
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yauheni Shastak | -- | 3507 | 2023-11-21 14:39:42 | | | |
| 2 | Lindsay Dong | + 2 word(s) | 3509 | 2023-11-22 01:57:36 | | |
Video Upload Options
Riboflavin, an essential B-vitamin, plays a crucial role in poultry metabolism, impacting energy production, growth, and immune regulation. Its role in redox reactions and energy metabolism is vital for optimal growth and development. Riboflavin is essential for adenosine triphosphate (ATP) production and the conversion of tryptophan into niacin. Deficiency can lead to skeletal deformities, impaired growth, and compromised immune function. Dietary riboflavin supplementation is necessary due to variable bioavailability in plant-derived sources. The vitamin is absorbed through specialized transport proteins, and its cellular uptake is facilitated by specific receptors. Riboflavin’s role in protein synthesis and its antioxidant properties influence poultry growth and defense against oxidative stress. Its impact on reproductive performance, hatchability, and overall poultry health underscores its significance in poultry nutrition.
1. Introduction
2. Biochemical Fundamentals of Riboflavin
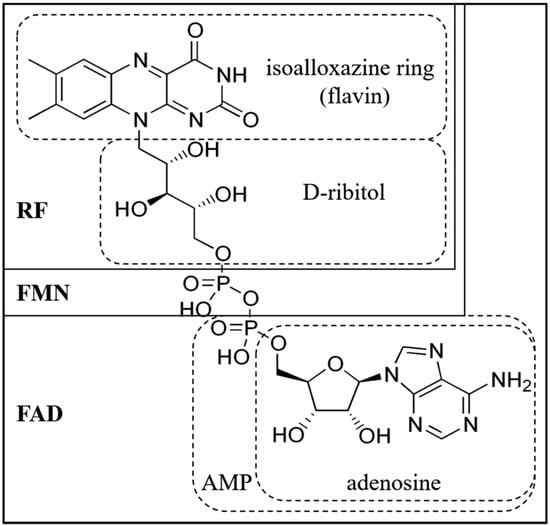
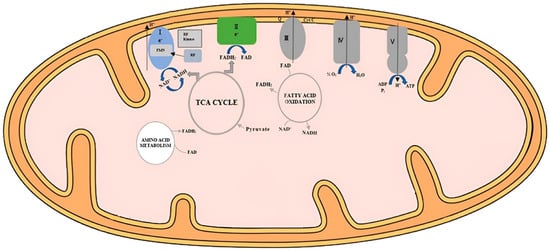
3. Riboflavin Metabolism in Poultry
4. Riboflavin and Poultry Growth
5. Oxidative Stress Defense
6. Reproductive Performance and Hatchability
7. Riboflavin Requirements for Poultry
8. Conclusions
References
- FAO (The Food and Agriculture Organization of the United Nations). OECD-FAO Agricultural Outlook 2022–2031: 6. Meat. 2022. Available online: https://www.oecd-ilibrary.org/sites/ab129327-en/index.html?itemId=/content/component/ab129327-en#:~:text=Poultry%20meat%20is%20projected%20to,by%20pig%2C%20sheep%20and%20bovine (accessed on 23 August 2023).
- Roth-Maier, D.A.; Paulicks, B.R. Effects of a suboptimal dietary intake of particular B-vitamins on the growth of fattening chicken. Arch. Geflügelkunde 2002, 66, 201–205.
- McDowell, L.R. Vitamin nutrition of livestock animals: Overview from vitamin discovery to today. Can. J. Anim. Sci. 2006, 86, 171–179.
- Jortner, B.S.; Cherry, J.; Lidsky, T.I.; Manetto, C.; Shell, L. Peripheral Neuropathy of Dietary Riboflavin Deficiency in Chickens. J. Neuropathol. Exp. Neurol. 1987, 46, 544–555.
- Johnson, W.D.; Storts, R.W. Peripheral Neuropathy Associated with Dietary Riboflavin Deficiency in the Chicken I. Light Microscopic Study. Vet. Pathol. 1988, 25, 9–16.
- Cai, Z.; Finnie, J.W.; Blumbergs, P.C. Avian Riboflavin Deficiency: An Acquired Tomaculous Neuropathy. Vet. Pathol. 2006, 43, 780–781.
- Leiber, F.; Amsler, Z.; Bieber, A.; Quander-Stoll, N.; Maurer, V.; Lambertz, C.; Früh, B.; Ayrle, H. Effects of riboflavin supplementation level on health, performance, and fertility of organic broiler parent stock and their chicks. Animal 2022, 16, 100433.
- Asplin, F. Riboflavin Deficiency in Poultry. Vet. J. 1941, 97, 16–26.
- Cai, Z.; Finnie, J.; Manavis, J.; Blumbergs, P. Avian riboflavin deficiency causes reliably reproducible peripheral nerve demyelination and, with vitamin supplementation, rapid remyelination. Hum. Exp. Toxicol. 2023, 42.
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950.
- Zhang, B.; Zhao, R.; Fouad, A.; Wu, Y.; Sun, P.; Wei, J.; Huang, W.; Xie, M.; Tang, J.; Hou, S. Research Note: Effects of riboflavin on reproductive performance and antioxidant status of duck breeders. Poult. Sci. 2020, 99, 1564–1570.
- Tang, J.; Hu, J.; Xue, M.; Guo, Z.; Xie, M.; Zhang, B.; Zhou, Z.; Huang, W.; Hou, S. Maternal diet deficient in riboflavin induces embryonic death associated with alterations in the hepatic proteome of duck embryos. Nutr. Metab. 2019, 16, 19.
- Lambertz, C.; Leopold, J.; Damme, K.; Vogt-Kaute, W.; Ammer, S.; Leiber, F. Effects of a riboflavin source suitable for use in organic broiler diets on performance traits and health indicators. Animal 2020, 14, 716–724.
- Cherian, G. Nutrition and metabolism in poultry: Role of lipids in early diet. J. Anim. Sci. Biotechnol. 2015, 6, 28.
- Abbas, C.A.; Sibirny, A.A. Genetic Control of Biosynthesis and Transport of Riboflavin and Flavin Nucleotides and Construction of Robust Biotechnological Producers. Microbiol. Mol. Biol. Rev. 2011, 75, 321–360.
- Udhayabanu, T.; Manole, A.; Rajeshwari, M.; Varalakshmi, P.; Houlden, H.; Ashokkumar, B. Riboflavin Responsive Mitochondrial Dysfunction in Neurodegenerative Diseases. J. Clin. Med. 2017, 6, 52.
- Brooks, A.; Martin, E. Riboflavin Deficiency in Broiler Chickens. AHL Newsletter 27(1):20 (Animal Health Laboratory, University of Guelph, Ontario, Canada). Available online: https://www.uoguelph.ca/ahl/riboflavin-deficiency-broiler-chickens (accessed on 23 August 2023).
- Cook, M.E.; Springer, W.T. Effect of Reovirus Infection and Dietary Levels of Selected Vitamins on Immunocompetence of Chickens. Avian Dis. 1983, 27, 367–377.
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front. Nutr. 2019, 6, 48.
- Scott, M.L.; Holm, E.R.; Reynolds, R.E. Studies on the Niacin, Riboflavin, Choline, Manganese and Zinc Requirements of Young Ringnecked Pheasants for Growth, Feathering and Prevention of Leg Disorders. Poult. Sci. 1959, 38, 1344–1350.
- Wyatt, R.D.; Tung, H.T.; Donaldson, W.E.; Hamilton, P.B. A New Description of Riboflavin Deficiency Syndrome in Chickens. Poult. Sci. 1973, 52, 237–244.
- Serafin, J. Studies on the Riboflavin, Niacin, Pantothenic Acid and Choline Requirements of Young Bobwhite Quail. Poult. Sci. 1974, 53, 1522–1532.
- Lee, D.J.W. Growth, erythrogyte glutathione redugtase and liver flavin as indicators of riboflavin status in Turkey poults. Br. Poult. Sci. 1982, 23, 263–272.
- Roth-Maier, D.A.; Kirchgessner, M. Investigations on riboflavin requirement of fattening chickens. Arch. Geflügelkunde 1997, 61, 14–16.
- Olkowski, A.; Classen, H.L. The study of riboflavin requirement in broiler chickens. Int. J. Vitam. Nutr. Res. 1998, 68, 316–327.
- Cogburn, L.A.; Smarsh, D.N.; Wang, X.; Trakooljul, N.; Carré, W.; White, H.B. Transcriptional profiling of liver in riboflavin-deficient chicken embryos explains impaired lipid utilization, energy depletion, massive hemorrhaging, and delayed feathering. BMC Genom. 2018, 19, 177.
- Gržinić, G.; Piotrowicz-Cieślak, A.; Klimkowicz-Pawlas, A.; Górny, R.L.; Ławniczek-Wałczyk, A.; Piechowicz, L.; Olkowska, E.; Potrykus, M.; Tankiewicz, M.; Krupka, M.; et al. Intensive poultry farming: A review of the impact on the environment and human health. Sci. Total Environ. 2023, 858 Pt 3, 160014.
- Szczuko, M.; Ziętek, M.; Kulpa, D.; Seidler, T. Riboflavin-properties, occurrence and its use in medicine. Pteridines 2019, 30, 33–47.
- Liu, S.; Hu, W.; Wang, Z.; Chen, T. Production of riboflavin and related cofactors by biotechnological processes. Microb. Cell Factories 2020, 19, 31.
- Rivero, M.; Boneta, S.; Novo, N.; Velázquez-Campoy, A.; Polo, V.; Medina, M. Riboflavin kinase and pyridoxine 5′-phosphate oxidase complex formation envisages transient interactions for FMN cofactor delivery. Front. Mol. Biosci. 2023, 10, 1167348.
- Huerta, C.; Borek, D.; Machius, M.; Grishin, N.V.; Zhang, H. Structure and Mechanism of a Eukaryotic FMN Adenylyltransferase. J. Mol. Biol. 2009, 389, 388–400.
- Friedmann, H.C. Flavin Mononucleotide. In Methods of Enzymatic Analysis, 2nd ed.; Bergmeyer, H.U., Ed.; Academic Press: Cambridge, MA, USA, 1974; pp. 2179–2181.
- Pinto, J.T.; Zempleni, J. Riboflavin. Adv. Nutr. Int. Rev. J. 2016, 7, 973–975.
- Poudel, S.; Tabler, G.T.; Lin, J.; Zhai, W.; Zhang, L. Riboflavin and Bacillus subtilis effects on growth performance and woody-breast of Ross 708 broilers with or without Eimeria spp. challenge. J. Anim. Sci. Technol. 2022, 64, 443–461.
- Oprian, D.D.; Coon, M.J. Oxidation-reduction states of FMN and FAD in NADPH-cytochrome P-450 reductase during reduction by NADPH. J. Biol. Chem. 1982, 257, 8935–8944.
- Mansoorabadi, S.O.; Thibodeaux, C.J.; Liu, H.-W. The Diverse Roles of Flavin Coenzymes—Nature’s Most Versatile Thespians. J. Org. Chem. 2007, 72, 6329–6342.
- Toyomizu, M.; Kikusato, M.; Kawabata, Y.; Azad, A.K.; Inui, E.; Amo, T. Meat-type chickens have a higher efficiency of mitochondrial oxidative phosphorylation than laying-type chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 75–81.
- Hubert, S.; Athrey, G. Transcriptomic signals of mitochondrial dysfunction and OXPHOS dynamics in fast-growth chicken. PeerJ 2022, 10, e13364.
- Balasubramaniam, S.; Yaplito-Lee, J. Riboflavin metabolism: Role in mitochondrial function. J. Transl. Genet. Genom. 2020, 4, 285–306.
- Qin, Y.; Zhou, J.; Xiong, X.; Huang, J.; Li, J.; Wang, Q.; Yang, H.; Yin, Y. Effect of riboflavin on intestinal development and intestinal epithelial cell function of weaned piglets. J. Anim. Physiol. Anim. Nutr. 2022, 107, 518–528.
- Russell, A.P.; Schrauwen, P.; Somm, E.; Gastaldi, G.; Hesselink, M.K.C.; Schaart, G.; Kornips, E.; Lo, S.K.; Bufano, D.; Giacobino, J.-P.; et al. Decreased Fatty Acid β-Oxidation in Riboflavin-Responsive, Multiple Acylcoenzyme A Dehydrogenase-Deficient Patients Is Associated with an Increase in Uncoupling Protein-3. J. Clin. Endocrinol. Metab. 2003, 88, 5921–5926.
- Parsons, H.G.; Dias, V.C. Intramitochondrial fatty acid metabolism: Riboflavin deficiency and energy production. Biochem. Cell Biol. 1991, 69, 490–497.
- Ruiz, N.; Harms, R.H. Conversion of Tryptophan into Niacin in the Turkey (Meleagris gallipavos). Poult. Sci. 1990, 69, 446–450.
- FNB (Food and Nutrition Board, Institute of Medicine, National Academy of Sciences). Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998.
- Cordona, N.; Payne, I. Absorption of Riboflavin in Chickens. Poult. Sci. 1967, 46, 1176–1179.
- Ruiz, N.; Harms, R. Riboflavin Requirement of Broiler Chicks Fed a Corn-Soybean Diet. Poult. Sci. 1988, 67, 794–799.
- Ruiz, N.; Harms, R. Riboflavin Requirement of Turkey Poults Fed a Corn-Soybean Meal Diet from 1 to 21 Days of Age. Poult. Sci. 1989, 68, 715–718.
- Banaszkiewicz, T. Nutritional Value of Soybean Meal. In Soybean and Nutrition; El-Shemy, H., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 1–20. Available online: https://www.intechopen.com/books/soybean-and-nutrition/nutritional-value-of-soybeanmeal (accessed on 26 August 2023).
- Merrill, A.H.; Lambeth, J.D.; Edmondson, D.E.; McCormick, D.B. Formation and mode of action of flavoproteins. Annu. Rev. Nutr. 1981, 1, 281–317.
- Chung, T.K.; Baker, D.H. Riboflavin Requirement of Chicks Fed Purified Amino Acid and Conventional Corn-Soybean Meal Diets. Poult. Sci. 1990, 69, 1357–1363.
- Kleyn, R.; Chrystal, P. Vitamins. In Broiler Nutrition: Masterclass; Context Products Ltd.: Leicestershire, UK, 2020; pp. 129–142.
- Witten, S. Characterisation of Organic Cereals and Grain Legumes as Feedstuffs for Monogastric Animals: Effects of Variety and Environmental Conditions on the Contents of Crude Nutrients, Amino Acids, Thiamine, Riboflavin, and In Vitro Digestibility of Crude Protein and Amino Acids. Ph.D. Thesis, Georg-August-Universität Göttingen, Göttingen, Germany, 2018.
- Dove, R.; Cook, D.A. Water-Soluble Vitamins in Swine Nutrition. In Swine Nutrition; Lewis, A.J., Southern, L.L., Eds.; CRC Press: New York, NY, USA, 2000; pp. 315–356.
- Sheraz, M.A.; Kazi, S.H.; Ahmed, S.; Anwar, Z.; Ahmad, I. Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 2014, 10, 1999–2012.
- White, H.B., III; Merrill, A.H., Jr. Riboflavin-Binding Proteins. Annu. Rev. Nutr. 1988, 8, 279–299.
- M’Clelland, D.A. The Refolding of Riboflavin Binding Protein. Ph.D. Thesis, Department of Biological and Molecular Sciences, University of Stirling, Stirling, UK, 1996.
- EFSA (the European Food Safety Authority). Tolerable Upper Intake Levels for Vitamins and Minerals. Scientific Committee on Food, Scientific Panel on Dietetic Products, Nutrition and Allergies. 2006. Available online: https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf (accessed on 28 August 2023).
- Zheng, D.B.; Lim, H.M.; Pène, J.J.; White, H.B., 3rd. Chicken riboflavin-binding protein. cDNA sequence and homology with milk folate-binding protein. J. Biol. Chem. 1988, 263, 11126–11129.
- Monaco, H.L. Crystal structure of chicken riboflavin-binding protein. EMBO J. 1997, 16, 1475–1483.
- Miller, M.S.; Benore-Parsons, M.; White, H.B. Dephosphorylation of chicken riboflavin-binding protein and phosvitin decreases their uptake by oocytes. J. Biol. Chem. 1982, 257, 6818–6824.
- Mac Lachlan, I.; Nimpf, J.; Schneider, W. Avian riboflavin binding protein binds to lipoprotein receptors in association with vitellogenin. J. Biol. Chem. 1994, 269, 24127–24132.
- Loch, J.I.; Lipowska, J.; Lewinski, K. Crystal Structure of Chicken Riboflavin Binding Protein in “Apo” Form at 2.5 A Resolution; Protein Data Bank, Brookhaven National Laboratory: New York, NY, USA, 2018.
- Combs, G.F.; McClung, J.P. Riboflavin. In The Vitamins; Combs, G.F., McClung, J.P., Eds.; Academic Press: London, UK, 2017; pp. 110–159, 315–329.
- Norioka, N.; Okada, T.; Hamazume, Y.; Mega, T.; Ikenaka, T. Comparison of the Amino Acid Sequences of Hen Plasma-, Yolk-, and White-Riboflavin Binding Proteins. J. Biochem. 1985, 97, 19–28.
- Kirchhausen, T.; Owen, D.; Harrison, S.C. Molecular Structure, Function, and Dynamics of Clathrin-Mediated Membrane Traffic. Cold Spring Harb. Perspect. Biol. 2014, 6, a016725.
- Carlsson, E.V.; Sherman, H.C. Riboflavin and a Further Growth Essential in the Tissues: Quantitative Distribution and the Influence of the Food, Two Figures. J. Nutr. 1938, 15, 57–65.
- Hodson, A.Z. The Influence of Dietary Riboflavin on the Content of This Vitamin in Chicken Tissue. J. Nutr. 1940, 20, 377–382.
- Leonhardt, M.; Wenk, C. Animal species and muscle related differences in thiamine and riboflavin contents of Swiss meat. Food Chem. 1997, 59, 449–452.
- Norris, L.; Heuser, C.G.F.; Wilgus, H.S. Is the chief value of milk for feeding poultry due to the presence of a new vitamin? Poult. Sci. 1930, 9, 133–140.
- Lepkovsky, S.; Jukes, T.H. The Response of Rats, Chicks and Turkey Poults to Crystalline Vitamin G (Flavin). J. Nutr. 1936, 12, 515–526.
- Norris, L.; Wilgus, C.H.S.; Ringrose, A.T.; Heiman, V.; Heuser, G.F. The vitamin G requirements of poultry. Cornell Agr. Exp. Stn. Bull. 1936, 600, 1.
- Bethke, R.M.; Record, P.R.; Wilder, O.H.M. Further studies on vitamin G in chick nutrition with special reference to flavins. Poult. Sci. 1937, 16, 175–182.
- Heuser, G.; Wilgus, H.; Norris, L. The Quantitative Vitamin-G Requirement of Chicks. Poult. Sci. 1938, 17, 105–108.
- Bethke, R.; Record, P. The Relation of Riboflavin to Growth and Curled-toe Paralysis in Chicks. Poult. Sci. 1942, 21, 147–154.
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991.
- Biagi, E.; Mengucci, C.; Barone, M.; Picone, G.; Lucchi, A.; Celi, P.; Litta, G.; Candela, M.; Manfreda, G.; Brigidi, P.; et al. Effects of Vitamin B2 Supplementation in Broilers Microbiota and Metabolome. Microorganisms 2020, 8, 1134.
- Northrop-Clewes, C.A.; Thurnham, D.I. The Discovery and Characterization of Riboflavin. Ann. Nutr. Metab. 2012, 61, 224–230.
- Chou, S.T.; Sell, J.L.; Kondra, P.A. Interrelationships between riboflavin and dietary energy and protein utilisation in growing chicks. Br. J. Nutr. 1971, 26, 323–333.
- De Oliveira, J.E.; Uni, Z.; Ferket, P.R. Important metabolic pathways in poultry embryos prior to hatch. World’s Poult. Sci. J. 2008, 64, 488–499.
- Van Every, H.A.; Schmidt, C.J. Transcriptomic and metabolomic characterization of post-hatch metabolic reprogramming during hepatic development in the chicken. BMC Genom. 2021, 22, 380.
- Tu, B.P.; Ho-Schleyer, S.C.; Travers, K.J.; Weissman, J.S. Biochemical Basis of Oxidative Protein Folding in the Endoplasmic Reticulum. Science 2000, 290, 1571–1574.
- Zhang, B.; Cao, J.-T.; Wu, Y.-B.; Gao, K.-X.; Xie, M.; Zhou, Z.-K.; Tang, J.; Hou, S.-S. Riboflavin (Vitamin B2) Deficiency Induces Apoptosis Mediated by Endoplasmic Reticulum Stress and the CHOP Pathway in HepG2 Cells. Nutrients 2022, 14, 3356.
- Cai, Z.; Blumbergs, P.C.; Finnie, J.W.; Manavis, J.; Thompson, P.D. Selective vulnerability of peripheral nerves in avian riboflavin deficiency demyelinating polyneuropathy. Vet. Pathol. 2009, 46, 88–96.
- Shastak, Y.; Gordillo, A.; Pelletier, W. The relationship between vitamin A status and oxidative stress in animal production. J. Appl. Anim. Res. 2023, 51, 546–553.
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902.
- Mishra, B.; Jha, R. Oxidative Stress in the Poultry Gut: Potential Challenges and Interventions. Front. Vet. Sci. 2019, 6, 60.
- King’ori, A.M. Review of the factors that influence egg fertility and hatchability in poultry. Int. J. Poult. Sci. 2011, 10, 483–484.
- Naber, E.C.; Squires, M.W. Research Note: Early Detection of the Absence of a Vitamin Premix in Layer Diets by Egg Albumen Riboflavin Analysis. Poult. Sci. 1993, 72, 1989–1993.
- Lepkovsky, S.; Taylor, L.W.; Jukes, T.H.; Almquist, H.J. The effect of riboflavin and the filtrate factor on egg production and hatchability. Hilgardia 1938, 11, 559–591.
- Squires, M.W.; Naber, E.C. Vitamin Profiles of Eggs as Indicators of Nutritional Status in the Laying Hen: Riboflavin Study. Poult. Sci. 1993, 72, 483–494.
- Davis, H.J.; Norris, L.C.; Heuser, G.F. The Rôle of Vitamin G in Reproduction in Poultry. Poult. Sci. 1938, 17, 81–86.
- Schumacher, A.; Heuser, G. The Importance of Riboflavin in Reproduction in Poultry. Poult. Sci. 1939, 18, 369–374.
- Abrams, V.A.; Han, C.-C.; White, H.B. Riboflavin-deficient chicken embryos: Hypoglycemia without dicarboxylic aciduria. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 111, 233–241.
- Engel, R.; Phillips, P.; Halpin, J.G. The Effect of a Riboflavin Deficiency in the Hen upon Embryonic Development of the Chick. Poult. Sci. 1940, 19, 135–142.
- Tuite, P.; Austic, R. Studies on a Possible Interaction between Riboflavin and Vitamin B12 as it Affects Hatchability of the Hen’s Egg. Poult. Sci. 1974, 53, 2125–2136.
- Juriloff, D.; Roberts, C. Genetics of Cleft Palate in Chickens and the Relationship Between the Occurrence of the Trait and Maternal Riboflavin Deficiency. Poult. Sci. 1975, 54, 334–346.
- Anisha, M.; Karnani, S. Choudhary and Manju. Nutritional Factors: Affecting Egg Quality and Hatchability in Poultry. Pashu Sandesh. Available online: https://pashusandesh.com/Nutritional-Factors-Affecting-Egg-Quality- (accessed on 28 August 2023).
- Wilson, H.R. Effects of maternal nutrition on hatchability. Poult. Sci. 1997, 76, 134–143.
- Folmes, C.D.L.; Terzic, A. Metabolic determinants of embryonic development and stem cell fate. Reprod. Fertil. Dev. 2015, 27, 82–88.
- Givisiez, P.E.N.; Moreira Filho, A.L.B.; Santos, M.R.B.; Oliveira, H.B.; Ferket, P.R.; Oliveira, C.J.B.; Malheiros, R.D. Chicken embryo development: Metabolic and morphological basis for in ovo feeding technology. Poult. Sci. 2020, 99, 6774–6782.
- Scanes, C.G.; Christensen, K.D. Fundamentals in Poultry Nutrition. In Poultry Science; Waveland Press, Inc.: Long Grove, IL, USA, 2020; pp. 109–131.
- Zhang, B.; Tang, J.; Wu, Y.; Cao, J.; Xing, G.; Sun, P.; Huang, W.; Xie, M.; Hou, S. Effects of riboflavin deficiency on the lipid metabolism of duck breeders and duck embryos. Poult. Sci. 2021, 100, 101342.
- Ribeiro, M.; Bittencourt, L.; Hermes, R.; Rönnau, M.; Rorig, A.; Lima, F.; Fernandes, J. Mineral Source and Vitamin Level in Broiler Diets: Effects on Performance, Yield, and Meat Quality. Braz. J. Poult. Sci. 2020, 22, 1–14.
- NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994.
- GfE (Gesellschaft für Ernährungsphysiologie). Empfehlungen zur Energie- und Nährstoffversorgung von Legehennen und Masthühnern (Broiler); Gesellschaft für Ernährungsphysiologie, DLG-Verlag: Frankfurt, Germany, 1999.
- Cobb 500 Broiler Performance & Nutrition Supplement. 2022. Available online: https://www.cobb-vantress.com/assets/Cobb-Files/product-guides/5502e86566/2022-Cobb500-Broiler-Performance-Nutrition-Supplement.pdf (accessed on 28 July 2023).
- ROSS Broiler: Nutrition Specifications. 2022. Available online: https://aviagen.com/eu/brands/ross/products/ross-308 (accessed on 28 July 2023).
- Nicholas and, B.U.T. Heavy Lines Feeding Guidelines. 2015. Available online: https://www.aviagenturkeys.com/uploads/2015/11/20/NU06%20Feeding%20Guidelines%20for%20Nicholas%20&%20BUT%20Heavy%20Lines%20EN.pdf (accessed on 28 July 2023).
- Lohmann LSL-Lite Management Guide. 2014. Available online: https://lohmann-breeders.com/media/strains/cage/management/LOHMANN-LSL-Lite-Cage-1.pdf (accessed on 25 August 2016).
- Shastak, Y.; Pelletier, W. Delving into vitamin A supplementation in poultry nutrition: Current knowledge, functional effects, and practical implications. World’s Poult. Sci. J. 2023, 1–23.
- Leeson, S.; Summers, J.D. Commercial Poultry Nutrition. In Scott’s Nutrition of the Chicken, 3rd ed.; Leeson, S., Ed.; Nottingham University Press: Nottingham, UK, 2001; p. 398.
- Motyl, K.J.; Guntur, A.R.; Carvalho, A.L.; Rosen, C.J. Energy Metabolism of Bone. Toxicol. Pathol. 2017, 45, 887–893.
- Summers, J.D.; Shen, H.; Leeson, S.; Julian, R.J. Influence of Vitamin Deficiency and Level of Dietary Protein on the Incidence of Leg Problems in Broiler Chicks. Poult. Sci. 1984, 63, 1115–1121.
- Costello, L.C.; Franklin, R.B.; Reynolds, M.A.; Chellaiah, M. The Important Role of Osteoblasts and Citrate Production in Bone Formation: “Osteoblast Citration” as a New Concept for an Old Relationship. Open Bone J. 2012, 4, 27–34.




