
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Faranak Mankavi | -- | 4863 | 2023-11-16 16:13:03 | | | |
| 2 | Lindsay Dong | Meta information modification | 4863 | 2023-11-20 02:56:34 | | |
Video Upload Options
Injuries to the peripheral nervous system are a common clinical issue, causing dysfunctions of the motor and sensory systems. Surgical interventions such as nerve autografting are necessary to repair damaged nerves. Even with autografting, i.e., the gold standard, malfunctioning and mismatches between the injured and donor nerves often lead to unwanted failure. Thus, there is an urgent need for a new intervention in clinical practice to achieve full functional recovery. Nerve guidance conduits (NGCs), providing physicochemical cues to guide neural regeneration, have great potential for the clinical regeneration of peripheral nerves. Typically, NGCs are tubular structures with various configurations to create a microenvironment that induces the oriented and accelerated growth of axons and promotes neuron cell migration and tissue maturation within the injured tissue. Once the native neural environment is better understood, ideal NGCs should maximally recapitulate those key physiological attributes for better neural regeneration.
1. Introduction
2. Physicochemical Properties and Regeneration Capacity of Peripheral Nerves
2.1. Structural, Compositional, and Physical Properties of Peripheral Nerves
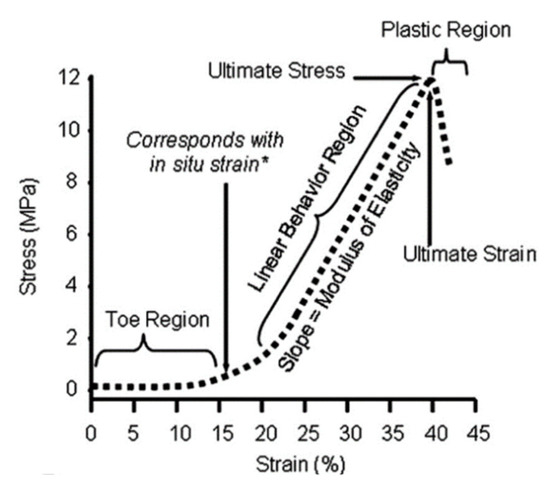
2.2. Regeneration of Peripheral Nerve Injuries
3. Design Considerations for Biomimetic Nerve Guidance Conduits
3.1. Compositional Considerations
3.1.1. Material Choice
Biomimetic Synthetic Materials
Biomimetic Natural Materials
Biomimetic Hybrid Materials
3.1.2. Cellular and Biomolecular Choices
Cellular Choices
Biomolecular Choices
3.2. Structural Considerations
3.2.1. Biomimetic Architectures
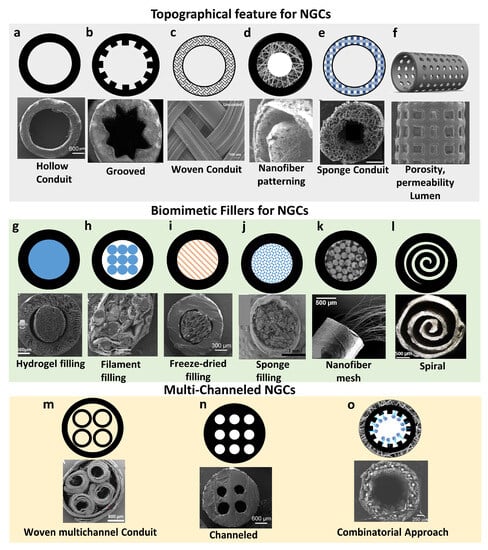
Biomimetic Topographical Features of NGCs
Biomimetic Fillers for NGCs
Multi-Channeled NGCs
3.2.2. Fabrication of Biomimetic Features
3.2.3. Mechanical Properties of Biomimetic NGCs
4. Conclusions
References
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918.
- Yan, Y.; Yao, R.; Zhao, J.; Chen, K.; Duan, L.; Wang, T.; Zhang, S.; Guan, J.; Zheng, Z.; Wang, X.; et al. Implantable nerve guidance conduits: Material combinations, multi-functional strategies and advanced engineering innovations. Bioact. Mater. 2022, 11, 57–76.
- Bhandari, P.S. Management of peripheral nerve injury. J. Clin. Orthop. Trauma 2019, 10, 862–866.
- Zhang, F.; Fischer, K.A. End-to-side neurorrhaphy. Microsurgery 2002, 22, 122–127.
- Yan, Z.; Qian, Y.; Fan, C. Biomimicry in 3D printing design: Implications for peripheral nerve regeneration. Regen. Med. 2021, 16, 683–701.
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.L.G.; et al. Current status of therapeutic approaches against peripheral nerve injuries: A detailed story from injury to recovery. Int. J. Biol. Sci. 2020, 16, 116–134.
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82, 160–167.
- Behtaj, S.; Ekberg, J.A.K.; St John, J.A. Advances in Electrospun Nerve Guidance Conduits for Engineering Neural Regeneration. Pharmaceutics 2022, 14, 219.
- Joung, D.; Lavoie, N.S.; Guo, S.Z.; Park, S.H.; Parr, A.M.; McAlpine, M.C. 3D Printed Neural Regeneration Devices. Adv. Funct. Mater. 2020, 30, 1906237.
- Wofford, K.L.; Shultz, R.B.; Burrell, J.C.; Cullen, D.K. Neuroimmune interactions and immunoengineering strategies in peripheral nerve repair. Prog. Neurobiol. 2022, 208, 102172.
- Rodríguez-Sánchez, D.N.; Pinto, G.B.A.; Cartarozzi, L.P.; de Oliveira, A.L.R.; Bovolato, A.L.C.; de Carvalho, M.; da Silva, J.V.L.; de Dernowsek, J.A.; Golim, M.; Barraviera, B.; et al. 3D-printed nerve guidance conduits multi-functionalized with canine multipotent mesenchymal stromal cells promote neuroregeneration after sciatic nerve injury in rats. Stem Cell Res. Ther. 2021, 12, 303.
- Sabongi, R.G.; Fernandes, M.; Dos Gomes Santos, J.B. Peripheral nerve regeneration with conduits: Use of vein tubes. Neural Regen. Res. 2015, 10, 529–533.
- Zargar Kharazi, A.; Dini, G.; Naser, R. Fabrication and evaluation of a nerve guidance conduit capable of Ca2+ ion release to accelerate axon extension in peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2018, 106, 2181–2189.
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, 323.
- Kang, N.U.; Lee, S.J.; Gwak, S.J. Fabrication Techniques of Nerve Guidance Conduits for Nerve Regeneration. Yonsei Med. J. 2022, 63, 114–123.
- Liu, Y.; Zhang, X.; Xiao, C.; Liu, B. Engineered hydrogels for peripheral nerve repair. Mater. Today Bio 2023, 20, 100668.
- Nadeau, S.; Filali, M.; Zhang, J.; Kerr, B.J.; Rivest, S.; Soulet, D.; Iwakura, Y.; de Vaccari, J.P.R.; Keane, R.W.; Lacroix, S. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: Implications for neuropathic pain. J. Neurosci. 2011, 31, 12533–12542.
- Topp, K.S.; Boyd, B.S. Structure and biomechanics of peripheral nerves: Nerve responses to physical stresses and implications for physical therapist practice. Phys. Ther. 2006, 86, 92–109.
- Dalton, P.D.; O’Neill, K.L.; Pêgo, A.P.; Plant, G.W.; Nisbet, D.R.; Oudega, M.; Brook, G.A.; Harvey, A.R. Tissue engineering of the nervous system. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 585–627.
- Dubový, P.; Jančálek, R.; Kubek, T. Role of Inflammation and Cytokines in Peripheral Nerve Regeneration. Int. Rev. Neurobiol. 2013, 108, 173–206.
- Martini, F.H.; Tallitsch, R.B.; Judi, L.; Nath, P.D. Human Anatomy; Pearson: London, UK, 2016; ISBN 9780134320762.
- Estrada, J.B.; Cramer, H.C.; Scimone, M.T.; Buyukozturk, S.; Franck, C. Neural cell injury pathology due to high-rate mechanical loading. Brain Multiphysics 2021, 2, 100034.
- Kwan, M.K.; Wall, E.J.; Massie, J.; Garfin, S.R. Strain, stress and stretch of peripheral nerve rabbit experiments in vitro and in vivo. Acta Orthop. 1992, 63, 267–272.
- Gaudin, R.; Knipfer, C.; Henningsen, A.; Smeets, R.; Heiland, M.; Hadlock, T. Approaches to peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed Res. Int. 2016, 2016, 3856262.
- Kaplan, B.; Levenberg, S. The Role of Biomaterials in Peripheral Nerve and Spinal Cord Injury: A Review. Int. J. Mol. Sci. 2022, 23, 1244.
- Ma, Y.; Gao, H.; Wang, H.; Cao, X. Engineering topography: Effects on nerve cell behaviors and applications in peripheral nerve repair. J. Mater. Chem. B 2021, 9, 6310–6325.
- Du, J.; Liu, J.; Yao, S.; Mao, H.; Peng, J.; Sun, X.; Cao, Z.; Yang, Y.; Xiao, B.; Wang, Y.; et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017, 55, 296–309.
- Ciardelli, G.; Chiono, V. Materials for peripheral nerve regeneration. Macromol. Biosci. 2006, 6, 13–26.
- Gan, L.; Zhao, L.; Zhao, Y.; Li, K.; Tong, Z.; Yi, L.; Wang, X.; Li, Y.; Tian, W.; He, X.; et al. Cellulose/soy protein composite-based nerve guidance conduits with designed microstructure for peripheral nerve regeneration. J. Neural Eng. 2016, 13, 056019.
- Xie, H.; Yang, W.; Chen, J.; Zhang, J.; Lu, X.; Zhao, X.; Huang, K.; Li, H.; Chang, P.; Wang, Z.; et al. A Silk Sericin/Silicone Nerve Guidance Conduit Promotes Regeneration of a Transected Sciatic Nerve. Adv. Healthc. Mater. 2015, 4, 2195–2205.
- Parker, B.J.; Rhodes, D.I.; O’Brien, C.M.; Rodda, A.E.; Cameron, N.R. Nerve guidance conduit development for primary treatment of peripheral nerve transection injuries: A commercial perspective. Acta Biomater. 2021, 135, 64–86.
- Suhar, R.A.; Marquardt, L.M.; Song, S.; Buabbas, H.; Doulames, V.M.; Johansson, P.K.; Klett, K.C.; Dewi, R.E.; Enejder, A.M.K.; Plant, G.W.; et al. Elastin-like Proteins to Support Peripheral Nerve Regeneration in Guidance Conduits. ACS Biomater. Sci. Eng. 2021, 7, 4209–4220.
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Kaplan, D.L.; Kim, H.W.; Kundu, S.C. Prospects of peripheral nerve tissue engineering using nerve guide conduits based on silk fibroin protein and other biopolymers. Int. Mater. Rev. 2017, 62, 367–391.
- Hu, Y.; Chen, Z.; Wang, H.; Guo, J.; Cai, J.; Chen, X.; Wei, H.; Qi, J.; Wang, Q.; Liu, H.; et al. Conductive Nerve Guidance Conduits Based on Morpho Butterfly Wings for Peripheral Nerve Repair. ACS Nano 2022, 16, 1868–1879.
- Farzan, A.; Borandeh, S.; Seppälä, J. Conductive polyurethane/PEGylated graphene oxide composite for 3D-printed nerve guidance conduits. Eur. Polym. J. 2022, 167, 111068.
- Fabbro, A.; Prato, M.; Ballerini, L. Carbon nanotubes in neuroregeneration and repair. Adv. Drug Deliv. Rev. 2013, 65, 2034–2044.
- Pan, X.; Sun, B.; Mo, X. Electrospun polypyrrole-coated polycaprolactone nanoyarn nerve guidance conduits for nerve tissue engineering. Front. Mater. Sci. 2018, 12, 438–446.
- Xu, B.; Bai, T.; Sinclair, A.; Wang, W.; Wu, Q.; Gao, F.; Jia, H.; Jiang, S.; Liu, W. Directed neural stem cell differentiation on polyaniline-coated high strength hydrogels. Mater. Today Chem. 2016, 1–2, 15–22.
- Wang, G.; Wu, W.; Yang, H.; Zhang, P.; Wang, J.Y. Intact polyaniline coating as a conductive guidance is beneficial to repairing sciatic nerve injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 128–142.
- Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N.I.; et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 2017, 3, e1602076.
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, 1806133.
- Choi, J.-S.; Kim, H.; An, H.-Y.; Shim, B.S.; Lim, J.-Y. Regeneration of Recurrent Laryngeal Nerve using Polycaprolactone (PCL) Nerve Guide Conduit Coated with Conductive Materials. J. Korean Thyroid Assoc. 2015, 8, 88.
- Du, J.; Chen, H.; Qing, L.; Yang, X.; Jia, X. Biomimetic neural scaffolds: A crucial step towards optimal peripheral nerve regeneration. Biomater. Sci. 2018, 6, 1299–1311.
- Hibbitts, A.J.; Kočí, Z.; Kneafsey, S.; Matsiko, A.; Žilić, L.; Dervan, A.; Hinton, P.; Chen, G.; Cavanagh, B.; Dowling, J.K.; et al. Multi-factorial nerve guidance conduit engineering improves outcomes in inflammation, angiogenesis and large defect nerve repair. Matrix Biol. 2022, 106, 34–57.
- Yang, Y.; Ding, F.; Wu, J.; Hu, W.; Liu, W.; Liu, J.; Gu, X. Development and evaluation of silk fibroin-based nerve grafts used for peripheral nerve regeneration. Biomaterials 2007, 28, 5526–5535.
- Nazeer, M.A.; Yilgor, E.; Yilgor, I. Electrospun polycaprolactone/silk fibroin nanofibrous bioactive scaffolds for tissue engineering applications. Polymer 2019, 168, 86–94.
- Cunha, C.; Panseri, S.; Antonini, S. Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 50–59.
- Furlani, F.; Montanari, M.; Sangiorgi, N.; Saracino, E.; Campodoni, E.; Sanson, A.; Benfenati, V.; Tampieri, A.; Panseri, S.; Sandri, M. Electroconductive and injectable hydrogels based on gelatin and PEDOT:PSS for a minimally invasive approach in nervous tissue regeneration. Biomater. Sci. 2022, 10, 2040–2053.
- Vallejo, F.A.; Diaz, A.; Errante, E.L.; Smartz, T.; Khan, A.; Silvera, R.; Brooks, A.E.; Lee, Y.S.; Burks, S.S.; Levi, A.D. Systematic review of the therapeutic use of Schwann cells in the repair of peripheral nerve injuries: Advancements from animal studies to clinical trials. Front. Cell. Neurosci. 2022, 16, 929593.
- Zhou, G.; Chen, Y.; Dai, F.; Yu, X. Chitosan-based nerve guidance conduit with microchannels and nanofibers promotes schwann cells migration and neurite growth. Colloids Surf. B Biointerfaces 2023, 221, 112929.
- Liu, S.; Xie, Y.Y.; Di Wang, L.; Tai, C.X.; Chen, D.; Mu, D.; Cui, Y.Y.; Wang, B. A multi-channel collagen scaffold loaded with neural stem cells for the repair of spinal cord injury. Neural Regen. Res. 2021, 16, 2284–2292.
- Ye, J.; Chen, Y.; Yang, H.; Chen, Q.; Huang, Y.; Zhao, J. Human IL12p80 and Neural Stem Cells Enhance Sciatic Nerve Regeneration. Lancent Pschch 2020, 23, 7002.
- Shalaby, S.M.; El-Shal, A.S.; Ahmed, F.E.; Shaban, S.F.; Wahdan, R.A.; Kandel, W.A.; Senger, M.S. Combined Wharton’s jelly derived mesenchymal stem cells and nerve guidance conduit: A potential promising therapy for peripheral nerve injuries. Int. J. Biochem. Cell Biol. 2017, 86, 67–76.
- Lu, Q.; Zhang, F.; Cheng, W.; Gao, X.; Ding, Z.; Zhang, X.; Lu, Q.; Kaplan, D.L. Nerve Guidance Conduits with Hierarchical Anisotropic Architecture for Peripheral Nerve Regeneration. Adv. Healthc. Mater. 2021, 10, 2100427.
- Ramburun, P.; Kumar, P.; Choonara, Y.E.; Bijukumar, D.; du Toit, L.C.; Pilay, V. A Review of Bioactive Release from Nerve Conduits as a Neurotherapeutic Strategy for Neuronal Growth in Peripheral Nerve Injury. BioMed Res. Int. 2014, 2014, 132350.
- Yan, L.; Yao, Z.; Lin, T.; Zhu, Q.; Qi, J.; Gu, L.; Fang, J.; Zhou, X.; Liu, X. The role of precisely matching fascicles in the quick recovery of nerve function in long peripheral nerve defects. Neuroreport 2017, 28, 1008–1015.
- Ni, H.C.; Tseng, T.C.; Chen, J.R.; Hsu, S.H.; Chiu, I.M. Fabrication of bioactive conduits containing the fibroblast growth factor 1 and neural stem cells for peripheral nerve regeneration across a 15 mm critical gap. Biofabrication 2013, 5, 035010.
- Vijayavenkataraman, S.; Zhang, S.; Thaharah, S.; Sriram, G.; Lu, W.F.; Fuh, J.Y.H. Electrohydrodynamic Jet 3D Printed Nerve Guide Conduits (NGCs) for peripheral Nerve Injury Repair. Polymers 2018, 10, 753.
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 337.
- Song, S.; Wang, X.; Wang, T.; Yu, Q.; Hou, Z.; Zhu, Z.; Li, R. Additive Manufacturing of Nerve Guidance Conduits for Regeneration of Injured Peripheral Nerves. Front. Bioeng. Biotechnol. 2020, 8, 590596.
- Bhatnagar, D.; Bushman, J.S.; Murthy, N.S.; Merolli, A.; Kaplan, H.M.; Kohn, J. Fibrin glue as a stabilization strategy in peripheral nerve repair when using porous nerve guidance conduits. J. Mater. Sci. Mater. Med. 2017, 28, 79.
- Sun, B.; Zhou, Z.; Wu, T.; Chen, W.; Li, D.; Zheng, H.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X.; Yu, Y. Development of Nanofiber Sponges-Containing Nerve Guidance Conduit for Peripheral Nerve Regeneration in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 26684–26696.
- Li, X.; Yang, W.; Xie, H.; Wang, J.; Zhang, L.; Wang, Z.; Wang, L. CNT/Sericin Conductive Nerve Guidance Conduit Promotes Functional Recovery of Transected Peripheral Nerve Injury in a Rat Model. ACS Appl. Mater. Interfaces 2020, 12, 36860–36872.
- Mohan, S.; Hernández, I.C.; Wang, W.; Yin, K.; Sundback, C.A.; Wegst, U.G.K.; Jowett, N. Fluorescent Reporter Mice for Nerve Guidance Conduit Assessment: A High-Throughput in vivo Model. Laryngoscope 2018, 128, E386–E392.
- Jenkins, P.M.; Laughter, M.R.; Lee, D.J.; Lee, Y.M.; Freed, C.R.; Park, D. A nerve guidance conduit with topographical and biochemical cues: Potential application using human neural stem cells. Nanoscale Res. Lett. 2015, 10, 264.
- Singh, A.; Asikainen, S.; Teotia, A.K.; Shiekh, P.A.; Huotilainen, E.; Kumar, A. Biomimetic Photocurable Three-Dimensional Printed Nerve Guidance Channels with Aligned Cryomatrix Lumen for Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 43327–43342.
- Pawar, K.; Welzel, G.; Haynl, C.; Schuster, S.; Scheibel, T. Recombinant Spider Silk and Collagen-Based Nerve Guidance Conduits Support Neuronal Cell Differentiation and Functionality in Vitro. ACS Appl. Bio Mater. 2019, 2, 4872–4880.
- Shah, M.B.; Chang, W.; Zhou, G.; Glavy, J.S.; Cattabiani, T.M.; Yu, X. Novel spiral structured nerve guidance conduits with multichannels and inner longitudinally aligned nano fi bers for peripheral nerve regeneration. J. Biomed. Mater. Res. 2018, 107, 1410–1419.
- Zhao, J.; Zhang, S.; Duan, L.; Yao, R.; Yan, Y.; Wang, T.; Wang, J.; Zheng, Z.; Wang, X.; Li, G. Preparation and mechanical optimization of a two-layer silk/magnesium wires braided porous artificial nerve guidance conduit. J. Biomed. Mater. Res. Part A 2022, 110, 1801–1812.
- Kim, S.M.; Lee, M.S.; Jeon, J.; Lee, D.H.; Yang, K.; Cho, S.W.; Han, I.; Yang, H.S. Biodegradable Nerve Guidance Conduit with Microporous and Micropatterned Poly(lactic-co-glycolic acid)-Accelerated Sciatic Nerve Regeneration. Macromol. Biosci. 2018, 18, 1800290.
- Stocco, E.; Barbon, S.; Emmi, A.; Tiengo, C.; Macchi, V.; De Caro, R.; Porzionato, A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. Int. J. Mol. Sci. 2023, 24, 9170.
- Tagandurdyyeva, N.A.; Trube, M.A.; Shemyakin, I.O.; Solomitskiy, D.N.; Medvedev, G.V.; Dresvyanina, E.N.; Nashchekina, Y.A.; Ivan’kova, E.M.; Dobrovol’skaya, I.P.; Kamalov, A.M.; et al. Properties of Resorbable Conduits Based on Poly(L-Lactide) Nanofibers and Chitosan Fibers for Peripheral Nerve Regeneration. Polymers 2023, 15, 3323.
- Aigner, T.B.; Haynl, C.; Salehi, S.; O’Connor, A.; Scheibel, T. Nerve guidance conduit design based on self-rolling tubes. Mater. Today Biol. 2020, 5, 100042.
- Teuschl, A.H.; Schuh, C.; Halbweis, R.; Pajer, K.; Márton, G.; Hopf, R.; Mosia, S.; Rünzler, D.; Redl, H.; Nógrádi, A.; et al. A New Preparation Method for Anisotropic Silk Fibroin Nerve Guidance Conduits and Its Evaluation in Vitro and in a Rat Sciatic Nerve Defect Model. Tissue Eng. Part C Methods 2015, 21, 945–957.
- Lee, Y.B.; Polio, S.; Lee, W.; Dai, G.; Menon, L.; Carroll, R.S.; Yoo, S.S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp. Neurol. 2010, 223, 645–652.
- Zhao, X.; Fan, C.; Wang, J.; Xiong, H.; Zhu, T.; Liu, Y.; Pan, H.; Weijia Lu, W. Bioinspired multichannel nerve guidance conduit based on shape memory nanofibers for potential application in peripheral nerve repair. ACS Nano 2020, 14, 12579–12595.
- Chang, Y.C.; Chen, M.H.; Liao, S.Y.; Wu, H.C.; Kuan, C.H.; Sun, J.S.; Wang, T.W. Multichanneled Nerve Guidance Conduit with Spatial Gradients of Neurotrophic Factors and Oriented Nanotopography for Repairing the Peripheral Nervous System. ACS Appl. Mater. Interfaces 2017, 9, 37623–37636.
- Wang, J.; Cheng, Y.; Wang, H.; Wang, Y.; Zhang, K.; Fan, C.; Wang, H.; Mo, X. Biomimetic and hierarchical nerve conduits from multifunctional nanofibers for guided peripheral nerve regeneration. Acta Biomater. 2020, 117, 180–191.
- Belanger, K.; Schlatter, G.; Hébraud, A.; Marin, F.; Testelin, S.; Dakpé, S.; Devauchelle, B.; Egles, C. A multi-layered nerve guidance conduit design adapted to facilitate surgical implantation. Health Sci. Rep. 2018, 1, e86.
- Grinberg, Y.; Schiefer, M.A.; Tyler, D.J.; Gustafson, K.J. Fascicular perineurium thickness, size, and position affect model predictions of neural excitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 572–581.
- Stewart, J.D. Peripheral nerve fascicles: Anatomy and clinical relevance. Muscle Nerve 2003, 28, 525–541.
- Li, Y.; Men, Y.; Wang, B.; Chen, X.; Yu, Z. Co-transplantation of Schwann cells and neural stem cells in the laminin-chitosan-PLGA nerve conduit to repair the injured recurrent laryngeal nerve in SD rats. J. Mater. Sci. Mater. Med. 2020, 31, 99.
- Anastasiou, E.; Lorentz, K.O.; Stein, G.J.; Mitchell, P.D. Prehistoric schistosomiasis parasite found in the Middle East. Lancet Infect. Dis. 2014, 14, 553–554.
- Das, S.R.; Lentner, M.T.; Hondred, J.A. Electrical Differentiation of Mesenchymal Stem Cells into Schwann-Cell-Like Phenotypes Using Inkjet-Printed Graphene Circuits Electrical Differentiation of Mesenchymal Stem Cells into Schwann-Cell-Like Phenotypes Using Inkjet-Printed Graphene. Adv. Healthc. Mater. 2017, 6, 1601087.
- Ilkhanizadeh, S.; Teixeira, A.I.; Hermanson, O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials 2007, 28, 3936–3943.
- Huang, W.C.; Lin, C.C.; Chiu, T.W.; Chen, S.Y. 3D Gradient and Linearly Aligned Magnetic Microcapsules in Nerve Guidance Conduits with Remotely Spatiotemporally Controlled Release to Enhance Peripheral Nerve Repair. ACS Appl. Mater. Interfaces 2022, 14, 46188–46200.
- Yucel, D.; Kose, G.T.; Hasirci, V. Polyester based nerve guidance conduit design. Biomaterials 2010, 31, 1596–1603.
- Zhang, D.; Yao, Y.; Duan, Y.; Yu, X.; Shi, H.; Nakkala, J.R.; Zuo, X.; Hong, L.; Mao, Z.; Gao, C. Surface-Anchored Graphene Oxide Nanosheets on Cell-Scale Micropatterned Poly(d, l-lactide-co-caprolactone) Conduits Promote Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 7915–7930.
- Kokai, L.E.; Lin, Y.C.; Oyster, N.M.; Marra, K.G. Diffusion of soluble factors through degradable polymer nerve guides: Controlling manufacturing parameters. Acta Biomater. 2009, 5, 2540–2550.
- Chen, Y.S.; Ng, H.Y.; Chen, Y.W.; Cho, D.Y.; Ho, C.C.; Chen, C.Y.; Chiu, S.C.; Jhong, Y.R.; Shie, M.Y. Additive manufacturing of Schwann cell-laden collagen/alginate nerve guidance conduits by freeform reversible embedding regulate neurogenesis via exosomes secretion towards peripheral nerve regeneration. Biomater. Adv. 2023, 146, 213276.
- Namini, M.S.; Ebrahimi-Barough, S.; Ai, J.; Jahromi, H.K.; Mikaeiliagah, E.; Azami, M.; Bahrami, N.; Lotfibakhshaiesh, N.; Saremi, J.; Shirian, S. Tissue-Engineered Core-Shell Silk-Fibroin/Poly-l-Lactic Acid Nerve Guidance Conduit Containing Encapsulated Exosomes of Human Endometrial Stem Cells Promotes Peripheral Nerve Regeneration. ACS Biomater. Sci. Eng. 2023, 9, 3496–3511.
- Ye, W.; Li, H.; Yu, K.; Xie, C.; Wang, P.; Zheng, Y.; Zhang, P.; Xiu, J.; Yang, Y.; Zhang, F.; et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 2020, 192, 108757.
- Liu, S.; Sun, L.; Zhang, H.; Hu, Q.; Wang, Y.; Ramalingam, M. High-resolution combinatorial 3D printing of gelatin-based biomimetic triple-layered conduits for nerve tissue engineering. Int. J. Biol. Macromol. 2021, 166, 1280–1291.
- Zhu, W.; Tringale, K.R.; Woller, S.A.; You, S.; Johnson, S.; Shen, H.; Schimelman, J.; Whitney, M.; Steinauer, J.; Xu, W.; et al. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater. Today 2018, 21, 951–959.




