, et al. "Role of Fibrinogen, Homocysteine and MetS in SSHL." Encyclopedia. Web. 22 November, 2023.
Copy Citation
Fibrinogen and homocysteine (HCY) are molecules known to play a role in vascular homeostasis, and their blood levels are often elevated in patients with metabolic syndrome. Recent evidence suggests that sudden sensorineural hearing loss (SSHL) may have a vascular origin. This has led many authors to advocate that fibrinogen, homocysteine, and metabolic syndrome (MetS) may play a direct role in SSHL.
sudden sensorineural hearing loss
fibrinogen
homocysteine
metabolic syndrome
therapies
1. Introduction
Sudden sensorineural hearing loss (SSHL) is defined as hearing loss occurring within 72 h with a loss of at least 30 decibels in three adjacent frequencies (The American Academy of Otolaryngology—Head and Neck Surgery) [1]. The incidence of SSHL is estimated to be 5–20 cases per 100,000 [2], but the actual incidence may exceed these estimates because individuals who experience rapid recovery from the disease often do not seek medical attention [3]. The exact cause of SSHL is unclear in many cases [4], but several potential factors and conditions have been associated with its development. The cause of SSHL can be determined in only 10 to 15% of patients [5]. Possible causes and contributing factors for sudden hearing loss include viral infections, vascular problems, autoimmune diseases, medications, trauma, tumors, neurological diseases, and genetic predisposition [6]. Some authors have suggested that idiopathic SSHL may have a vascular origin, indicating that a local ischemic event leading to hypoperfusion of the cochlea may play a pathogenetically relevant role. The reason why the cochlea appears so vulnerable to vascular insults is to be found in its vascularization: the cochlea is supplied with blood by two small terminal arteries that are small in diameter and lack a collateral blood supply [7]. It is interesting to notice how unilateral SSHL has a clinical presentation comparable to ischemic vascular diseases such as amaurosis fugax and transient ischemic episodes [8].
Sudden sensorineural hearing loss (SSHL) is a medical emergency that requires immediate ENT medical attention. Much of the literature indicates that 32% to 65% of cases of SSHL can recover spontaneously. However, clinical experience indicates that these numbers may be overestimated [9]. Various hypotheses have been proposed in the scientific literature to explain sudden sensorineural hearing loss (SSHL), although specific triggers can only be described for a relatively small proportion of SSHL cases, ranging from 10% to 30% [10]. In fact, it is estimated that up to 90 percent of cases of SSHL are idiopathic at presentation, and approximately 1% to 3% of SSHL cases can be attributed to retrocochlear disorders, which may include conditions such as acoustic neuromas, meningiomas, demyelinating diseases, or strokes [11].
According to a review of the evidence regarding the etiology of sudden sensorineural hearing loss (SSHL) in adults conducted by Chau et al. [12], they discovered that the suspected etiologies included idiopathic (71.0%), infectious diseases (12.8%), otologic diseases (4.7%), trauma (4.2%), vascular or hematologic issues (2.8%), neoplastic causes (2.3%), and other factors (2.2%). The establishment of a direct causal link between SSHL and these etiologies remains elusive. The low incidence of vascular or hematologic issues could be related to the challenges in demonstrating a causal-effect link. In fact, there is still no established method for detecting vascular damage in the cochlea, and it’s possible that several idiopathic cases could be related to vascular issues.
Several lines of research have focused on the concept that altered blood flow and/or oxygenation lead to cochlear hair cell damage and hearing loss [13]. According to the vascular theory, hearing loss may be caused by various factors such as sudden vascular hemorrhage, blockage due to emboli, vasospasm, changes in blood viscosity, and other risk factors including hypercholesterolemia, hyperfibrinogenemia, and microembolism [14]. This suggests that a localized ischemic event resulting in reduced blood flow to the cochlea could be a significant factor in its pathogenesis. In a review by Doo et al., the authors examined various hemostatic parameters and tested the hypothesis that certain blood molecules may play a role in altering blood flow, particularly in the labyrinthine artery [15].
Given the theory of microvascular occlusion, it is worthwhile to investigate hypercoagulability in patients with idiopathic SSHL, especially when thrombophilia-related abnormalities or predominant venous factors such as oral contraceptive use, pregnancy or puerperium, and cardiovascular factors such as arterial hypertension, hyperlipidemia, diabetes, and cigarette smoking are present. Thrombophilia abnormalities can be divided into two categories: inherited and acquired. Inherited thrombophilia includes conditions such as deficiency of natural anticoagulant proteins such as antithrombin, protein C, and protein S, and gain-of-function mutations in the genes of factor V and prothrombin. On the other hand, acquired thrombophilia is due to factors such as the presence of antiphospholipid antibodies. In addition, there are two other thrombophilia-related disorders for which there is no clear evidence of inheritance. These are elevated plasma levels of coagulation factor VIII and mild to moderate hyperhomocysteinemia (HHcy). Finally, circulating microparticles, tiny phospholipid-rich particles released by various blood cells such as platelets, endothelial cells, leukocytes, and erythrocytes, are also considered potential risk factors for sudden sensorineural hearing loss (SSHL) because they are associated with an increased risk of thrombosis [13] (Figure 1).
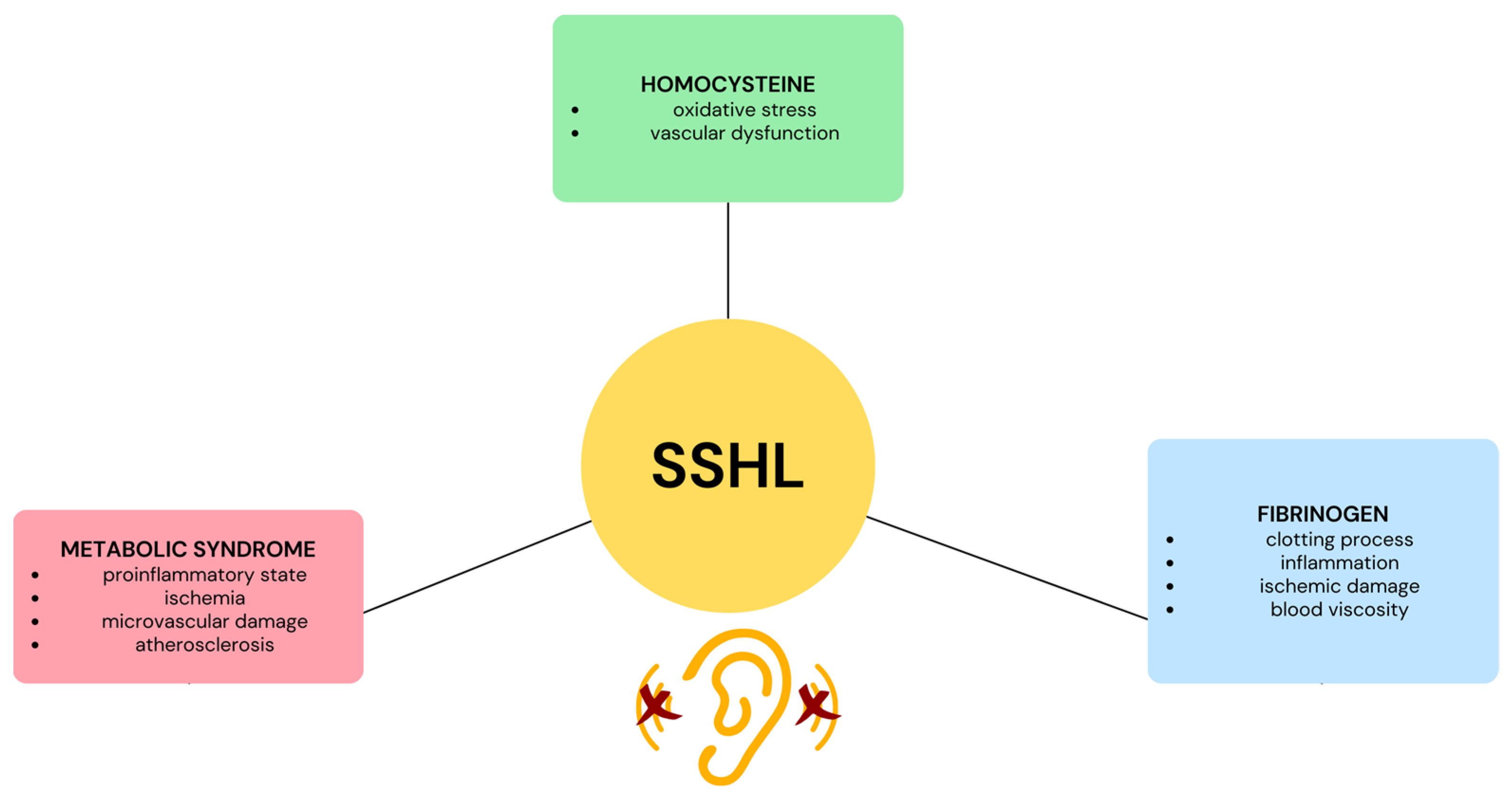
Figure 1. Illustration depicting the influence of fibrinogen, homocysteine, and metabolic syndrome on sudden sensorineural hearing loss (SSHL).
2. Fibrinogen
Fibrinogen is a glycoprotein normally present in human blood plasma. It is mainly produced by the liver and released into the bloodstream. It plays a central role in several biological functions, including hemostasis and inflammation [16]. Although fibrinogen exists as a soluble macromolecule, it undergoes a crucial transformation in response to vascular injury. Through a series of enzymatic reactions, it is converted to fibrin, forming a mesh-like structure that effectively traps blood cells and facilitates the formation of a stable clot at the site of injury. This clotting process is important for preventing excessive bleeding and maintaining the integrity of the circulatory system. In addition, the involvement of fibrinogen in inflammation is noteworthy, as it interacts with various molecules and cells, contributing to the body’s response to injury and infection [17]. Fibrinogen may suggest a link between vascular factors and SSHL and shed light on underlying mechanisms.
In a study in guinea pigs, an association was found between elevated serum fibrinogen levels and a decrease in blood flow in the cochlea. It was also observed that administration of a defibrinogenating drug improved blood flow in the cochlea [18]. In a prospective cohort study by Okada [19] et al., the authors investigated the prognostic significance of fibrinogen and inflammatory cytokines in sudden sensorineural hearing loss (SSHL). The study revealed a remarkable inverse relationship between SSHL recovery rate and serum fibrinogen level. This decrease in blood flow, due to thrombosis in the inner ear, leads to oxygen starvation and apoptosis of the hair cells of the cochlea. Consequently, individuals with elevated fibrinogen levels, potentially indicative of ischemic damage to the cochlea, have a limited response to treatment, independent of concurrent inflammatory conditions. These findings underscore the role of ischemic changes in the inner ear as a possible cause of SSHL, ultimately leading to a suboptimal response to steroid therapy.
Indeed, fibrinogen levels may indicate increased blood viscosity, which in turn could lead to decreased blood flow in the affected regions. In 2018, Oya et al. performed a literature review on the relationship between SSHL and fibrinogen and analyzed 1577 patients. They found that a high serum fibrinogen level correlated with a poor prognosis [4]. These results were also confirmed by Kanzaki et al. [20]. Moreover, they suggested that hyperfibrinogenemia may not be directly related to the cause of SSHL, but rather to the severity of the disease. Also, Sun et al. [21] in 2023 found that blood fibrinogen level is a reliable indicator of the prognosis of SSHL when all frequencies are affected. In addition, recent studies have confirmed that thrombosis occurring in the inner ear can lead to a reduction in blood flow. This reduction may ultimately lead to inadequate oxygenation and apoptosis of the hair cells of the cochlea. Therefore, thrombosis appears to be the most likely cause of SSHL at all frequencies.
As noted by Hira et al., plasma fibrinogen levels increase susceptibility to atherosclerosis due to two main factors: increased plasma viscosity and thrombotic activation triggered by fibrin during its degradation [22]. Numerous studies have indicated that this mechanism may lead to idiopathic sudden hearing loss (SSHL) when it occurs in the inner ear. Oreskovic et al. investigated the prevalence of abnormal lipoprotein and fibrinogen levels in patients with SSHL [23]. They suggested that these findings could serve as a basis for developing new treatments or prevention strategies in the future [23]. In a recent literature review by Linthicum [11], the authors recommend a complete blood count and coagulation test (fibrinogen) for all cases of sudden SSHL. Thus, these tests are inexpensive, readily available, and provide as much information about the diagnosis and prognosis of sudden SSHL as any other biomarker.
However, it is important to note that the pathophysiology of SSHL at both low and high frequencies is not yet confirmed to be due to only thrombosis. Consequently, the role of fibrinogen remains unclear, and its impact on prognosis is currently not fully understood. To date, it is not possible to definitively determine whether hyperfibrinogenemia directly causes SSHL. In fact, it remains unclear whether hyperfibrinogenemia is the cause or the consequence of SSHL [4].
3. Homocysteine
Homocysteine (HCY) is an amino acid produced in the body as a byproduct of the metabolism of another amino acid called methionine. It is also a homolog of cysteine [24]. Several factors can lead to an increase in HCY levels, including genetic influences, dietary habits, lifestyle factors, certain medications, and more.
A laboratory study showed that when homocysteine levels are elevated, a condition known as hyperhomocysteinemia (hHCY) can be associated with an increased risk of neurovascular disease, dementia, migraine, developmental disabilities, and epilepsy [25]. Mechanisms underlying the neurotoxic effects of HCY include oxidative stress, DNA damage, protein thiolation, and protein homocysteinylation, which may ultimately trigger processes such as apoptosis and excitotoxicity [24]. Elevated homocysteine levels, in turn, have been associated with impaired blood flow and vascular dysfunction, possibly contributing to SSHL.
As mentioned above, HCY is a risk factor for increased vascular injury, and its abnormal increase impairs endothelial function, reduces vascular flexibility, and leads to microvascular dysfunction, resulting in ischemia and hypoxia-related changes [26]. Currently, two main factors are widely recognized for the development of hyperhomocysteinemia: (I) nutritional elements characterized by a deficiency of metabolic cofactors such as vitamin B6, vitamin B12, and/or folic acid, and (II) genetic factors, including mutations in genes such as MTHFR and CBS, which may reduce enzyme activity. These factors may contribute to the accumulation of HCY in the body [27].
A retrospective case–control study by Passamonti et al. showed that HCY was associated with an increased risk of idiopathic SSHL, and hyperhomocysteinemia was reported as a negative prognostic factor for hearing recovery [10]. In addition, Fasano et al. found that HCY levels were higher in severe SSHL than in mild SSHL, demonstrating that HCY is associated with the degree of deafness in SSHL, as has been found for fibrinogen [4][28]. In a recent study, Wang et al. investigated the factors involved in the development of bilateral sudden sensorineural hearing loss. They focused specifically on HCY and confirmed the evidence found by Passamonti et al. and Fasano et al. [29].
Niu et al. conducted a literature review on the association between SSHL and HCY in 2023 [30]. The study included nine articles and found that serum/plasma concentrations of HCY were higher in SSHL patients than in the control group. According to the results, elevated serum or plasma levels of HCY could be a risk factor for SSHL. They also suggest that vascular factors could be one of the causes of SSHL. To confirm the theory that hyperhomocysteinemia is a risk factor for SSHL due to vascular damage in the cochlea, further studies on pathophysiology are needed [30].
There are also some authors who do not believe in the role of HCY in SSHL. Cadoni et al. found no correlation between these two factors [31]. Also, Berner et al. conducted a study with 91 Danish adults and found no correlation between serum levels of folic acid, vitamin B12, and HCY and the occurrence of hearing loss [31]. Furthermore, in a cross-sectional study, Gopinath et al. reported that serum levels of folic acid, vitamin B12, and HCY were not significantly related to sudden hearing loss. However, they found that serum levels of folic acid and HCY were associated with age-related hearing loss [32]. Regarding the clinical effects of HCY on SSHL patients, Huang et al. conducted a study to investigate the association between plasma HCY concentration, serum folic acid concentration, and treatment occurrence and response in adult patients with total frequency deafness. The aim of this study was to test the hypothesis that higher plasma HCY levels, in association with lower serum folic acid levels, may be associated with a higher risk of sudden total frequency deafness [27]. Indeed, the researchers found that plasma HCY levels were elevated, and serum folic acid levels were decreased in patients with sudden hearing loss compared with the normal control group, with statistically significant differences (p < 0.05). Huang et al. attempted to administer folic acid, vitamin B6, and B12 to patients with SSHL, but their results showed no improvement in clinical outcomes. Nevertheless, they suggest that maintaining low levels of HCY in daily life may prove beneficial for the clinical prevention and treatment of hearing loss [27].
To date, there is insufficient evidence to definitively clarify the exact relationship between fibrinogen and HCY levels and their effects on hearing loss. Although many studies postulate this hypothesized relationship, this is not sufficient to develop therapeutic and preventive strategies based solely on these assessments.
4. Metabolic Syndrome (MetS)
While metabolic syndrome (MetS) itself does not involve single molecules like fibrinogen and homocysteine (HCY), it plays a significant role in causing various molecular dysfunctions that can potentially lead to vascular damage, which, in turn, affects the cochlea. As a condition with a high global prevalence, it is valuable to comprehend its implications, including a comprehensive analysis of its individual components.
Metabolic syndrome (MetS) is a set of metabolic disorders that include central obesity, insulin resistance or impaired glucose uptake and utilization, dyslipidemia, and hypertension [33]. Metabolic syndrome (MetS) has been defined as when a person has three or more of the following criteria. (1) Increased waist circumference (EWC): waist circumference ≥ 102 cm in men and ≥88 cm in women; (2) increased blood pressure: blood pressure ≥ 130/85 mmHg or drug treatment for previously diagnosed hypertension; (3) decreased HDL-C: <40 mg/dL in men and <50 mg/dL in women or specific treatment for decreased HDL-C; (4) increased TGs: TG level ≥ 150 mg/dL or drug treatment for elevated TG; and (5) elevated fasting glucose: fasting glucose level of ≥100 mg/L or drug treatment for elevated glucose and previously diagnosed type 2 diabetes [34].
Its multisystemic pattern arises from a confluence of factors including pro-inflammatory states, oxidative stress, hemodynamic dysfunction, and ischemia in ‘dysmetabolic’ patients. This convergence leads to the emergence of various clinical conditions, encompassing cardiovascular diseases (CVD), non-alcoholic steatohepatitis, liver dysfunctions, chronic kidney disease, numerous types of cancer, and neurodegenerative disorders [35].
Metabolic syndrome and its associated components have the potential to lead to several comorbidities, one of which is inner ear dysfunction. The exact mechanism linking metabolic syndrome and hearing loss is not yet clear, but there is evidence that it may be related to peripheral blood vessel dysfunction [36]. It is known that subjects with metabolic syndrome had significantly higher plasma fibrinogen and homocysteine levels than subjects without metabolic syndrome. This could contribute to damage to the vascularization of the cochlea, as the primary mechanism responsible for hearing loss appears to be vascular damage within the cochlea [37]. Chien et al. reported that metabolic syndrome increases the risk of SSNHL by 3.5-fold [38]. These findings were confirmed by a study by Park et al. [39], which found that metabolic syndrome was more prevalent in SSNHL patients and that patients with metabolic syndrome responded worse to treatment than patients without this syndrome.
Metabolic syndrome encompasses a number of disorders that include insulin resistance and abnormal fat deposition. The combination of these factors can lead to microvascular damage and endothelial dysfunction [40]. Numerous studies in the existing literature have investigated the relationship between metabolic syndrome and recovery from sudden sensorineural hearing loss. However, there is a notable lack of studies directly investigating metabolic syndrome as a primary cause of SSHL. Zand et al. conducted a study that specifically looked at the causal relationship between MetS and SSHL [40]. A study by Chien et al. found that metabolic syndrome plays a role in the development of idiopathic SSNHL but has no impact on the prognosis of idiopathic SSNHL [38]. The study by Zand et al. highlighted that both insulin resistance, which seems to be the main cause of metabolic syndrome, and hyperlipidemia cause atherosclerotic changes and endothelial dysfunction, leading to cochlear microangiopathy [40]. Hence, SSHL in patients with metabolic syndrome (MetS) appears to be associated with a range of molecular alterations. These alterations involve factors such as fibrinogen, HCY, hyperlipidemia, and elevated blood glucose levels. Currently, it is still not possible to definitively determine which molecule plays a decisive role in the pathophysiology of SSHL. Therefore, metabolic syndrome (MetS) should be regarded as a complex entity.
MetS has also been shown to be associated with poorer recovery in SSHL. Indeed, metabolic syndrome had a negative impact on hearing improvement in patients with idiopathic SSNHL. Lower initial hearing threshold, absence of diabetes mellitus and hypertension, and BMI < 25 were associated with improvement in hearing after treatment, according to the results of Zand et al. [40]. These findings were confirmed by Jung et al., who conducted a study showing that individuals with metabolic syndrome had lower rates of complete and partial improvement, while the rate of no improvement was higher. This study provides further evidence of the negative impact of metabolic syndrome on the prognosis of patients with sudden sensorineural hearing loss [41]. The authors also discovered that correction of dyslipidemia in patients with SSHL in the chronic phase led to an improvement in hearing, providing further evidence of the association between dyslipidemia and the occurrence and prognosis of hearing impairment [41]. Jung et al. emphasized that hypertension is a risk factor for SSNHL. People with hypertension are more likely to develop SSNHL and have a lower cure rate than people without hypertension. Elevated blood pressure contributes to decreased elasticity of blood vessels in the inner ear, leading to atherosclerotic changes that cause narrowing of the vessels, decreased blood flow, and increased damage to the cochlea. It has been widely demonstrated in the literature that microangiopathy is a pathological mechanism associated with all five factors of metabolic syndrome, as well as vascular mechanisms that are one of the causes of SSHL. To date, the metabolic syndrome appears to play a dual role in the development of SSHL. On the one hand, it contributes to vascular damage that directly affects the cochlea; on the other hand, it leads to poorer recovery from SSHL, slows down the healing process, and impairs the improvement of hearing.
References
- Khater, A.; El-Anwar, M.W.; Nofal, A.A.; Elbahrawy, A.T. Sudden sensorineural hearing loss: Comparative study of different treatment modalities. Int. Arch. Otorhinolaryngol. 2018, 22, 245–249.
- Hughes, G.B.; Freedman, M.A.; Haberkamp, T.J.; Guay, M.E. Sudden sensorineural hearing loss. Otolaryngol. Clin. N. Am. 1996, 29, 393–405.
- Byl, F.M., Jr. Sudden hearing loss: Eight years’ experience and suggested prognostic table. Laryngoscope 1984, 94 5 Pt 1, 647–661.
- Oya, R.; Takenaka, Y.; Imai, T.; Sato, T.; Osaki, Y.; Ohta, Y.; Inohara, H. Serum Fibrinogen as a Prognostic Factor in Sudden Sensorineural Hearing Loss: A Meta-analysis. Otol. Neurotol. 2018, 39, e929–e935.
- Conlin, A.E.; Parnes, L.S. Treatment of sudden sensorineural hearing loss, I: A systematic review. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 573–581.
- Kuhn, M.; Heman-Ackah, S.E.; Shaikh, J.A.; Roehm, P.C. Sudden sensorineural hearing loss: A review of diagnosis, treatment, and prognosis. Trends Amplif. 2011, 15, 91–105.
- Ballesteros, F.; Alobid, I.; Tassies, D.; Reverter, J.C.; Scharf, R.E.; Guilemany, J.M.; Bernal-Sprekelsen, M. Is there an overlap between sudden neurosensorial hearing loss and cardiovascular risk factors? Audiol. Neurootol. 2009, 14, 139–145.
- Tripathi, P.; Deshmukh, P. Sudden Sensorineural Hearing Loss: A Review. Cureus 2022, 14, e29458.
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol. Head Neck Surg. 2019, 161 (Suppl. S1), S1–S45.
- Passamonti, S.M.; Di Berardino, F.; Bucciarelli, P.; Berto, V.; Artoni, A.; Gianniello, F.; Ambrosetti, U.; Cesarani, A.; Pappalardo, E.; Martinelli, I. Risk factors for idiopathic sudden sensorineural hearing loss and their association with clinical outcomes. Thromb. Res. 2015, 135, 508–512.
- Linthicum, F.H.; Doherty, J.; Berliner, K.I. Idiopathic sudden sensorineural hearing loss. Otolaryngol. Head Neck Surg. 2013, 149, 914–917.
- Chau, J.K.; Lin, J.R.; Atashband, S.; Irvine, R.A.; Westerberg, B.D. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 2010, 120, 1011–1021.
- Seidman, M.D.; Quirk, W.S.; Shirwany, N.A. Mechanisms of alterations in the microcirculation of the cochlea. Ann. N. Y. Acad. Sci. 1999, 884, 226–232.
- Capaccio, P.; Ottaviani, F.; Cuccarini, V.; Bottero, A.; Schindler, A.; Cesana, B.M.; Censuales, S.; Pignataro, L. Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope 2007, 117, 547–551.
- Doo, J.G.; Kim, D.; Kim, Y.; Yoo, M.C.; Kim, S.S.; Ryu, J.; Yeo, S.G. Biomarkers Suggesting Favorable Prognostic Outcomes in Sudden Sensorineural Hearing Loss. Int. J. Mol. Sci. 2020, 21, 7248.
- Takeda, Y. Studies of the metabolism and distribution of fibrinogen in healthy men with autologous 125-I-labeled fibrinogen. J. Clin. Investig. 1966, 45, 103–111.
- Weisel, J.W. Fibrinogen and fibrin. Adv. Protein Chem. 2005, 70, 247–299.
- Ihler, F.; Strieth, S.; Pieri, N.; Göhring, P.; Canis, M. Acute hyperfibrinogenemia impairs cochlear blood flow and hearing function in guinea pigs in vivo. Int. J. Audiol. 2012, 51, 210–215.
- Okada, M.; Ogawa, H.; Takagi, T.; Nishihara, E.; Yoshida, T.; Hyodo, J.; Shinomori, Y.; Honda, N.; Fujiwara, T.; Teraoka, M.; et al. A double-blinded, randomized controlled clinical trial of hydrogen inhalation therapy for idiopathic sudden sensorineural hearing loss. Front. Neurosci. 2022, 16, 1024634.
- Kanzaki, S.; Sakagami, M.; Hosoi, H.; Murakami, S.; Ogawa, K. High fibrinogen in peripheral blood correlates with poorer hearing recovery in idiopathic sudden sensorineural hearing loss. PLoS ONE 2014, 9, e104680.
- Sun, H.; Jiang, W.; Wang, J. The prognostic value of peripheral blood parameters on all-frequency sudden sensorineural hearing loss. Braz. J. Otorhinolaryngol. 2023, 89, 101302.
- Hıra, İ.; Yaşar, M.; Kaya, A.; Bayram, A.; Özcan, İ. Prognostic Value of Fibrinogen/HDL Ratio in Idiopathic Sudden Sensorineural Hearing Loss. J. Int. Adv. Otol. 2021, 17, 91–95.
- Oreskovic, Z.; Shejbal, D.; Bicanic, G.; Kekic, B. Influence of lipoproteins and fibrinogen on pathogenesis of sudden sensorineural hearing loss. J. Laryngol. Otol. 2011, 125, 258–261.
- Hermann, A.; Sitdikova, G. Homocysteine: Biochemistry, Molecular Biology and Role in Disease. Biomolecules 2021, 11, 737.
- Elsherbiny, N.M.; Sharma, I.; Kira, D.; Alhusban, S.; Samra, Y.A.; Jadeja, R.; Martin, P.; Al-Shabrawey, M.; Tawfik, A. Homocysteine Induces Inflammation in Retina and Brain. Biomolecules 2020, 10, 393.
- Lai, W.K.; Kan, M.Y. Homocysteine-induced endothelial dysfunction. Ann. Nutr. Metab. 2015, 67, 1–12.
- Huang, Y.; Lv, T.; Xie, M.; He, J.; Pei, J.; Guan, Y.; Jiang, H.; Gong, S.; Cao, X. Blood homocysteine and folic acid levels may provide reference value for the treatment of sudden total frequency deafness. Ann. Palliat. Med. 2019, 8, 604–610.
- Fasano, T.; Pertinhez, T.A.; Tribi, L.; Lasagni, D.; Pilia, A.; Vecchia, L.; Baricchi, R.; Bianchin, G. Laboratory assessment of sudden sensorineural hearing loss: A case-control study. Laryngoscope 2017, 127, 2375–2381.
- Wang, Y.; Xiong, W.; Sun, X.; Liu, W.; Fan, Z.; Wang, H.; Wang, M. Characteristics and prognosis analysis of bilateral sudden sensorineural hearing loss: A retrospective case-control study. Clin. Otolaryngol. 2022, 47, 732–740.
- Niu, X.; Chen, Y.; Zhong, Y.; Xiao, X. The relationship between serum homocysteine levels and sudden sensorineural hearing loss: A meta-analysis. Eur. Arch Otorhinolaryngol. 2023, 280, 2091–2097.
- Cadoni, G.; Scipione, S.; Agostino, S.; Addolorato, G.; Cianfrone, F.; Leggio, L.; Paludetti, G.; Lippa, S. Coenzyme Q 10 and cardiovascular risk factors in idiopathic sudden sensorineural hearing loss patients. Otol. Neurotol. 2007, 28, 878–883.
- Gopinath, B.; Flood, V.M.; Rochtchina, E.; McMahon, C.M.; Mitchell, P. Serum homocysteine and folate concentrations are associated with prevalent age-related hearing loss. J. Nutr. 2010, 140, 1469–1474.
- Pan, W.H.; Yeh, W.T.; Weng, L.C. Epidemiology of metabolic syndrome in Asia. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 37–42.
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752.
- Rossi, J.L.S.; Barbalho, S.M.; de Araujo, R.R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic Syndrome and Cardiovascular Diseases: Going Beyond Traditional Risk Factors. Diabetes Metab. Res. Rev. 2022, 38, e3502.
- Jalali, M.M.; Dalili, S.; Koohmanaee, S.; Rad, S. The Role of Metabolic Syndrome Components in Sensorineural Hearing Loss in Adolescents: A Case-Control Study. Int. Arch. Otorhinolaryngol. 2023, 27, e393–e399.
- Zhou, Y.; Qiu, S.; Liu, D. Impact of metabolic syndrome on recovery of idiopathic sudden sensorineural hearing loss. Am. J. Otolaryngol. 2019, 40, 573–576.
- Chien, C.Y.; Tai, S.Y.; Wang, L.F.; Hsi, E.; Chang, N.C.; Wu, M.T.; Ho, K.Y. Metabolic Syndrome Increases the Risk of Sudden Sensorineural Hearing Loss in Taiwan: A Case-Control Study. Otolaryngol. Head Neck Surg. 2015, 153, 105–111.
- Park, J.S.; Kim, S.H.; Kim, I.; Kim, H.; Kim, J.H.; Lee, J.B. Does Metabolic Syndrome Affect the Incidence and Prognosis of Sudden Sensorineural Hearing Loss? Life 2022, 12, 930.
- Zand, V.; Dadgarnia, M.; Baradaranfar, M.; Meybodian, M.; Vaziribozorg, S.; Fazilati, M. The association between metabolic syndrome and the prognosis of idiopathic sudden sensorineural hearing loss. Eur. Arch. Otorhinolaryngol. 2023, 280, 1411–1415.
- Jung, S.Y.; Shim, H.S.; Hah, Y.M.; Kim, S.H.; Yeo, S.G. Association of Metabolic Syndrome With Sudden Sensorineural Hearing Loss. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 308–314.
More
Information
Subjects:
Otorhinolaryngology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
501
Revisions:
2 times
(View History)
Update Date:
23 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




