
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adalberto Benavides-Mendoza | -- | 3057 | 2023-11-18 23:46:08 | | | |
| 2 | Peter Tang | Meta information modification | 3057 | 2023-11-20 02:49:01 | | |
Video Upload Options
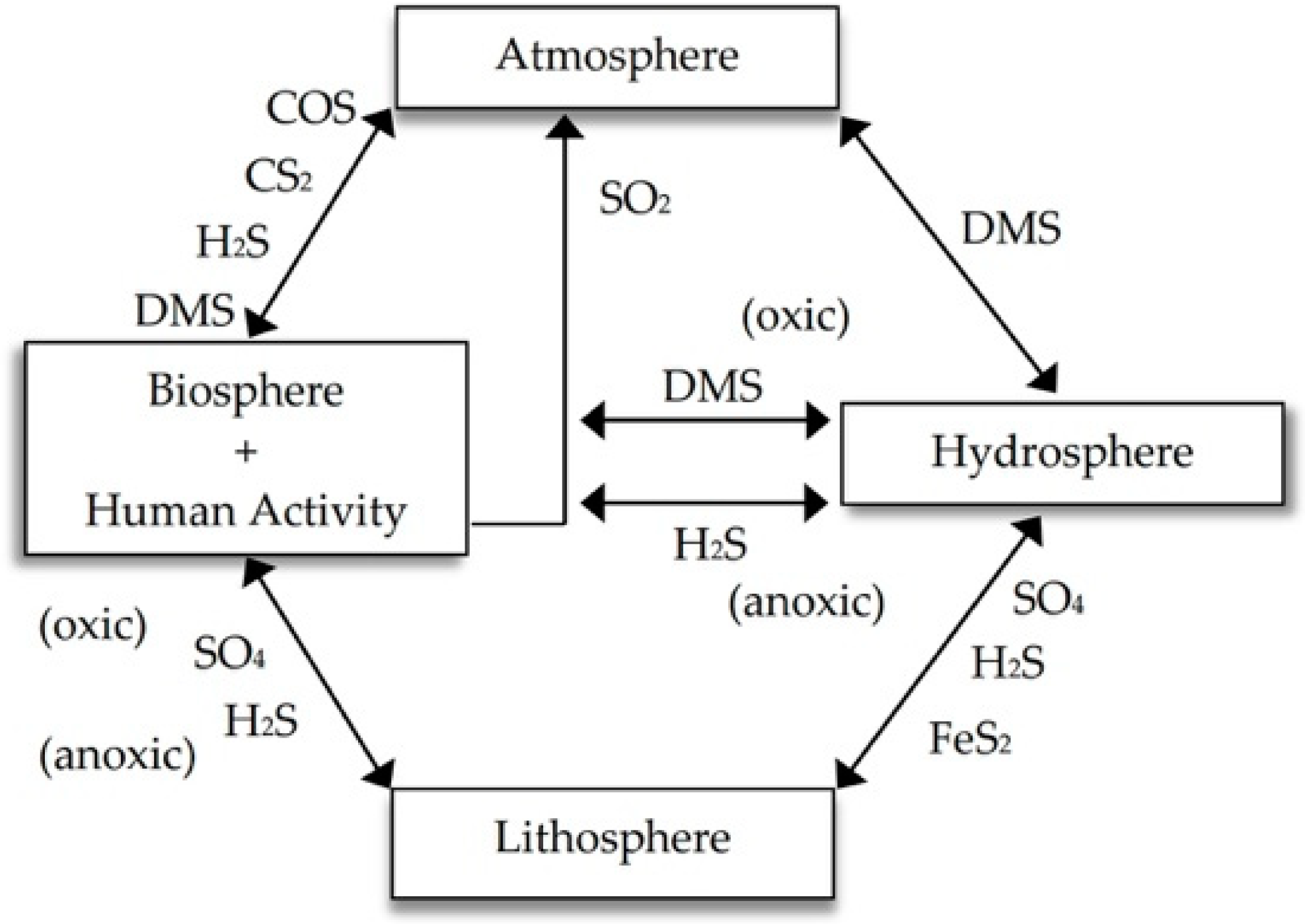
Sulfur is an essential element in determining the productivity and quality of agricultural products. It is also an element associated with tolerance to biotic and abiotic stress in plants. In agricultural practice, sulfur has broad use in the form of sulfate fertilizers and, to a lesser extent, as sulfite biostimulants.
1. Introduction
|
Oxidation State |
Representative Compound and Formula |
Oxidation State |
Representative Compound and Formula |
|---|---|---|---|
|
+6 |
Sulfate, SO42− |
0 |
S0, elemental sulfur. Sulfoxide (R-S(-O)-R such as dimethyl sulfoxide (DMSO). Oxidized derivatives of sulfide and sulfenic acid (RSOH). |
|
+6 and −2 |
Thiosulfate, S2O32− |
−1 |
Disulfide (R-S-S-R) is a persulfide found in the linkages between two cysteine residues in proteins. RSSH denotes persulfides (or hydrosulfides) obtained by the action of H2S on cysteine residues (R-SH). Thioethers and thiols can be oxidized to disulfides. Major products of decomposition of persulfides are polysulfanes. Thiyl-radical RS*. |
|
+5 and −2 |
Polythionates (−O3S-Sn-SO3−): Dithionate, S2O62−; Trithionate, S3O62−; Tetrathionate, S4O62− |
−2 |
Sulfide, S2−, polysulfides, S22−, S32−, S52−; carbon disulfide (CS2); FeS2; NaHS and Na2S are sources of S2− and of its conjugated acids SH− and H2S. Polysulfides (with Sn > 2) contain S0 atoms, which allows a diversity of oxidation states. |
|
+4 |
Sulfur dioxide, SO2; Sulfite, SO32−; Disulfite, S2O52−; Sulfone, OS(S) the oxidation product of sulfoxides |
−2 |
Hydrogen sulfide (H2S), disulfane (H2S2), and polysulfanes (RSSnSR, n > 2). Polysulfanes contain S0 atoms, which allows a diversity of oxidation states. |
|
+3 |
Dithionite, S2O42− |
−2 |
Thioethers (C-S-C) such as dimethyl sulfide (DMS), CH3-S-CH3 and dimethyl disulfide (DMDS), CH3-S-S-CH3. |
|
+2 |
Carbonyl sulfide (COS), OCS |
−2 |
Thiols (R-SH) such as glutathione (GSH) and methyl mercaptan, CH3-SH. Thiols are derived from the sulfhydryl group -SH of cysteine, which enables multiple oxidation states (−2 to +6). Thiolates are derivatives of thiols in which a metal or other cation replaces H. |
|
0 |
Elementary sulfur (S0), mainly S8 (cycloocta-S) |
−2 |
Carbon disulfide, CS2. |
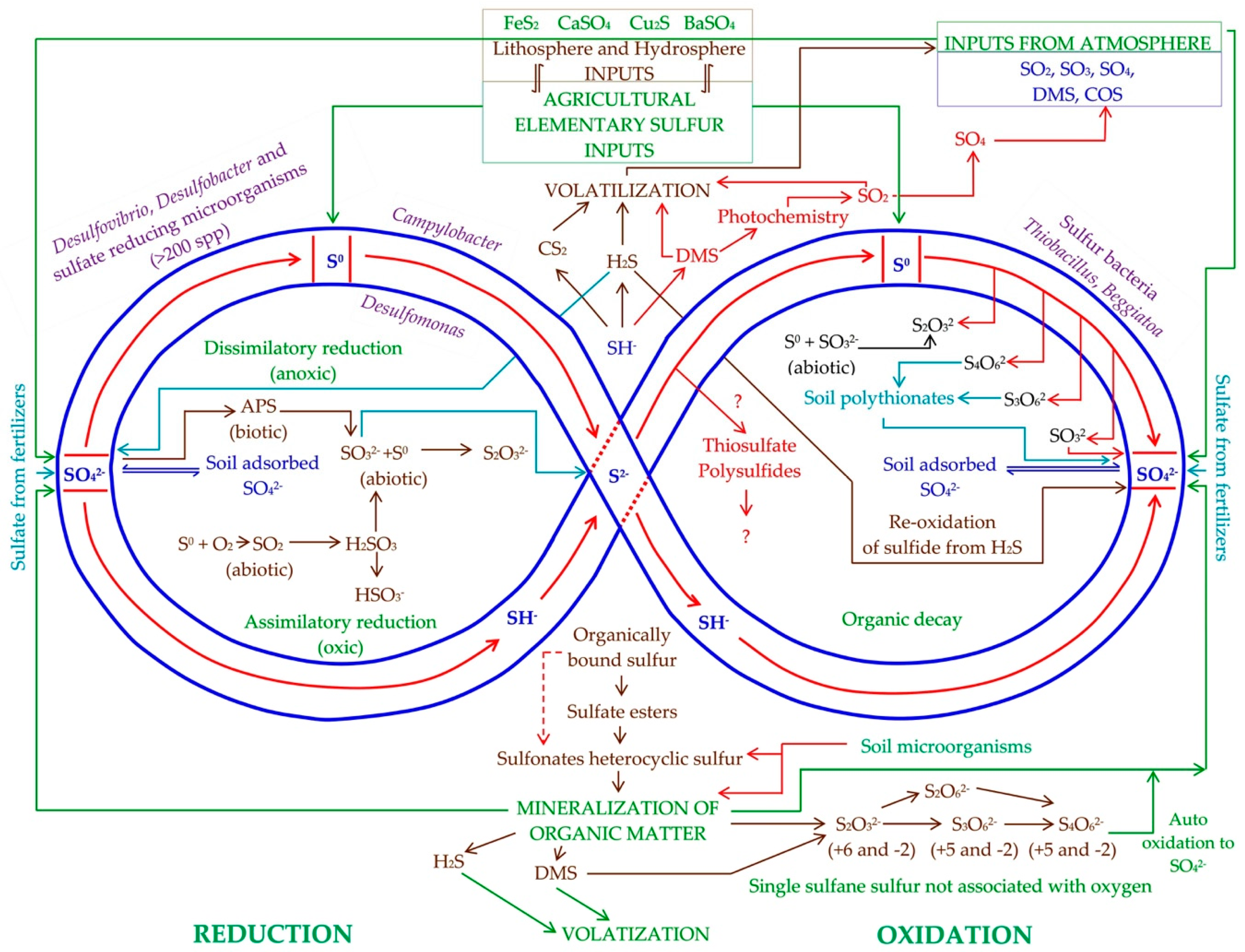
2. Transformations of Elemental Sulfur in Soil
References
- De Kok, L.J.; Castro, A.; Durenkamp, M.; Koralewska, A.; Posthumus, F.S.; Stuiver, C.E.E.; Yang, L.; Stulen, I. Pathways of plant sulfur uptake and metabolism—An overview. In Proceedings of the 1st Sino-German Workshop on Aspects of Sulfur Nutrition of Plants, Shenyang, China, 23–27 May 2004; Schnug, E., de Kok, L.J., Eds.; FAL Agricultural Research: Braunschweig, Germany, 2005; pp. 5–13, ISBN 3-86576-007-4.
- Huxtable, R.J. Biochemistry of Sulfur; Springer: Boston, MA, USA, 1986; ISBN 978-1-4757-9438-0.
- Rendig, V.V.; Oputa, C.; McComb, E.A. Effects of sulfur deficiency on non-protein nitrogen, soluble sugars, and N/S ratios in young corn (Zea mays L.) plants. Plant Soil 1976, 44, 423–437.
- Reuveny, Z.; Dougall, D.K.; Trinity, P.M. Regulatory coupling of nitrate and sulfate assimilation pathways in cultured tobacco cells. Proc. Natl. Acad. Sci. USA 1980, 77, 6670–6672.
- Rennenberg, H. The fate of excess sulfur in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 121–153.
- Andreae, M.O. Ocean-atmosphere interactions in the global biogeochemical sulfur cycle. Mar. Chem. 1990, 30, 1–29.
- Tolocka, M.P.; Turpin, B. Contribution of organosulfur compounds to organic aerosol mass. Environ. Sci. Technol. 2012, 46, 7978–7983.
- Mikkelsen, R.; Norton, R. Soil and fertilizer sulfur. Better Crop. 2013, 97, 7–9.
- Chao, T.T.; Harward, M.E.; Fang, S.C. Cationic effects on sulfate adsorption by soils. Soil Sci. Soc. Am. J. 1963, 27, 35–38.
- Chao, T.T.; Harward, M.E.; Fang, S.C. Adsorption and desorption phenomena of sulfate ions in soils. Soil Sci. Soc. Am. J. 1962, 26, 234–237.
- Przygocka-Cyna, K.; Biber, M.; Przygocka-Cyna, K.; Grzebisz, W.; Pluta, M.; Grzebisz, W. Mineral density of onion bulbs as affected by fertilizers based on elemental sulfur. J. Elem. 2016, 21, 485–499.
- González-Morales, S.; Pérez-Labrada, F.; García-Enciso, E.L.; Leija-Martínez, P.; Medrano-Macías, J.; Dávila-Rangel, I.E.; Juárez-Maldonado, A.; Rivas-Martínez, E.N.; Benavides-Mendoza, A. Selenium and sulfur to produce Allium functional crops. Molecules 2017, 22, 558.
- Tea, I.; Genter, T.; Naulet, N.; Boyer, V.; Lummerzheim, M.; Kleiber, D. Effect of foliar sulfur and nitrogen fertilization on wheat storage protein composition and dough mixing properties. Cereal Chem. J. 2004, 81, 759–766.
- TSI Sulphur Fertilizer Types. Available online: https://www.sulphurinstitute.org/fertilizer/sulphate.cfm (accessed on 5 May 2019).
- Johnson, D.W.; Cole, D.W. Anion mobility in soils: Relevance to nutrient transport from forest ecosystems. Environ. Int. 1980, 3, 79–90.
- Hutt, L.P. Taxonomy, Physiology and Biochemistry of the Sulfur Bacteria; Plymouth University: Plymouth, UK, 2017.
- Montesinos-Pereira, D.; de la Torre-González, A.; Blasco, B.; Ruiz, J.M. Hydrogen sulphide increase the tolerance to alkalinity stress in cabbage plants (Brassica oleracea L. ’Bronco’). Sci. Hortic. (Amsterdam) 2018, 235, 349–356.
- Steiger, A.K.; Zhao, Y.; Pluth, M.D. Emerging roles of carbonyl sulfide in chemical biology: Sulfide transporter or gasotransmitter? Antioxid. Redox Signal. 2018, 28, 1516–1532.
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146.
- Wong, M.W. Quantum-chemical calculations of sulfur-rich compounds. Top. Curr. Chem. 2003, 231, 1–29.
- Mayer, R. Elemental sulfur and its reactions. In Organic Chemistry of Sulfur; Oae, S., Ed.; Plenum Press: New York, NY, USA, 1977; p. 681. ISBN 978-1-4684-2049-4.
- Reusch, W. Nucleophilicity of Sulfur Compounds. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Thiols_and_Sulfides/Nucleophilicity_of_Sulfur_Compounds (accessed on 5 May 2019).
- Chapman, S.J. Powdered elemental sulphur: Oxidation rate, temperature dependence and modelling. Nutr. Cycl. Agroecosyst. 1996, 47, 19–28.
- Lucheta, A.R.; Lambais, M.R. Sulfur in agriculture. Rev. Bras. Ciência do Solo 2012, 36, 1369–1379.
- Wörner, S.; Zecchin, S.; Dan, J.; Todorova, N.H.; Loy, A.; Conrad, R.; Pester, M. Gypsum amendment to rice paddy soil stimulated bacteria involved in sulfur cycling but largely preserved the phylogenetic composition of the total bacterial community. Environ. Microbiol. Rep. 2016, 8, 413–423.
- DeLuca, T.H.; Skogley, E.O.; Engel, R.E. Band-applied elemental sulfur to enhance the phytoavailability of phosphorus in alkaline calcareous soils. Biol. Fertil. Soils 1989, 7, 346–350.
- Degryse, F.; Ajiboye, B.; Baird, R.; da Silva, R.C.; McLaughlin, M.J. Oxidation of elemental sulfur in granular fertilizers depends on the soil-exposed surface area. Soil Sci. Soc. Am. J. 2016, 80, 294.
- Zhao, C.; Degryse, F.; Gupta, V.; McLaughlin, M.J. Low effective surface area explains slow oxidation of co-granulated elemental sulfur. Soil Sci. Soc. Am. J. 2016, 80, 911–918.
- Lee, A.; Boswell, C.C.; Watkinson, J.H. Effect of particle size on the oxidation of elemental sulphur, thiobacilli numbers, soil sulphate, and its availability to pasture. N. Z. J. Agric. Res. 1988, 31, 179–186.
- Haneklaus, S.; Bloem, E.; Schnug, E. Sulfur interactions in crop ecosystems. In Sulfur in Plants An Ecological Perspective; Hawkesford, M.J., De Kok, L.J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 16–58. ISBN 978-1-4020-5887-5.
- Gupta, V.V.S.R.; Lawrence, J.R.; Germida, J.J. Impact of elemental sulfur fertilization on agricultural soils. I. Effects on microbial biomass and enzyme activities. Can. J. Soil Sci. 1988, 68, 463–473.
- Cooper, R.M.; Williams, J.S. Elemental sulphur as an induced antifungal substance in plant defence. J. Exp. Bot. 2004, 55, 1947–1953.
- Bloem, E.; Haneklaus, S.; Schnug, E. Milestones in plant sulfur research on sulfur-induced-resistance (SIR) in Europe. Front. Plant Sci. 2015, 5, 779.
- Terán, G.E.; Benavides, A.; Hernández, F.; Quero, E. New Technologies for Horticultural Crops. In Plant Production on the Threshold of a New Century; Struik, P.C., Vredenberg, W.J., Renkema, J.A., Parlevliet, J.E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 375–380. ISBN 0792329031.
- Almutairi, K.F.; Machado, R.M.A.; Bryla, D.R.; Strik, B.C. Chemigation with micronized sulfur rapidly reduces soil pH in a new planting of northern highbush blueberry. HortScience 2017, 52, 1413–1418.
- Roy Choudhury, S.; Ghosh, M.; Mandal, A.; Chakravorty, D.; Pal, M.; Pradhan, S.; Goswami, A. Surface-modified sulfur nanoparticles: An effective antifungal agent against Aspergillus niger and Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2011, 90, 733–743.
- Rao, K.J.; Paria, S. Use of sulfur nanoparticles as a green pesticide on Fusarium solani and Venturia inaequalis phytopathogens. RSC Adv. 2013, 3, 10471.
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162.
- Williams, J.S.; Cooper, R.M. Elemental sulphur is produced by diverse plant families as a component of defence against fungal and bacterial pathogens. Physiol. Mol. Plant Pathol. 2003, 63, 3–16.
- Wainwright, M. Sulfur oxidation in soils. Adv. Agron. 1984, 37, 349–396.
- Zhao, C.; Gupta, V.V.S.R.; Degryse, F.; McLaughlin, M.J. Effects of pH and ionic strength on elemental sulphur oxidation in soil. Biol. Fertil. Soils 2017, 53, 247–256.
- Kumar, U.; Panneerselvam, P.; Gupta, V.V.S.R.; Manjunath, M.; Priyadarshinee, P.; Sahoo, A.; Dash, S.R.; Kaviraj, M.; Annapurna, K. Diversity of sulfur-oxidizing and sulfur-reducing microbes in diverse ecosystems. In Advances in Soil Microbiology: Recent Trends and Future Prospects. Microorganisms for Sustainability; Adhya, T., Lal, B., Mohapatra, B., Paul, D., Das, S., Eds.; Springer: Singapore, 2018; Volume 3, pp. 65–89. ISBN 978-981-10-6178-3.
- Rennenberg, H. Synthesis and emission of hydrogen sulfide by higher plants. In Biogenic Sulfur in the Environment; Saltzman, E.S., Cooper, W.J., Eds.; American Chemical Society: Washington, DC, USA, 1989; pp. 44–57.




