Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tamirat Solomon | -- | 3311 | 2023-11-21 15:47:53 | | | |
| 2 | Camila Xu | Meta information modification | 3311 | 2023-11-22 02:13:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Solomon, T.; Gupta, V.; Ncho, C.M. Environmental Impacts of Livestock Production. Encyclopedia. Available online: https://encyclopedia.pub/entry/51867 (accessed on 02 March 2026).
Solomon T, Gupta V, Ncho CM. Environmental Impacts of Livestock Production. Encyclopedia. Available at: https://encyclopedia.pub/entry/51867. Accessed March 02, 2026.
Solomon, Tamirat, Vaishali Gupta, Chris Major Ncho. "Environmental Impacts of Livestock Production" Encyclopedia, https://encyclopedia.pub/entry/51867 (accessed March 02, 2026).
Solomon, T., Gupta, V., & Ncho, C.M. (2023, November 21). Environmental Impacts of Livestock Production. In Encyclopedia. https://encyclopedia.pub/entry/51867
Solomon, Tamirat, et al. "Environmental Impacts of Livestock Production." Encyclopedia. Web. 21 November, 2023.
Copy Citation
Livestock production, as one of the oldest and most significant human activities, plays a vital role in fulfilling the global demand for human nutrition and other animal-related products while contributing to poverty reduction. However, it is also important to address the environmental impact of livestock animals.
livestock
environment
forestry
greenhouse gases
water and land use
1. Introduction
The first known domesticated livestock animal, which was used as a food-producing animal by our ancestors, was the sheep, with this domestication dating back to around 11,000 years ago [1]. This domestication was even before our ancestors started cultivating plants [2]. Several other species of livestock are now reared across the globe which serve food, hide, and milk to humankind. Today, the livestock sector is one of the most flourishing and highly organized sectors in the world [3][4]. According to the World Bank report, meat production, which was around 45 million tons in 1980, jumped to 134 million tons in 2002, witnessing a three-times boom within 22 years [5]. Similarly, the recent forecast for meat production predicts approximately 360 million tons, reflecting a 1.2 percent increase compared with the 2021 estimates [6]. The world population is forecasted to touch the 10 billion mark by 2050. An increase in livestock farming ensures food availability to the ever-growing population. However, the other side of “animal agriculture” is now coming to light and creating an upcoming issue of environmental health. There are several pros and cons of livestock production on social equity, economic growth, and natural resources [5]. The detrimental effects of livestock farming impact the global temperature, biodiversity, and quality of natural resources (air, water, and soil) [7]. These effects are attributable to the permuted biogeochemical cycles of carbon, nitrogen, and phosphorous [8][9]. The calamitous impact of livestock production on soil health [10][11], global warming [12][13][14], air quality [15][16], water pollution [7], and environmental stability [3] are well documented (Figure 1).
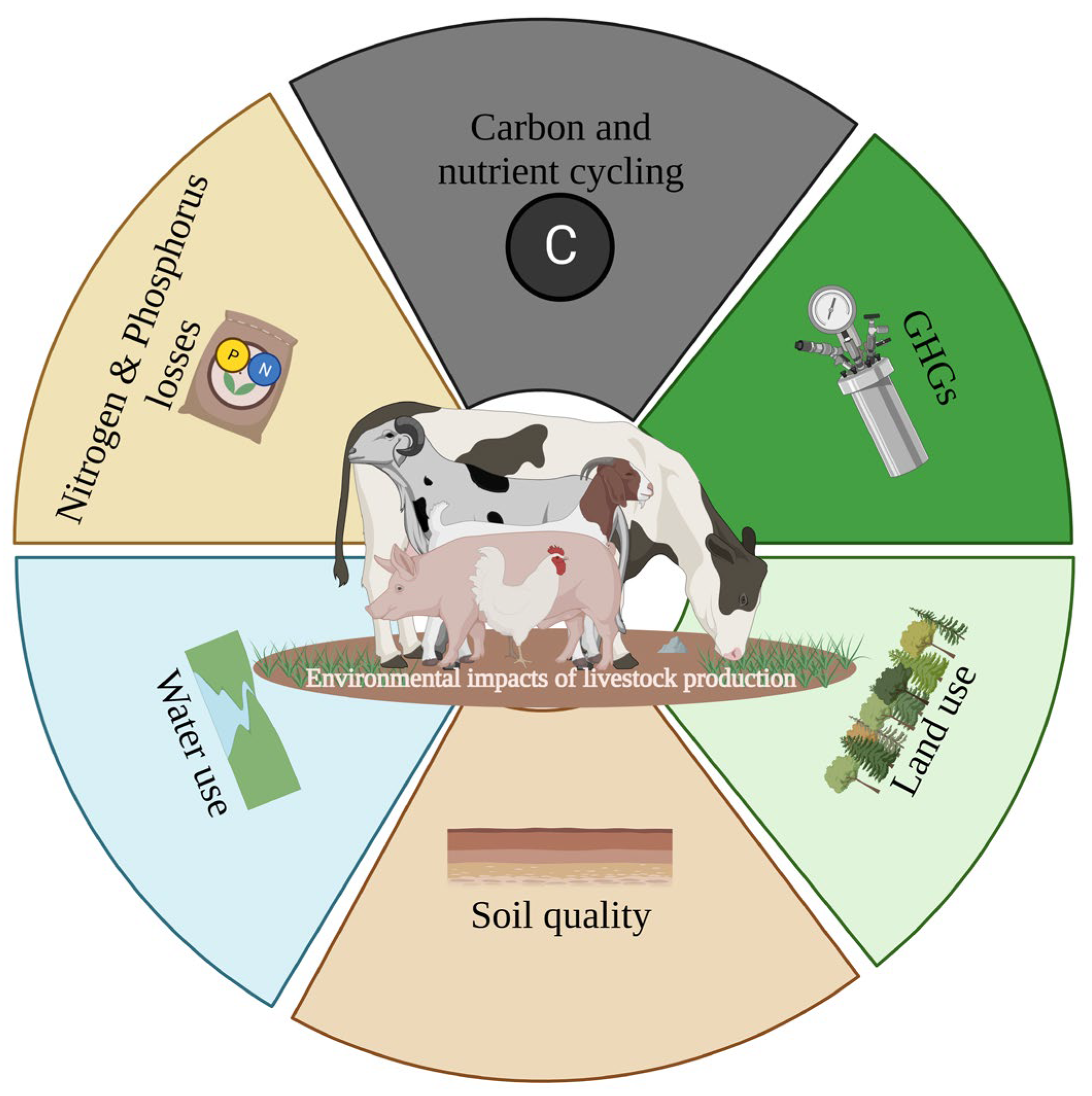
Figure 1. Summary of the potential impacts of livestock production on the environment. The figure was created with created with BioRender.com (accessed on 15 August 2020). The environmental footprint of livestock is discernible through their impact on various factors, including carbon and nutrient cycling, greenhouse gases (GHGs), nutrient losses, water and land use, and, ultimately, soil quality.
It is imperative to recognize the detrimental impacts of livestock farming on the environment and explore viable solutions to mitigate them. Reducing the consumption of meat could be one possible option [17] that aims to decrease the rearing and breeding of animals on a large scale. However, such a shift in diet cannot be based only on nutritional choices as there are multifaceted rationales for choosing food consumed by an individual [18]. Hence, more globally applicable, sustainable remedies are crucial. Forests, vital for life, produce oxygen, making them indispensable. For instance, a model developed in Turkey estimated that a forest management approach focused on timber could result in the production of 2.6 million tons of oxygen over 100 years [19]. Moreover, forests play a vital role in conserving natural resources, subduing several types of pollution (air, water, soil, noise, etc.), regulating biogeochemical cycles, and providing habitats for several species of plants and animals [20]. Past studies have highlighted the effectiveness of urban forests in Beijing, China in removing over 772 tons of PM10 (particulate matter with an aerodynamic diameter smaller than 10 μm) and storing approximately 0.2 million tons of carbon biomass [21]. Similarly, earlier research in the UK demonstrated that good forestry management practices could significantly limit soil erosion, preventing unacceptable increases in turbidity and sedimentation in watercourses [22].
2. Overview of Environmental Impacts of Livestock Production
2.1. Impacts of Livestock Production on Greenhouse Gas (GHG) Emissions
In the current literature, six main gases are generally considered GHGs: perfluorocarbons, sulfur hexafluoride, hydrofluorocarbons, carbon dioxide, methane, and nitrous oxide [23]. Of these, over 50% of the greenhouse effect is directly linked to the last three [24]. The interaction between the sun’s energy and these gases generates the greenhouse effect, as their ability to retain and capture heat is one of the main reasons for their special attention from researchers [25]. For example, after absorbing infrared radiation, carbon dioxide molecules vibrate and emit their radiation, which is then absorbed by another GHG molecule [26]. This cycle, known as absorption-emission-absorption, maintains heat close to the earth’s surface [23]. Similarly, methane and nitrous oxide molecules, due to their constitutions, are also able to vibrate with the absorption of heat [27]. While the greenhouse gases in our atmosphere are essential for keeping living organisms on the planet, the continuous increase in their concentration is likely to be detrimental [28]. As a result, the latest meetings of the Intergovernmental Panel on Climate Change (IPCC) concluded that governments should not only assess their overall emissions but also find solutions to reduce further emissions [29]. In this sense, methane and nitrous oxide were found to be the most relevant GHGs in the livestock production sector.
2.1.1. Methane
Methane is a significant concern due to its global warming potential, which is estimated to be 25 times greater than that of carbon dioxide [30][31]. Different types of livestock, production regions, and systems contribute to varying degrees of overall methane emissions [30]. In modern agriculture, ruminants are the largest source of methane emissions, accounting for approximately one-third of global anthropogenic methane emissions [32]. This significant contribution is attributed to their large share in animal biomass and unique digestive system. Generally, ruminants generate methane through two primary channels [33]. Earlier studies have demonstrated that methane emissions arising from forage crops are minimal (less than 5%), and can therefore be disregarded [32]. While there is evidence of methane emissions from micro anaerobic soil conditions during grazing, soils with higher drainage can significantly slow down this process [34]. Therefore, the first channel, which accounts for the highest proportion of emissions, is directly through enteric fermentation [35]. This results in methane being emitted through flatulence and eructation [36]. The process of methane production during enteric fermentation is quite complex and involves actors of microbial origin [32][33][34][35][37]. Unlike monogastric animals, ruminants have the ability to digest fibrous plant material, mainly due to their stomach, which consists of four distinct compartments [38]. A large anaerobic chamber in the gut of cattle called the rumen, is responsible for fermenting plant materials [39][40][41][42][43]. Methane is produced by microorganisms in the rumen known as methanogens [44][45][46]. These microorganisms have unique characteristics that classify them as archaea. Methanogens are responsible for the final step of methanogenesis, which produces methane from hydrogen and carbon dioxide [47]. The production of methane is influenced by biological factors in the rumen, such as pH and microbial composition, which are linked to the type of feed provided to the animals [42][48][49][50]. In pastoral systems, natural pasture is the only feed source, resulting in higher methane output [51]. Intensive systems, which feed animals concentrate-based diets rich in grains and soybeans, generate lower methane emissions per animal [52]. However, overall, intensive systems have higher emissions when considering the livestock population in each system. Countries such as Brazil, China, and the United States, which rely on intensive systems, are the largest contributors to agricultural methane emissions [53].
The second channel in which ruminant animals contribute to methane emissions is through manure [54]. Specifically, the anaerobic decomposition of manure content and the use of lagoons and holding tanks to manage the liquid phase of manure are localized areas of methane production [55]. In addition to odor pollution and hygiene hazards, poorly managed manure can also have negative consequences. Manure, which is composed of carbohydrates, proteins, and fats, is a complex type of substrate that is easily subjected to fermentation [56]. In an anaerobic digestion process, organic matter from manure is successively hydrolyzed and fermented, resulting in alcohols, fatty acids, hydrogen, and carbon dioxide [57]. Like in enteric fermentation, these two last components are used to generate methane, as ruminant manure is rich in microbes capable of methanogenesis [58]. However, the outputs are significantly lower due to moderate anaerobic biodegradability below the threshold of 50%. In particular, the rate can be even lower in cattle manure, depending on the levels of leftover lignin complexes from the diet [59]. Temperature is a factor that can influence methanogenesis from manure. More precisely, low temperatures can significantly decrease methane outputs [60]. In fact, when ambient temperatures fall below the optimal range for methanogen activity, it significantly decelerates methane production [61][62]. Previous studies have also shown a decrease in the decomposition rate of organic matter within manure linked with temperature, and directly impacting overall methane yield [63][64]. As such, it was proposed that in temperate and cold climates, methane emissions could be decreased at a low cost by frequent removal and outdoor storage [65]. Methane can also be oxidized by methane-oxidizing bacteria (MOB), which were found to be present in solid manure [66]. Previous reports highlighted the need to keep manure in a solid state to enhance the activity of MOB, as they might play a role in methane mitigation [67]. However, it is critical to mention that manure containing substantial water proportions with a relatively high buffer capacity can enhance anaerobic digestion [68]. This is the case with lagoons and holding tanks. During the filling of these facilities, there is a fast inoculation of new slurry material, which, depending on its chemical properties, can intensify methanogenic activity.
Fortunately, the IPCC developed a methodology to estimate and potentially limit methane emissions from liquid manure [69]. This methodology utilizes equations of varying complexity, which are categorized into tiers. To date, three tiers have been defined. The methods for estimating methane emissions from livestock require definitions of livestock subcategories, annual populations, and, for higher-tier methods, feed intake and characterization. The Tier 1 approach for estimating manure from livestock is based on a default emission factor per unit of volatile solid by livestock category and manure storage system. The Tier 2 method, which is more advanced, requires additional variables. This tier is country-specific and considers the impact of the interaction between manure management systems and livestock category during both excretion and storage. The Tier 3 method goes beyond country-specific methodology and is a measurement-based approach to quantify emission factors, which is the most accurate form of estimation but is not yet widely adopted due to the high input needed.
2.1.2. Nitrous Oxide
Nitrous oxide is a greenhouse gas that is emitted in large quantities, ranking third after carbon dioxide and methane [70]. The increasing concentration of nitrous oxide in the atmosphere has become a concern due to its role in regulating stratospheric ozone and the planetary radiation balance [71]. Previous research has also shown that nitrous oxide is involved in the formation of acid rain [72]. Human activities such as land use, fossil fuel combustion, wastewater treatment, and agriculture are sources of nitrous oxide emissions [73]. In the United States, agricultural and soil management activities were estimated to be the largest source of nitrous oxide emissions [74]. Livestock production, however, contributes only minimally to nitrous oxide emissions, with manure being the primary contributor [75]. The storage and management of manure lead to the formation of nitrous oxide through alternative nitrification and denitrification processes, and both aerobic and anaerobic reactions are involved [55]. The conversion of nitrogen from its oxidized form to its gaseous state during the application and handling of manure also contributes to nitrous oxide emissions [76]. Nitrification, which yields nitrate from ammonia via nitrite in a two-step conversion reaction, is catalyzed by two categories of microorganisms: ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) [77]. While the presence of ammonia-oxidizing archaea has been detected in manure and soil during composting, their role in nitrification has not yet been established [78]. On the other hand, denitrification involves a wide variety of microorganisms, with fungi, archaea, and especially heterotrophic bacteria being directly involved [79]. Denitrification can occur in either oxic or anoxic conditions, depending on oxygen availability, and microorganisms can produce nitrous oxide through different pathways [55]. Therefore, during storage and after field application of manure, factors such as oxygen gradients can influence the production rate of nitrous oxide [80]. Additionally, there is a correlation between chemical gradients and emission levels of nitrous oxide from manure [81]. The influence of decomposer activity on the development of chemical gradients, particularly the loss of carbon dioxide and ammonia near air-liquid interfaces, can cause a reduction in alkalinity and pH. This is primarily due to the acidifying process of ammonia oxidation, which may result in decreased rates of nitrification and denitrification [80]. However, it can also lead to an increased nitrite-to-nitrogen ratio in denitrification [82]. Overall, the current evidence suggests that livestock impact on atmospheric nitrous oxide concentration is mostly related to manure. Furthermore, net emissions not only depend on manure composition but also ambient climatic conditions. Therefore, specific attention should be given to manure properties and environmental conditions in order to develop appropriate mitigation solutions.
2.2. Impacts of Livestock Production on Water and Land Use
2.2.1. Water Use
The increasing demand for livestock-related products not only results in impacts on GHG emissions and nutrient cycles but also puts substantial pressure on natural resources such as land and water [8]. Large-scale feed crop cultivation, grazing land, and water use are among the factors that directly link livestock production with detrimental impacts on natural resource availability [83]. Given the finite and essential nature of water as a resource for life on the planet, the overall water usage of livestock production systems is a topic of ongoing debate [84]. Globally, freshwater resources are relatively scarce, accounting for only about 2.5% of the total water reserves, with a significant portion of these resources being inaccessible due to their storage in glaciers and permanent ice formations [85]. Addressing water use in livestock production appears to be vital for sustainable agriculture and environmental conservation. Water utilization within the livestock industry extends beyond direct consumption for hydration to a range of auxiliary functions and product processing, as well as the crucial need for water in feed crop growth [86]. While these additional water requirements are significant, direct consumption and feed crop growth remain the central components. Previous reports have provided a quantitative assessment of water usage in the livestock sector, shedding light on both direct and indirect water consumption [87][88]. It was highlighted that the water footprint of feedlot beef cattle ranged from 3.3 to 221 L H2Oe kg−1 of live weight [89]. Throughout the life cycle of a single broiler, at least 1 L of water is needed to maintain homeostasis [90], with the potential for even greater water requirements as temperatures rise [91][92][93][94][95]. Similarly, swine production under confined and dry feeding conditions had a water turnover rate of 120 mL/kg during the growing phase and 80 mL/kg of body weight for non-lactating adult pigs [96]. As a result, it appears that direct water consumption contributes to the overall water use in livestock production, primarily due to physiological functions such as growth, production, reproduction, and temperature regulation, which require substantial amounts of water. The use of water required for growing livestock feed seems to contribute the most to the overall water demands in the industry [97]. As the demand for livestock products continues to increase, the water demand will also increase since the livestock population will need to grow. However, there is not yet a standardized method to estimate the exact amount of evapotranspired water needed to produce 1 kg of feedstuff, and variations exist in the quantities of feedstuff that can be produced per cubic meter of water [98]. Studies have shown that between 0.5 and 8 kg of dry matter forage can be generated per cubic meter of water, depending on the production system [98]. More specifically, forage maize, one of the most common feedstuffs, has been estimated to yield around 2.9 to 3.7 kg of dry matter per cubic meter [99]. Major differences exist among livestock production systems in various regions with differing levels of intensification, indicating the need for systematic consideration of livestock-water interactions and site-specific interventions. This approach is crucial for ensuring sustainable and productive use of water resources.
2.2.2. Land Use
Livestock primarily consumes food items that can otherwise be made available for human consumption. Additionally, there is a recent consensus that the production of feedstuff diverts arable land from food production [100]. Hence, finding the right balance between direct consumption of plant production and feedstuff is crucial to global food availability. Livestock production directly impacts land use through feed crops, grazing land, and to a lesser extent, land conversion. Most of the agricultural land is directed towards livestock nutrition, which is about twice as much as that for arable crops. Unfortunately, the majority of livestock production systems have a protein conversion efficiency of less than 0.5, which indirectly translates to the land needed to maintain specific livestock systems [100]. Milk and egg production have efficiencies estimated at around 0.25 [101], while lamb and beef have the lowest efficiency at 0.06 and 0.04, respectively [102]. Humans would thus benefit more from consuming grain-based protein directly rather than consuming grain-fed animal protein [103]. Despite the increasing interest in the use of inedible feed in livestock production, using land to produce such products would not necessarily eliminate competition if crops were simply grown instead [98]. In livestock production, inedible feed encompasses materials unfit for human consumption yet valuable as sources of nutrition for animals [104][105]. They consist of but are not limited to, straws, hays, hulls, and crop residues, as well as certain weeds and invasive plants. These materials are primarily utilized in ruminant nutrition as it is preferred that the use of potentially edible feed resources by livestock be restricted to those with the highest daily nutrient requirements [104]. Therefore, it appears that the large-scale cultivation of crops needed for animal nutrition necessitates extensive areas of land, causing habitat loss and a decline in biodiversity.
Grazing land, which can also be referred to as pastureland or grazing pasture, consists of natural or cultivated vegetation that serves as a food source for ruminant animals [106]. Like feed crops, grazing land has a significant impact on land use. Indeed, managed grazing covers more than 33 million square kilometers of the global land surface, making it the single most extensive form of land use on Earth [107]. It is important to consider the intensity and extent to which farm animals consume plants in a specific area over a given period to limit their impacts on the environment. In fact, the continuous presence of livestock on pastureland can hinder the natural generation of biomass, often resulting in vegetation degradation [108]. Additionally, certain plant species are often targeted by animals during grazing due to their nutritional profile and flavor, which can lead to negative impacts on biodiversity and disrupt ecosystems [109]. Changes are noticed in the abundance, cover, and configuration of life forms. These structural alterations are often associated with vegetal species invasion, water drainage, erosion, and the alteration of soil biochemical characteristics [110]. Grasslands have a natural ability to store large amounts of carbon dioxide. Recent research indicates that a diverse plant community contributes to increased soil organic carbon (SOC) levels by promoting the growth of belowground biomass and enhancing the contribution of microbial necromass to SOC storage [111][112][113]. Conversely, intensive livestock grazing has been found to significantly reduce plant and microbe-mediated SOC formation [110]. Eze, et al. [114] reported that grazing tends to decrease SOC stocks by an average of 15% globally, with the most significant reductions occurring in tropical regions and the least in temperate climates. Likewise, sheep grazing has a generally stronger impact on SOC than cattle grazing, with SOC reduction occurring mostly in topsoil [115]. These findings suggest that livestock species, water availability, and temperature are confounding factors in the impacts of grazing on SOC storage.
The relationship between livestock production and land conversion is evident from the current literature. In this context, land conversion can be defined as the transformation of natural ecosystems, such as forests or grasslands, into agricultural zones [116]. The continuous expansion of these crops and pastures is the primary driver of livestock-related land conversion. In fact, the demand for animal products has been increasing globally, with meat production almost quadrupling between 1963 and 2015, and milk production more than doubling (from 340 to 818 Mt) over the same period [117]. As a result, more land is needed to meet these increasing demands. Indeed, not only more feedstuffs are required, but a significant number of infrastructures need to be built in order to accommodate the new herds [118]. This has led to deforestation in areas such as the Amazon, where cultivated soybean areas are expanding [119]. Hence, the business-as-usual approach of land conversion for livestock production is not sustainable, and strategies should instead focus on improving efficiency through higher product output with limited inputs.
References
- Turnbull, P.F.; Reed, C.A. The fauna from the terminal Pleistocene of Palegawra Cave, a Zarzian occupation site in northeastern Iraq. Fieldiana Anthropol. 1974, 63, 81–146.
- Hartung, J. A short history of livestock production. In Livestock Housing: Modern Management to Ensure Optimal Health and Welfare of Farm Animals; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 81–146.
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2853–2867.
- Randolph, T.F.; Schelling, E.; Grace, D.; Nicholson, C.F.; Leroy, J.L.; Cole, D.C.; Demment, M.W.; Omore, A.; Zinsstag, J.; Ruel, M. Invited review: Role of livestock in human nutrition and health for poverty reduction in developing countries. J. Anim. Sci. 2007, 85, 2788–2800.
- Meadowcroft, J. Minding the Stock: Bringing Public Policy to Bear on Livestock Sector Development; The World Bank: Washington, DC, USA, 2009.
- FAO. Meat Market Review: Emerging Trends and Outlook; FAO: Rome, Italy, 2021.
- Sakadevan, K.; Nguyen, M.L. Livestock Production and Its Impact on Nutrient Pollution and Greenhouse Gas Emissions. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 141, pp. 147–184.
- Leip, A.; Billen, G.; Garnier, J.; Grizzetti, B.; Lassaletta, L.; Reis, S.; Simpson, D.; Sutton, M.A.; De Vries, W.; Weiss, F. Impacts of European livestock production: Nitrogen, sulphur, phosphorus and greenhouse gas emissions, land-use, water eutrophication and biodiversity. Environ. Res. Lett. 2015, 10, 115004.
- Bartley, R.; Roth, C.H.; Ludwig, J.; McJannet, D.; Liedloff, A.; Corfield, J.; Hawdon, A.; Abbott, B. Runoff and erosion from Australia’s tropical semi-arid rangelands: Influence of ground cover for differing space and time scales. Hydrol. Process. 2006, 20, 3317–3333.
- Bell, L.W.; Kirkegaard, J.A.; Swan, A.; Hunt, J.R.; Huth, N.I.; Fettell, N.A. Impacts of soil damage by grazing livestock on crop productivity. Soil Tillage Res. 2011, 113, 19–29.
- Whitmore, A. Impact of livestock on soil. In Proceedings of the Workshop 4 on Sustainable Animal Production, Hannover, Germany, 28 September 2000; pp. 39–41.
- Moumen, A.; Azizi, G.; Chekroun, K.B.; Baghour, M. The effects of livestock methane emission on the global warming: A review. Int. J. Glob. Warm. 2016, 9, 229–253.
- Sejian, V.; Hyder, I.; Ezeji, T.; Lakritz, J.; Bhatta, R.; Ravindra, J.; Prasad, C.S.; Lal, R. Global warming: Role of livestock. In Climate Change Impact on Livestock: Adaptation and Mitigation; Springer: Berlin/Heidelberg, Germany, 2015; pp. 141–169.
- Caro, D.; Davis, S.J.; Bastianoni, S.; Caldeira, K. Global and regional trends in greenhouse gas emissions from livestock. Clim. Chang. 2014, 126, 203–216.
- Casey, K.D.; Bicudo, J.R.; Schmidt, D.R.; Singh, A.; Gay, S.W.; Gates, R.S.; Jacobson, L.D.; Hoff, S.J. Air quality and emissions from livestock and poultry production/waste management systems. In Animal Agriculture and the Environment: National Center for Manure and Animal Waste Management White Papers; ASABE: St. Joseph, MI, USA, 2006.
- Banhazi, T.; Aland, A.; Hartung, J. Air Quality and Livestock Farming; CRC Press: Boca Raton, FL, USA, 2018.
- Baroni, L.; Cenci, L.; Tettamanti, M.; Berati, M. Evaluating the environmental impact of various dietary patterns combined with different food production systems. Eur. J. Clin. Nutr. 2007, 61, 279–286.
- Richardson, N.; Shepherd, R.; Elliman, N. Current attitudes and future influence on meat consumption in the UK. Appetite 1993, 21, 41–51.
- Başkent, E.Z.; Keleş, S.; Kadıoğulları, A.İ.; Bingöl, Ö. Quantifying the Effects of Forest Management Strategies on the Production of Forest Values: Timber, Carbon, Oxygen, Water, and Soil. Environ. Model. Assess. 2011, 16, 145–152.
- Pawar, K.; Rothkar, R.V. Forest conservation & environmental awareness. Procedia Earth Planet. Sci. 2015, 11, 212–215.
- Yang, J.; McBride, J.; Zhou, J.; Sun, Z. The urban forest in Beijing and its role in air pollution reduction. Urban For. Urban Green. 2005, 3, 65–78.
- Nisbet, T.R. The role of forest management in controlling diffuse pollution in UK forestry. For. Ecol. Manag. 2001, 143, 215–226.
- Kweku, D.; Bismark, O.; Maxwell, A.; Desmond, K.; Danso, K.; Oti-Mensah, E.; Quachie, A.; Adormaa, B. Greenhouse Effect: Greenhouse Gases and Their Impact on Global Warming. J. Sci. Res. Rep. 2018, 17, 1–9.
- Wang, W.; Guo, L.; Li, Y.; Su, M.; Lin, Y.; de Perthuis, C.; Ju, X.; Lin, E.; Moran, D. Greenhouse gas intensity of three main crops and implications for low-carbon agriculture in China. Clim. Chang. 2014, 128, 57–70.
- Arias, P.; Bellouin, N.; Coppola, E.; Jones, R.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.; Plattner, G.-K.; Rogelj, J. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Summary; Cambridge University Press: Cambridge, UK, 2021.
- Lacis, A.A.; Schmidt, G.A.; Rind, D.; Ruedy, R.A. Atmospheric CO2: Principal control knob governing Earth’s temperature. Science 2010, 330, 356–359.
- Nelson, D.D.; McManus, B.; Urbanski, S.; Herndon, S.; Zahniser, M.S. High precision measurements of atmospheric nitrous oxide and methane using thermoelectrically cooled mid-infrared quantum cascade lasers and detectors. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 3325–3335.
- Kolstad, C.D. Learning and Stock Effects in Environmental Regulation: The Case of Greenhouse Gas Emissions. J. Environ. Econ. Manag. 1996, 31, 1–18.
- Cai, W.; Ng, B.; Wang, G.; Santoso, A.; Wu, L.; Yang, K. Increased ENSO sea surface temperature variability under four IPCC emission scenarios. Nat. Clim. Chang. 2022, 12, 228–231.
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013.
- Havran, V.; Duduković, M.P.; Lo, C.S. Conversion of Methane and Carbon Dioxide to Higher Value Products. Ind. Eng. Chem. Res. 2011, 50, 7089–7100.
- Broucek, J. Production of Methane Emissions from Ruminant Husbandry: A Review. J. Environ. Prot. 2014, 5, 1482–1493.
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, S2–S16.
- Ellis, J.L.; Dijkstra, J.; Kebreab, E.; Bannink, A.; Odongo, N.E.; McBride, B.W.; France, J. Aspects of rumen microbiology central to mechanistic modelling of methane production in cattle. J. Agric. Sci. 2008, 146, 213–233.
- Boadi, D.; Benchaar, C.; Chiquette, J.; Massé, D. Mitigation strategies to reduce enteric methane emissions from dairy cows: Update review. Can. J. Anim. Sci. 2004, 84, 319–335.
- Honan, M.; Feng, X.; Tricarico, J.M.; Kebreab, E. Feed additives as a strategic approach to reduce enteric methane production in cattle: Modes of action, effectiveness and safety. Anim. Prod. Sci. 2021, 62, 1303–1317.
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27.
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492.
- McAllister, T.A.; Cheng, K.J.; Okine, E.K.; Mathison, G.W. Dietary, environmental and microbiological aspects of methane production in ruminants. Can. J. Anim. Sci. 1996, 76, 231–243.
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253.
- Joblin, K.N. Ruminal acetogens and their potential to lower ruminant methane emissions. Aust. J. Agric. Res. 1999, 50, 1307–1314.
- McAllister, T.A.; Newbold, C.J. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 2008, 48, 7–13.
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2010, 4, 351–365.
- Danielsson, R.; Dicksved, J.; Sun, L.; Gonda, H.; Muller, B.; Schnurer, A.; Bertilsson, J. Methane Production in Dairy Cows Correlates with Rumen Methanogenic and Bacterial Community Structure. Front. Microbiol. 2017, 8, 226.
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7.
- Ungerfeld, E.M. Inhibition of Rumen Methanogenesis and Ruminant Productivity: A Meta-Analysis. Front. Vet. Sci. 2018, 5, 113.
- Balch, W.E.; Fox, G.E.; Magrum, L.J.; Woese, C.R.; Wolfe, R.S. Methanogens: Reevaluation of a unique biological group. Microbiol. Rev. 1979, 43, 260–296.
- Lopez, S.; McIntosh, F.M.; Wallace, R.J.; Newbold, C.J. Effect of adding acetogenic bacteria on methane production by mixed rumen microorganisms. Anim. Feed Sci. Technol. 1999, 78, 1–9.
- Asanuma, N.; Iwamoto, M.; Hino, T. Effect of the addition of fumarate on methane production by ruminal microorganisms in vitro. J. Dairy Sci. 1999, 82, 780–787.
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261.
- Buddle, B.M.; Denis, M.; Attwood, G.T.; Altermann, E.; Janssen, P.H.; Ronimus, R.S.; Pinares-Patino, C.S.; Muetzel, S.; Neil Wedlock, D. Strategies to reduce methane emissions from farmed ruminants grazing on pasture. Vet. J. 2011, 188, 11–17.
- Haque, M.N. Dietary manipulation: A sustainable way to mitigate methane emissions from ruminants. J. Anim. Sci. Technol. 2018, 60, 15.
- Tarazkar, M.H.; Kargar Dehbidi, N.; Ansari, R.A.; Pourghasemi, H.R. Factors affecting methane emissions in OPEC member countries: Does the agricultural production matter? Environ. Dev. Sustain. 2020, 23, 6734–6748.
- Dalby, F.R.; Hafner, S.D.; Petersen, S.O.; VanderZaag, A.C.; Habtewold, J.; Dunfield, K.; Chantigny, M.H.; Sommer, S.G. Understanding methane emission from stored animal manure: A review to guide model development. J. Environ. Qual. 2021, 50, 817–835.
- Petersen, S.O.; Blanchard, M.; Chadwick, D.; Del Prado, A.; Edouard, N.; Mosquera, J.; Sommer, S.G. Manure management for greenhouse gas mitigation. Animal 2013, 7 (Suppl. S2), 266–282.
- Osada, T.; Kuroda, K.; Yonaga, M. Determination of nitrous oxide, methane, and ammonia emissions from a swine waste composting process. J. Mater. Cycles Waste Manag. 2000, 2, 51–56.
- Liu, L.; Li, C.; Li, H. Long-term microbial community succession and mechanisms of regulation of dissolved organic matter derivation in livestock manure fermentation system. Chemosphere 2023, 329, 138588.
- Kim, S.Y.; Pramanik, P.; Bodelier, P.L.; Kim, P.J. Cattle Manure Enhances Methanogens Diversity and Methane Emissions Compared to Swine Manure under Rice Paddy. PLoS ONE 2014, 9, e113593.
- Li, Y.; Achinas, S.; Zhao, J.; Geurkink, B.; Krooneman, J.; Willem Euverink, G.J. Co-digestion of cow and sheep manure: Performance evaluation and relative microbial activity. Renew. Energy 2020, 153, 553–563.
- Sommer, S.G.; Petersen, S.O.; Søgaard, H.T. Greenhouse Gas Emission from Stored Livestock Slurry. J. Environ. Qual. 2000, 29, 744–751.
- Kettunen, R.H.; Rintala, J.A. The effect of low temperature (5-29 degrees C) and adaptation on the methanogenic activity of biomass. Appl. Microbiol. Biotechnol. 1997, 48, 570–576.
- Elsgaard, L.; Olsen, A.B.; Petersen, S.O. Temperature response of methane production in liquid manures and co-digestates. Sci. Total Environ. 2016, 539, 78–84.
- Hashimoto, A.G.; Varel, V.H.; Chen, Y.R. Ultimate methane yield from beef cattle manure: Effect of temperature, ration constituents, antibiotics and manure age. Agric. Wastes 1981, 3, 241–256.
- Massé, D.I.; Masse, L.; Claveau, S.; Benchaar, C.; Thomas, O. Methane Emissions from Manure Storages. Trans. ASABE 2008, 51, 1775–1781.
- Sommer, S.G.; Olesen, J.E.; Petersen, S.O.; Weisbjerg, M.R.; Valli, L.; Rodhe, L.; BÉLine, F. Region-specific assessment of greenhouse gas mitigation with different manure management strategies in four agroecological zones. Glob. Chang. Biol. 2009, 15, 2825–2837.
- Sharma, R.; Ryan, K.; Hao, X.; Larney, F.J.; McAllister, T.A.; Topp, E. Real-time quantification of mcrA, pmoA for methanogen, methanotroph estimations during composting. J. Environ. Qual. 2011, 40, 199–205.
- Wilshusen, J.H.; Hettiaratchi, J.P.; De Visscher, A.; Saint-Fort, R. Methane oxidation and formation of EPS in compost: Effect of oxygen concentration. Environ. Pollut. 2004, 129, 305–314.
- Fan, Y.; Lei, Z.; Yang, X.; Kobayashi, M.; Adachi, Y.; Zhang, Z.; Shimizu, K. Effect of nano-bubble water on high solid anaerobic digestion of pig manure: Focus on digestion stability, methanogenesis performance and related mechanisms. Bioresour. Technol. 2020, 315, 123793.
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006.
- Schulze, E.D.; Luyssaert, S.; Ciais, P.; Freibauer, A.; Janssens, I.A.; Soussana, J.F.; Smith, P.; Grace, J.; Levin, I.; Thiruchittampalam, B.; et al. Importance of methane and nitrous oxide for Europe’s terrestrial greenhouse-gas balance. Nat. Geosci. 2009, 2, 842–850.
- Crutzen, P.J. The Influence of Nitrogen Oxides on Atmospheric Ozone Content. In Paul J. Crutzen: A Pioneer on Atmospheric Chemistry and Climate Change in the Anthropocene; Crutzen, P.J., Brauch, H.G., Eds.; Springer Briefs on Pioneers in Science and Practice; Springer International Publishing: Cham, Switzerland, 2016; pp. 108–116.
- Badr, O.; Probert, S.D. Environmental impacts of atmospheric nitrous oxide. Appl. Energy 1993, 44, 197–231.
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Chang. 2012, 2, 410–416.
- Lu, Q.; Wu, J.; Wang, M.; Zhou, C.; Han, X.; Odongo, E.N.; Tan, Z.; Tang, S. Effects of dietary addition of cellulase and a Saccharomyces cerevisiae fermentation product on nutrient digestibility, rumen fermentation and enteric methane emissions in growing goats. Arch. Anim. Nutr. 2016, 70, 224–238.
- Xu, P.; Houlton, B.Z.; Zheng, Y.; Zhou, F.; Ma, L.; Li, B.; Liu, X.; Li, G.; Lu, H.; Quan, F.; et al. Policy-enabled stabilization of nitrous oxide emissions from livestock production in China over 1978–2017. Nat. Food 2022, 3, 356–366.
- Rotz, C.A. Management to reduce nitrogen losses in animal production. J. Anim. Sci. 2004, 82, E119–E137.
- Jia, Z.; Conrad, R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671.
- Yamamoto, N.; Otawa, K.; Nakai, Y. Diversity and abundance of ammonia-oxidizing bacteria and ammonia-oxidizing archaea during cattle manure composting. Microb. Ecol. 2010, 60, 807–815.
- Wallenstein, M.D.; Myrold, D.D.; Firestone, M.; Voytek, M. Environmental controls on denitrifying communities and denitrification rates: Insights from molecular methods. Ecol. Appl. 2006, 16, 2143–2152.
- Petersen, S.O.; Nielsen, A.L.; Haarder, K.; Henriksen, K. Factors controlling nitrification and denitrification: A laboratory study with gel-stabilized liquid cattle manure. Microb. Ecol. 1992, 23, 239–255.
- Husted, S.; Jensen, L.S.; Jørgensen, S.S. Reducing ammonia loss from cattle slurry by the use of acidifying additives: The role of the buffer system. J. Sci. Food Agric. 1991, 57, 335–349.
- Baggs, E.M.; Smales, C.L.; Bateman, E.J. Changing pH shifts the microbial sourceas well as the magnitude of N2O emission from soil. Biol. Fertil. Soils 2010, 46, 793–805.
- Heinke, J.; Lannerstad, M.; Gerten, D.; Havlík, P.; Herrero, M.; Notenbaert, A.M.O.; Hoff, H.; Müller, C. Water Use in Global Livestock Production—Opportunities and Constraints for Increasing Water Productivity. Water Resour. Res. 2020, 56, e2019WR026995.
- Bogardi, J.J.; Dudgeon, D.; Lawford, R.; Flinkerbusch, E.; Meyn, A.; Pahl-Wostl, C.; Vielhauer, K.; Vörösmarty, C. Water security for a planet under pressure: Interconnected challenges of a changing world call for sustainable solutions. Curr. Opin. Environ. Sustain. 2012, 4, 35–43.
- Halla, C.; Blöthe, J.H.; Tapia Baldis, C.; Trombotto Liaudat, D.; Hilbich, C.; Hauck, C.; Schrott, L. Ice content and interannual water storage changes of an active rock glacier in the dry Andes of Argentina. Cryosphere 2021, 15, 1187–1213.
- Ran, Y.; Lannerstad, M.; Herrero, M.; Van Middelaar, C.E.; De Boer, I.J.M. Assessing water resource use in livestock production: A review of methods. Livest. Sci. 2016, 187, 68–79.
- Boulay, A.-M.; Drastig, K.; Amanullah; Chapagain, A.; Charlon, V.; Civit, B.; DeCamillis, C.; De Souza, M.; Hess, T.; Hoekstra, A.Y.; et al. Building consensus on water use assessment of livestock production systems and supply chains: Outcome and recommendations from the FAO LEAP Partnership. Ecol. Indic. 2021, 124, 107391.
- Kebebe, E.G.; Oosting, S.J.; Haileslassie, A.; Duncan, A.J.; de Boer, I.J. Strategies for improving water use efficiency of livestock production in rain-fed systems. Animal 2015, 9, 908–916.
- Ridoutt, B.G.; Sanguansri, P.; Freer, M.; Harper, G.S. Water footprint of livestock: Comparison of six geographically defined beef production systems. Int. J. Life Cycle Assess. 2011, 17, 165–175.
- Pesti, G.M.; Amato, S.V.; Minear, L.R. Water consumption of broiler chickens under commercial conditions. Poult. Sci. 1985, 64, 803–808.
- Ncho, C.M.; Goel, A.; Gupta, V.; Jeong, C.M.; Choi, Y.H. Effect of in ovo feeding of gamma-aminobutyric acid combined with embryonic thermal manipulation on hatchability, growth, and hepatic gene expression in broilers. Anim. Biosci. 2023, 36, 284–294.
- Ncho, C.M.; Gupta, V.; Choi, Y.H. Effects of Dietary Glutamine Supplementation on Heat-Induced Oxidative Stress in Broiler Chickens: A Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 570.
- Ncho, C.M.; Gupta, V.; Goel, A. Effect of thermal conditioning on growth performance and thermotolerance in broilers: A systematic review and meta-analysis. J. Therm. Biol. 2021, 98, 102916.
- Ncho, C.M.; Jeong, C.; Gupta, V.; Goel, A. The effect of gamma-aminobutyric acid supplementation on growth performances, immune responses, and blood parameters of chickens reared under stressful environment: A meta-analysis. Environ. Sci. Pollut. Res. Int. 2021, 28, 45019–45028.
- Goel, A.; Ncho, C.M.; Choi, Y.H. Regulation of gene expression in chickens by heat stress. J. Anim. Sci. Biotechnol. 2021, 12, 11.
- Schlink, A.C.; Nguyen, M.L.; Viljoen, G.J. Water requirements for livestock production: A global perspective. Rev. Sci. Tech. 2010, 29, 603–619.
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163.
- Thornton, P.; Herrero, M. The Inter-Linkages between Rapid Growth in Livestock Production, Climate Change, and the Impacts on Water Resources, Land Use, and Deforestation; World Bank Policy Research Working Papers; The World Bank: Washington, DC, USA, 2010.
- Alkhamisi, S.A.; Abdelrahman, H.A.; Ahmed, M.; Goosen, M.F.A. Assessment of reclaimed water irrigation on growth, yield, and water-use efficiency of forage crops. Appl. Water Sci. 2011, 1, 57–65.
- Manceron, S.; Ben-Ari, T.; Dumas, P. Feeding proteins to livestock: Global land use and foodvs.feed competition. OCL 2014, 21, D408.
- Wilkinson, J.M. Re-defining efficiency of feed use by livestock. Animal 2011, 5, 1014–1022.
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Rounsevell, M.D.A. Human appropriation of land for food: The role of diet. Glob. Environ. Chang. 2016, 41, 88–98.
- Bakhsh, A.; Lee, E.-Y.; Ncho, C.M.; Kim, C.-J.; Son, Y.-M.; Hwang, Y.-H.; Joo, S.-T. Quality Characteristics of Meat Analogs through the Incorporation of Textured Vegetable Protein: A Systematic Review. Foods 2022, 11, 1242.
- Wilkinson, J.M.; Lee, M.R.F. Review: Use of human-edible animal feeds by ruminant livestock. Animal 2018, 12, 1735–1743.
- Salami, S.A.; Luciano, G.; O’Grady, M.N.; Biondi, L.; Newbold, C.J.; Kerry, J.P.; Priolo, A. Sustainability of feeding plant by-products: A review of the implications for ruminant meat production. Anim. Feed Sci. Technol. 2019, 251, 37–55.
- Reid, R.S.; Galvin, K.A.; Kruska, R.S. Global significance of extensive grazing lands and pastoral societies: An introduction. In Fragmentation in Semi-Arid and Arid Landscapes: Consequences for Human and Natural Systems; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–24.
- Asner, G.P.; Elmore, A.J.; Olander, L.P.; Martin, R.E.; Harris, A.T. Grazing Systems, Ecosystem Responses, and Global Change. Annu. Rev. Environ. Resour. 2004, 29, 261–299.
- Pei, S.; Fu, H.; Wan, C. Changes in soil properties and vegetation following exclosure and grazing in degraded Alxa desert steppe of Inner Mongolia, China. Agric. Ecosyst. Environ. 2008, 124, 33–39.
- Filazzola, A.; Brown, C.; Dettlaff, M.A.; Batbaatar, A.; Grenke, J.; Bao, T.; Peetoom Heida, I.; Cahill, J.F., Jr. The effects of livestock grazing on biodiversity are multi-trophic: A meta-analysis. Ecol. Lett. 2020, 23, 1298–1309.
- Byrnes, R.C.; Eastburn, D.J.; Tate, K.W.; Roche, L.M. A Global Meta-Analysis of Grazing Impacts on Soil Health Indicators. J. Environ. Qual. 2018, 47, 758–765.
- Bai, Y.; Cotrufo, M.F. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608.
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, L.E.; Johnson, D.; et al. Combatting global grassland degradation. Nat. Rev. Earth Environ. 2021, 2, 720–735.
- Conant, R.T.; Cerri, C.E.; Osborne, B.B.; Paustian, K. Grassland management impacts on soil carbon stocks: A new synthesis. Ecol. Appl. 2017, 27, 662–668.
- Eze, S.; Palmer, S.M.; Chapman, P.J. Soil organic carbon stock in grasslands: Effects of inorganic fertilizers, liming and grazing in different climate settings. J. Environ. Manag. 2018, 223, 74–84.
- Zhou, G.; Zhou, X.; He, Y.; Shao, J.; Hu, Z.; Liu, R.; Zhou, H.; Hosseinibai, S. Grazing intensity significantly affects belowground carbon and nitrogen cycling in grassland ecosystems: A meta-analysis. Glob. Chang. Biol. 2017, 23, 1167–1179.
- Deng, X.; Huang, J.; Rozelle, S.; Uchida, E. Cultivated land conversion and potential agricultural productivity in China. Land Use Policy 2006, 23, 372–384.
- Michalk, D.L.; Kemp, D.R.; Badgery, W.B.; Wu, J.; Zhang, Y.; Thomassin, P.J. Sustainability and future food security—A global perspective for livestock production. Land Degrad. Dev. 2018, 30, 561–573.
- van Zanten, H.H.E.; Mollenhorst, H.; Klootwijk, C.W.; van Middelaar, C.E.; de Boer, I.J.M. Global food supply: Land use efficiency of livestock systems. Int. J. Life Cycle Assess. 2015, 21, 747–758.
- Naylor, R.; Steinfeld, H.; Falcon, W.; Galloway, J.; Smil, V.; Bradford, E.; Alder, J.; Mooney, H. Agriculture. Losing the links between livestock and land. Science 2005, 310, 1621–1622.
More
Information
Subjects:
Green & Sustainable Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
9.5K
Revisions:
2 times
(View History)
Update Date:
22 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





