Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Antonia De Francesco | -- | 1910 | 2023-11-16 13:59:36 | | | |
| 2 | Jason Zhu | -4 word(s) | 1906 | 2023-11-17 03:42:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
De Francesco, M.A. Antifungal-Resistance Mechanisms of Aspergillus spp. Encyclopedia. Available online: https://encyclopedia.pub/entry/51684 (accessed on 07 February 2026).
De Francesco MA. Antifungal-Resistance Mechanisms of Aspergillus spp. Encyclopedia. Available at: https://encyclopedia.pub/entry/51684. Accessed February 07, 2026.
De Francesco, Maria Antonia. "Antifungal-Resistance Mechanisms of Aspergillus spp" Encyclopedia, https://encyclopedia.pub/entry/51684 (accessed February 07, 2026).
De Francesco, M.A. (2023, November 16). Antifungal-Resistance Mechanisms of Aspergillus spp. In Encyclopedia. https://encyclopedia.pub/entry/51684
De Francesco, Maria Antonia. "Antifungal-Resistance Mechanisms of Aspergillus spp." Encyclopedia. Web. 16 November, 2023.
Copy Citation
Infections due to the Aspergillus species constitute an important challenge for human health. Invasive aspergillosis represents a life-threatening disease, mostly in patients with immune defects. Drugs used for fungal infections comprise amphotericin B, triazoles, and echinocandins. However, an increased emergence of azole-resistant Aspergillus strains has been reported, principally belonging to Aspergillus fumigatus species. Therefore, both the early diagnosis of aspergillosis and its epidemiological surveillance are very important to establish the correct antifungal therapy and to ensure a successful patient outcome.
drug
resistance
Aspergillus
azole
1. Introduction
Aspergillus spp. are filamentous fungi found ubiquitously in the environment, in places such as soil, decaying vegetative material, and dust [1]. Furthermore, the fungi might colonize oligotrophic water systems: more than 400 different species have been found to inhabit different water sources [2], underlining that as well as air, water might also be a potential source of the transmission of filamentous fungi [3].
In different geographical areas, climatic factors such as humidity, rainy season, and temperature influence the prevalence of Aspergillus spp. [4].
The inhalation of Aspergillus conidia gives rise to different respiratory infections, affecting immunocompromised patients more severely.
Despite great advances in the diagnosis and treatment of aspergillosis, mortality remains high, particularly in subjects with important immune defects and invasive diseases.
Infections are generally due to Aspergillus fumigatus, even if other species are increasingly detected as etiological agents [5].
Recently, Aspergillus fumigatus was added to the list of the 19 fungal pathogens to be prioritized by the World Health Organization (WHO) and inserted into the critical group together with Cryptococcus neoformans, Candida auris, and Candida albicans [6].
Antifungal resistance is an emerging and important challenge in different parts of the world, with up to 20% of Aspergillus isolates displaying de novo resistance to commonly used antifungal drugs [7].
2. Amphotericin B Resistance
Amphotericin B acts by interacting with sterols, in particular with ergosterol, the principal component of the fungal cell membrane. The integration of amphotericin B into the fungal membrane leads to the formation of channels (Figure 1). This formation impairs barrier membrane function, increasing the permeability responsible for the leakage of potassium, protons, cations, and cytoplasmic materials that induce cell death [8]. Furthermore, amphotericin B can produce reactive oxygen species (ROS) that give rise to cellular damage [9]. Among Aspergillus spp., Aspergillus terreus is the strain harboring intrinsic resistance to amphotericin B [10].
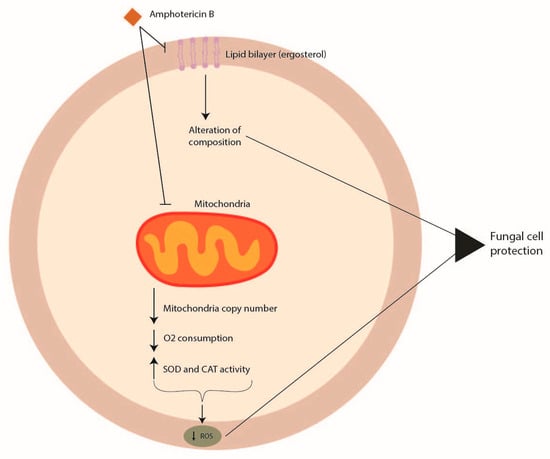
Figure 1. Mechanisms of Aspergillus spp. antifungal drug resistance: amphotericin B resistance.
The mode of action of amphotericin B in Aspergillus terreus has not been well elucidated, and no genomic features have been identified to date that might be linked to amphotericin B resistance. However, amphotericin B resistance in section Terrei seems to be associated with the modulation of molecular chaperones, targeting ROS by mitochondria and influencing cellular redox homeostasis, with an increase in the level of catalase and superoxide dismutase with respect to other Aspergillus species [10][11][12] (Figure 1).
Following the increasing rate of azole resistance [13], the use of amphotericin B has enhanced, and this might be the reason why, recently, an increase in MIC values for amphotericin B in different Aspergillus species has been reported [14][15][16][17], even if resistance to this drug remains extremely rare [18].
In a recent study [19], among 26,909 Aspergillus isolates analyzed, resistance to amphotericin B was detected in 36.8% of Aspergillus terreus, 14.9% of Aspergillus flavus, 5.2% of Aspergillus niger, and 2.01% of Aspergillus fumigatus isolates. Furthermore, some Aspergillus lentulus and Aspergillus ustus isolates have been reported to show amphotericin B resistance [20][21]. Additionally, an increasing trend in amphotericin B resistance was observed in Aspergillus fumigatus between 2016 and 2020, together with a decreasing trend in amphotericin B resistance in Aspergillus terreus and Aspergillus flavus [19].
3. Azole Resistance
Azole antifungal drugs act by interfering with the synthesis of ergosterol, mediated by the fungal sterol 14 alpha-demethylases (Cyp51A and Cyp51B) (Figure 2).
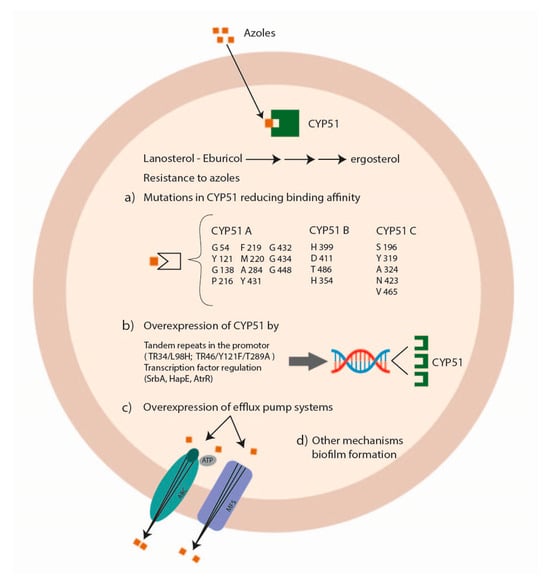
Figure 2. Mechanisms of Aspergillus spp. antifungal drug resistance: azole resistance.
The principal mechanisms of azole resistance involve (a) mutations in the sterol-demethylase gene cyp51A reducing the affinity between the azole drug and its target; (b) the overexpression of the sterol-demethylase gene cyp51A leading to an increase of the azole concentration able to inhibit fungal growth; (c) the overexpression of efflux pump systems decreasing the intracellular drug concentration [22][23]. Alternate mechanisms associated with azole resistance are the modification of HapE [24], the involvement of the mitochondrial complex 1 and the cytochrome b5-CybE redox systems [25][26], the transcription factors SrbA and AtrR [27][28] and biofilm formation (Figure 3) [29].
The acquisition of azole resistance occurs in two ways: in vivo, by selection of resistant isolates during long therapy with azoles, and in vitro, by the selection of resistant isolates as a consequence of an extensive use of azole fungicide in agriculture [30][31][32].
3.1. Mutations in the Sterol-Demethylase Gene Cyp51
Cyp51 genes are the major targets studied for azole resistance in fungal pathogens—resistance that might also be acquired by horizontal gene transfer (HGT) [33]. Different species of Aspergillus exhibit several numbers of cyp51 paralogs in their genome. This genetic redundancy gives the fungus an advantage to survive when exposed to fungicides, increasing its azole resistance [34]. In Aspergillus fumigatus, Aspergillus terreus, and Aspergillus niger, there are only two paralogs (cyp51A and cyp51B), while in Aspergillus flavus, three paralogs exist (cyp51A, cyp51B and cyp51C) [35]. However, only specific mutations in Cyp51A and Cyp51C proteins have been shown to have an impact on azole resistance, while the Cyp51B protein might play other roles that need to be studied in detail [36].
In Aspergillus fumigatus, amino-acid mutations in the Cyp51A protein related to azole resistance were G54, Y121, G138, P216, F219, M220, A284, Y431, G432, G434, and G448 [37][38][39][40][41][42][43]. No mutations were associated with azole resistance in the CypB protein [22][44].
Also, in Aspergillus lentulus, it was demonstrated by targeted cyp51A gene knockout that intrinsic azole resistance was related to this gene [45].
In Aspergillus flavus with reduced voriconazole susceptibility, different mutations were reported in Cyp51A, Cyp51B, and Cyp51C proteins, even if their role in azole resistance needs to be clarified by further studies. Amino-acid changes in Cyp51A protein were identified at positions R450S, K197N/D282E/M288L, and Y132N/T469S [46]; in Cyp51B protein, detected mutations were H399P, D411N, T454P, T486P 105, and Q354K [47]; in Cyp51C protein, the Y319H mutation was identified from an azole-resistant clinical isolate [48] and the mutations S196F, A324P, N423D, and V465M [49][50].
In section Nigri, in Cyp51A protein, many mutations were identified, but their role in inducing azole resistance is still uncertain [51].
In particular, in Aspergillus tubingensis, a recent study found the amino-acid change H467Q in combination with the mutations K64E or V377I exclusively in non-wildtype isolates [52], while in Aspergillus niger, no mutation is associated with azole resistance [52].
However, it has been found that single-gene deletions of cyp51A and cyp51B genes in Aspergillus tubingensis and Aspergillus niger decrease the voriconazole MIC values below the ECV established by CLSI [53].
In Aspergillus braziliensis with reduced azole susceptibility, there were no mutations present in Cyp51A protein [52].
Regarding the Terrei section, few studies are available in the literature about their azole resistance; only a mutation of methionine in position 217 in Aspergillus terreus has been reported [54][55].
In the Usti section, the intrinsically azole-resistant species Aspergillus calidoustus exhibited a mutation M220V in Cyp51A protein, a position already associated with azole resistance in Aspergillus fumigatus [22].
3.2. Overexpression of the Sterol-Demethylase Cyp51
The overexpression of Cyp51 is considered another mechanism of azole resistance in Aspergillus spp., in particular in Aspergillus fumigatus.
Regarding Aspergillus flavus, the overexpression of Cyp51A and Cyp51B was not related to azole resistance because the levels of gene expression were the same both in wild and non-wildtype strains [56][57]. Also, in the Nigri section, this mechanism seems not to be related to azole resistance, even if Cyp51A is upregulated after azole exposure [52][58].
Changes in the promoter region of cyp51 have been described as a mechanism to contrast azole toxicity, mostly in Aspergillus fumigatus, such as the insertion of tandem repeats (TR) of 34bp, 46bp, and 53 bp leading to upregulation of cyp51 [59]. These insertions were often associated with amino-acid substitutions in Cyp51A and have been observed in strains that exhibit reduced susceptibility to azoles [60].
In particular, the TR34/L98H mutation was found principally in resistant environmental isolates, linked to total resistance to itraconazole and reduced susceptibility to voriconazole and posaconazole [23][61][62].
Furthermore, the TR46/Y121F/T289A mutation was found in isolates with high levels of resistance to voriconazole and other azoles [63]. For the tandem repeat of 53 bp, no amino-acid substitution has been found to date [62][64].
Other transcription factors were found able to regulate cyp51 expression in Aspergillus fumigatus, such as SrbA, HapE, and AtrR.
SrbA is a transcriptional regulator that is involved in different processes, as well as sterol biosynthesis [65][66][67], and its deletion leads to greater azole susceptibility [27].
The mutation P88L in heme activator protein E (HapE), a subunit of the CCAAT-binding transcription factor complex (CBC), determines an upregulation of the cyp51A gene and confers azole resistance [68]. A decrease in CBC activity, a negative regulator of the ergosterol pathway, increases the expression of the enzymes involved in its biosyntheses, such as HMG-CoA-synthase, HMG-CoA-reductase, and sterol C14-demethylase contributing to azole resistance [24].
Furthermore, a Zn2Cys6 cluster-containing transcription factor, called ABC transporter regulator or AtrR, was found to be important for azole tolerance both in Aspergillus fumigatus and Aspergillus flavus [69][70]. Additionally, cytochrome b5CybE has been found to be able to regulate the expression level of cyp51A [26].
Furthermore, biofilm production was also hypothesized to play a role in the azole resistance of Aspergillus fumigatus. The cell density reached in a mature biofilm, together with the production of polysaccharide extracellular matrix, protects the mold by the action of the immune system and the antifungal drugs [30].
3.3. Overexpression of Efflux Pump Systems
Efflux pumps are transmembrane proteins that expel drugs from the cell, reducing their intracellular concentration. Therefore, the overexpression of these proteins might be related to azole resistance. The principal types of efflux pumps are the ATP-binding cassette (ABC) transporter and the major facilitator superfamily (MFS) transporter, which differ in structure and activity [71]. ABC transporters use ATP as an energy source, while MFS transporters use a proton gradient to pump out the drugs [72].
The more studied ABC transporters involved in azole resistance are cdr1B, mdr1, mdr2, mdr3, mdr4, abcD, abcE, atrI, atrB, atrC, and atrF. In Aspergillus fumigatus and Aspergillus flavus, only the ABC transporter Cdr1B has been found to be related to azole resistance [73][74].
MFS transporters were less studied, and among them, only mdrA has been associated with an increase of itraconazole and voriconazole susceptibility in Aspergillus fumigatus [75].
4. Echinocandin Resistance
Echinocandins (caspofungin, anidulafungin, and micafungin) act by inhibiting the glucan synthase, an enzyme codified by the FKS1 and FSK2 genes, important for the synthesis of the beta1, 3 glucan (Figure 3). However, because echinocandins have only a fungistatic activity against Aspergillus spp., they are used only in combination with a polyene or an azole to obtain an important synergistic effect. To date, the echinocandin resistance is rarely found in Aspergillus spp. [76].
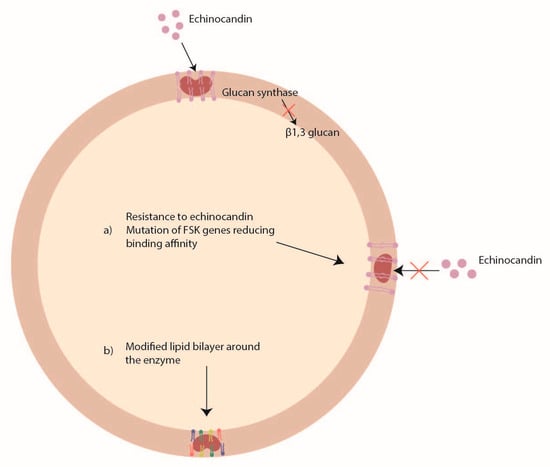
Figure 3. Mechanisms of Aspergillus spp. antifungal drug resistance: echinocandin resistance.
Some studies have reported mutations in FSK genes [77] and changes in the lipid profile around the enzyme [78] as possible mechanisms of echinocandin resistance (Figure 3). In Aspergillus fumigatus, two mutations (S678P and E671Q) in the FSK1 gene were associated with echinocandin resistance [79][80], while in Aspergillus flavus it was shown that P-type ATPase and ubiquinone biosynthesis methyltransferase COQ5 might be involved in caspofungin resistance [81].
References
- Denning, D.W. Invasive aspergillosis. Clin. Infect. Dis. 1998, 26, 781–803.
- Babic, N.; Gunde-Cimerman, N.; Vargha, M.; Tischnner, Z.; Magyar, D.; Verissimo, C.; Sabino, R.; Viegas, C.; Meyer, W.; Brandão, J. Fungal contaminants in drinlink water regulaton? A tle of ecology, exposure, purification and clinical relevance. Int. J. Environ. Res. Public Health 2017, 14, 636.
- Warris, A.; Klaassen, C.H.W.; Meis, J.F.G.M.; de Ruiter, M.T.; de Valk, H.A.; Abrahamsen, T.G.; Gaustad, P.; Verweij, P.E. Molecular epidemiology of Aspergillus fumigatus isolates recovered from water, air, and patients shows two clusters of genetically distinct strains. J. Clin. Microbiol. 2003, 41, 4101–4106.
- Panackai, A.A.; Li, H.; Kontoyiannis, D.P.; Mori, M.; Perego, C.A.; Boeckh, M.; Marr, K.A. Geoclimatic influences on invasive aspergillosis after hematopoietic stem cell transplantation. Clin. Infect. Dis. 2010, 50, 1588–1597.
- Zanganeh, E.; Zarrinfar, H.; Rezaeetalab, F.; Fata, A.; Tohidi, M.; Najafzadeh, M.J.; Alizadeh, M.; Seyedmousavi, S. Predominance of non-fumigatus Aspergillus species among patients suspectedto pulmonary aspergillosis in a tropical and subtropical region of the MiddleEast. Microb. Pathog. 2018, 116, 296–300.
- Cadena, J.; Thompson, G.R.; Patterson, T.F. Aspergillosis. Epidemiology, diagnosis and treatment. Infect. Dis. Clin. N. Am. 2021, 3, 415–434.
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022.
- Seo, K.; Akiyoshi, H.; Ohnishi, Y. Alteration of cell wall components leads to amphotericin B resistance in Aspergillus flavus. Microbiol. Immunol. 1999, 4, 1017–1025.
- Stone, N.R.; Bicanic, T.; Salim, R.; Hope, W. Liposomal amphotericin B (AmBisome®): A review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 2016, 76, 485–500.
- Blum, G.; Perkhofer, S.; Haas, H.; Schrettl, M.; Würzner, R.; Dierich, M.P.; Lass-Flörl, C. Potential basis for amphotericin B resistance in Aspergillus terreus. Antimicrob. Agents Chemother. 2008, 52, 1553–1555.
- Jukic, E.; Blatzer, M.; Posch, W.; Steger, M.; Binder, U.; Lass-Flörl, C.; Wilflingseder, D. Oxidative stress response tips the balance in Aspergillus terreus amphotericin B resistance. Antimicrob. Agents Chemother. 2017, 61, e00670-17.
- Posch, W.; Blatzer, M.; Wilflingseder, D.; Lass-Flörl, C. Aspergillus terreus: Novel lessons learned on amphotericin B resistance. Med. Mycol. 2018, 56, 73–82.
- Ostrosky-Zeichner, L.; Marr, K.A.; Rex, J.H.; Cohen, S.H. Amphotericin B: Time for a new “gold standard”. Clin. Infect. Dis. 2003, 37, 415–425.
- Reichert-Lima, F.; Lyra, L.; Pontes, L.; Moretti, M.L.; Pham, C.D.; Lockhart, S.R.; Zaninelli Scrhreiber, A. Surveillance for azoles resistance in Aspergillus spp. highlights a high number of amphotericin B-resistant isolates. Mycoses 2018, 61, 360–365.
- Hadrich, I.; Makni, F.; Neji, S.; Cheikhrouhou, F.; Bellaaj, H.; Elloumi, M.; Ayadi, A.; Ranque, S. Amphotericin B in vitro resistance is associated with fatal Aspergillus flavus infection. Med. Mycol. 2012, 50, 829–834.
- Rudramurthy, S.M.; Chakrabarti, A.; Geertsen, E.; Mouton, J.W.; Meis, J.F. In vitro activity of isavuconazole against 208 Aspergillus flavus isolates in comparison with 7 other antifungal agents: Assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Diagn. Microbiol. Infect. Dis. 2011, 71, 370–377.
- Gonçalves, S.S.; Stchigel, A.M.; Cano, J.; Guarro, J.; Colombo, A.L. In vitro antifungal susceptibility of clinically relevant species belonging to Aspergillus section Flavi. Antimicrob. Agents Chemother. 2013, 57, 1944–1947.
- Gray, K.C.; Palacios, D.S.; Dailey, I.; Endo, M.M.; Uno, B.E.; Wilcock, B.C.; Burke, M.D. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239.
- Fakhim, H.; Badali, H.; Dannaoui, E.; Nasirian, M.; Jahangiri, F.; Raei, M.; Vaseghi, N.; Ahmaadikia, K.; Vaezi, A. Trends in the prevalence of amphotericin B resistance (AmBR) among clinical isolates of Aspergillus species. J. Mycol. Med. 2022, 32, 101310.
- Balajee, S.A.; Gribskov, J.L.; Hanley, E.; Nickle, D.; Marr, K.A. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot. Cell 2005, 4, 625–632.
- Azzola, A.; Passweg, J.; Habicht, J.; Bubendorf, L.; Tamm, M.; Gratwohl, A.; Eich, G. Use of lung resection and voriconazole for successful treatment of invasive pulmonary Aspergillus ustus infection. J. Clin. Microbiol. 2004, 42, 4805–4808.
- Hagiwara, D.; Watanabe, A.; Kamei, K.; Goldman, G.H. Epidemiological and genomic landscape of azole resistance mechanisms in Aspergillus fungi. Front. Microbiol. 2016, 7, 1382.
- Chowdhary, A.; Sharma, C.; Meis, J.F. Azole-resistant aspergillosis: Epidemiology, molecular mechanisms, and treatment. J. Infect. Dis. 2017, 216, S436–S444.
- Gsaller, F.; Hortschansky, P.; Furukawa, T.; Carr, P.D.; Rash, B.; Capilla, J.; Müller, C.; Bracher, F.; Bowyer, P.; Haas, H.; et al. Sterol biosynthesis and azole tolerance is governed by the opposing actions of SrbA and the CCAAT binding complex. PLoS Pathog. 2016, 12, e1005775.
- Bromley, M.; Johns, A.; Davies, E.; Fraczek, M.; Mabey Gilsenan, J.; Kurbatova, N.; Keays, M.; Kapushesky, M.; Gut, M.; Gut, I.; et al. Mitochondrial complex I is a global regulator of secondary metabolism virulence and azole sensitivity in fungi. PLoS ONE 2016, 11, e0158724.
- Misslinger, M.; Gsaller, F.; Hortschansky, P.; Muller, C.; Bracher, F.; Bromley, M.J.; Haas, H. The cytochrome b5 CybE is regulated by iron availability and is crucial for azole resistance in A. fumigatus. Metallomics 2017, 9, 1655–1665.
- Hagiwara, D.; Watanabe, A.; Kamei, K. Sensitisation of an azole resistant Aspergillus fumigatus strain containing the Cyp51A-related mutation by deleting the SrbA gene. Sci. Rep. 2016, 6, 38833.
- Hagiwara, D.; Miura, D.; Shimizu, K.; Paul, S.; Ohba, A.; Gonoi, T.; Watanabe, A.; Kamei, K.; Shintani, T.; Moye-Rowley, W.S.; et al. A novel Zn2-Cys6 transcription factor AtrR plays a key role in an azole resistance mechanism of Aspergillus fumigatus by co-regulating cyp51A and cdr1B expressions. PLoS Pathog. 2017, 13, e1006096.
- Morelli, K.A.; Kerkaert, J.D.; Cramer, R.A. Aspergillus fumigatus biofilms: Toward understanding how growth as a multicellular network increases antifungal resistance and disease progression. PLoS Pathog. 2021, 17, e1009794.
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.; Meis, J.F. Azole Resistance in Aspergillus fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin. Infect. Dis. 2016, 62, 362–368.
- Verweij, P.E.; Snelders, E.; Kema, G.H.; Mellado, E.; Melchers, W.J. Azole resistance in Aspergillus fumigatus: A side-effect of environmental fungicide use? Lancet Infect. Dis. 2009, 9, 789–795.
- Buil, J.B.; Hare, R.K.; Zwaan, B.J.; Arendrup, M.C.; Melchers, W.J.G.; Verweij, P.E. The fading boundaries between patient and environmental routes of triazole resistance selection in Aspergillus fumigatus. PLoS Pathog. 2019, 15, e1007858.
- Morogovsky, A.; Handelman, M.; Kandil, A.A.; Shadkchan, Y.; Osherov, N. Horizontal gene transfer of triazole resistance in Aspergillus fumigatus. Microbiol. Spectr. 2022, 10, e0111222.
- Hawkins, N.J.; Cools, H.J.; Sierotzki, H.; Shaw, M.W.; Knogge, W.; Kelly, S.L.; Kelly, D.E.; Fraaije, B.A. Paralog re-emergence: A novel, historically contigent mechanism in the evolution of antimicrobial resistance. Mol. Biol. Evol. 2014, 31, 1793–1802.
- Mellado, E.; Diaz-Guerra, T.M.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Identification of two different 14-alpha-sterol-demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 2001, 39, 2431–2438.
- Warrilow, A.G.S.; Parker, J.E.; Price, C.L.; Nes, W.D.; Kelly, S.L.; Kelly, D.E. In vitro bio-chemical study of CYP51-mediated azole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2015, 59, 7771–7778.
- Mann, P.A.; Parmegiani, R.M.; Wei, S.Q.; Mendrick, C.A.; Li, X.; Loebenberg, D.; DiDomenico, B.; Hare, R.S.; Walker, S.S.; McNicholas, P.M. Mutations in Aspergillus fumigatus resulting in reduced susceptibility to posaconazole appear to be restricted to a single amino acid in the cytochrome P450 14 α-demethylase. Antimicrob. Agents Chemother. 2003, 47, 577–581.
- Nascimento, A.M.; Goldman, G.H.; Park, S.; Marras, S.A.E.; Delmas, G.; Oza, U.; Dudley, M.N.; Mann, P.A.; Perlin, D.S. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob. Agents Chemother. 2003, 47, 1719–1726.
- Bader, O.; Weig, M.; Reichard, U.; Lugert, R.; Kuhns, M.; Christner, M.; Hels, J.; Peter, S.; Schumacher, Y.; Bucheidt, D.; et al. cyp51A—Based mechanisms of Aspergillus fumigatus azole drug resistance present in clinical samples from Germany. Antimicrob. Agents Chemother. 2013, 57, 3513–3517.
- Lescar, J.; Meyer, I.; Akshita, K.; Srinivasaraghavan, K.; Verma, C.; Palous, M.; Mazier, D.; Datry, A.; Fekkar, A. Aspergillus fumigatus harbouring the sole Y121F mutation shows decreased susceptibility to voriconazole but maintained susceptibility to itraconazole and posaconazole. J. Antimicrob. Chemother. 2014, 69, 3244–3247.
- Mellado, E.; Garcia-Effron, G.; Alcazar-Fuoli, L.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Substitutions at methionine 220 in the 14 α-sterol demethylase (Cyp51A) of Aspergillus fumigatus are responsible for resistance in vitro to azole antifungal drugs. Antimicrob. Agents Chemother. 2004, 48, 2747–2750.
- Howard, S.J.; Cerar, D.; Anderson, M.J.; Albarrag, A.; Fisher, M.C.; Pasqualotto, A.C.; Arendrup, M.C.; Perlin, D.S.; Denning, D.W. Frequency and evolution of azole resistance in Aspergillus fumigatus associ-ated with treatment failure. Emerg. Infect. Dis. 2009, 15, 1068–1076.
- Albarrag, A.M.; Anderson, M.J.; Howard, S.J.; Robson, G.D.; Warn, P.A.; Sanglard, D.; Denning, D.W. Interrogation of related clinical pan-azole-resistant Aspergillus fumigatus strains: G138C, Y431C, and G434C single nucleotide polymorphisms in cyp51A, upregulation of cyp51A, and integration and activation of transposon Atf1 in the cyp51A promoter. Antimicrob. Agents Chemother. 2011, 55, 5113–5121.
- Dudakova, A.; Spiess, B.; Tangwattanachuleeporn, M.; Sasse, C.; Buchheidt, D.; Weig, M.; Grob, U.; Bader, O. Molecular tools for the detection and deduction of azole antifungal drug resistance phenotypes in Aspergillus species. Clin. Microbiol. Rev. 2017, 30, 1065–1091.
- Mellado, E.; Alcazar-Fuoli, L.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Role of Aspergillus lentulus 14-a sterol demethylase (Cyp51A) in azole drug susceptibility. Antimicrob. Agents Chemother. 2011, 55, 5459–5468.
- Pérez-Cantero, A.; Lopez-Fernandez, L.; Guarro, J.; Capilla, J. Azole resistance mechanisms in Aspergillus: Update and recent advances. Int. J. Antimicrob. Agents 2020, 55, 105807.
- Krishnan, S.; Manavathu, E.K.; Chandrasekar, P.H. Aspergillus flavus: An emerging non-fumigatus Aspergillus species of significance. Mycoses 2009, 52, 206–222.
- Paul, R.A.; Rudramurthy, S.M.; Meis, J.F.; Mouton, J.W.; Chakrabarti, A. A Novel Y319H substitution in CYP51C associated with azole resistance in Aspergillus flavus. Antimicrob. Agents Chemother 2015, 59, 6615–6619.
- Krishnan-Natesan, S.; Chandrasekar, P.H.; Alangaden, G.J.; Manavathu, E.K. Molecular characterisation of cyp51A and cyp51B genes coding for P450 14 α-lanosterol demethylases A (CYP51Ap) and B (CYP51Bp) from voriconazole-resistant laboratory isolates of Aspergillus flavus. Int. J. Antimicrob. Agents 2008, 32, 519–524.
- Sharma, C.; Kumar, R.; Kumar, N.; Masih, A.; Gupta, D.; Chowdhary, A. Investigation of multiple resistance mechanisms in voriconazole-resistant Aspergillus flavus clinical isolates from a chest hospital surveillance in Delhi. India. Antimicrob. Agents Chemother. 2018, 62, e01928-17.
- Howard, S.J.; Harrison, E.; Bowyer, P.; Varga, J.; Denning, D.W. Cryptic species and azole resistance in the Aspergillus niger complex. Antimicrob. Agents Chemother. 2011, 55, 4802–4809.
- Pérez-Cantero, A.; López-Fernández, L.; Guarro, J.; Capilla, J. New insights into the Cyp51 contribution to azole resistance in Aspergillus section Nigri. Antimicrob. Agents Chemother. 2019, 63, e00543-19.
- Pérez-Cantero, A.; Martin-Vicente, A.; Guarro, J.; Fortwendel, J.R.; Capilla, J. Analysis of the contribution of cyp51 genes to azole resistance in Aspergillus section Nigri with the CRISPR-Cas9 technique. Antimicrob. Agents Chemother. 2021, 65, e01996-20.
- Arendrup, M.C.; Jensen, R.H.; Grif, K.; Skov, M.; Pressler, T.; Johansen, H.K.; Lass-Flörl, C. In vivo emergence of Aspergillus terreus with reduced azole susceptibility and a Cyp51a M217I alteration. J. Infect. Dis 2012, 206, 981–985.
- Zoran, T.; Sartori, B.; Sappl, L.; Aigner, M.; Sánchez-Reus, F.; Rezusta, A.; Chowdhary, A.; Taj-Aldeen, S.J.; Arendrup, M.C.; Oliveri, S.; et al. Azole-resistance in Aspergillus terreus and related species: An emerging problem or a rare phenomenon? Front. Microbiol. 2018, 9, 516.
- Paul, R.A.; Rudramurthy, S.M.; Dhaliwal, M.; Singh, P.; Ghosh, A.K.; Kaur, H.; Varma, S.; Agarwal, R.; Chakrabarti, A. Magnitude of voriconazole resistance in clinical and environmental isolates of Aspergillus flavus and investigation into the role of multidrug efflux pumps. Antimicrob. Agents Chemother. 2018, 62, e01022-18.
- Liu, W.; Sun, Y.; Chen, W.; Liu, W.; Wan, Z.; Bu, D.; Varma, S.; Agarwal, R.; Chakrabarti, A. The T788G mutation in the cyp51C gene confers voriconazole resistance in Aspergillus flavus causing aspergillosis. Antimicrob. Agents Chemother. 2012, 56, 2598–2603.
- Hashimoto, A.; Hagiwara, D.; Watanabe, A.; Yahiro, M. Drug sensitivity and resis-tance mechanism in Aspergillus section Nigri strains from Japan. Antimicrob. Agents Chemother. 2017, 61, e02583-16.
- Price, C.L.; Parker, J.E.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Azole fungicides—Under-standing resistance mechanisms in agricultural fungal pathogens. Pest Manag. Sci. 2015, 71, 1054–1058.
- Hodiamont, C.J.; Dolman, K.M.; Ten Berge, I.J.M.; Melchers, W.J.G.; Verweij, P.E.; Pajkrt, D. Multiple-azole-resistant Aspergillus fumigatus osteomyelitis in a patient with chronic granulomatous disease successfully treated with long-term oral posaconazole and surgery. Med. Mycol. 2009, 47, 217–220.
- Chowdhary, A.; Sharma, C.; Hagen, F.; Meis, J.F. Exploring azole antifungal drug resis-tance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol. 2014, 9, 697–711.
- Rhodes, J.; Abdolrasouli, A.; Dunne, K.; Sewell, T.R.; Zhang, Y.; Ballard, E.; Brackin, A.P.; van Rhijn, N.; Chown, H.; Tsitsopoulou, A.; et al. Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment. Nat. Microbiol. 2022, 7, 663–674.
- Snelders, E.; Camps, S.M.; Karawajczyk, A.; Rijs, A.J.; Zoll, J.; Verweij, P.E.; Melchers, W.J.G. Genotype-phenotype complexity of the TR46/Y121F/T289A cyp51A azole resistance mechanism in Aspergillus fumigatus. Fungal Genet. Biol. 2015, 82, 129–135.
- Garcia-Rubio, R.; Escribano, P.; Gomez, A.; Guinea, J.; Mellado, E. Comparison of two highly discriminatory typing methods to analyze Aspergillus fumigatus azole resistance. Front. Microbiol. 2018, 9, 1626.
- Willger, S.D.; Puttikamonkul, S.; Kim, K.H.; Burritt, J.B.; Grahl, N.; Metzel, L.J.; Barbuch, R.; Bard, M.; Lawrence, C.B.; Cramer, R.A., Jr. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008, 4, e1000200.
- Chung, D.; Barker, B.M.; Carey, C.C.; Merriman, B.; Werner, E.R.; Lechner, B.E.; Dhingra, S.; Cheng, C.; Xu, W.; Blosser, S.J.; et al. ChIP-seq and in vivo transcriptome analyses of the Aspergillus fumigatus SREBP SrbA reveals a new regulator of the fungal hypoxia response and virulence. PLoS Pathog. 2014, 10, e1004487.
- Dhingra, S.; Cramer, R.A. Regulation of sterol biosynthesis in the human fungal pathogen Aspergillus fumigatus: Opportunities for therapeutic development. Front. Microbiol. 2017, 8, 92.
- Camps, S.M.T.; Dutilh, B.E.; Arendrup, M.C.; Rijs, A.J.M.M.; Snelders, E.; Huynen, M.A.; Melchers, W.J.G. Discovery of a hapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS ONE 2012, 7, e50034.
- Paul, S.; Stamnes, M.; Thomas, G.H.; Liu, H.; Hagiwara, D.; Gomi, K.; Filler, S.G.; Moye-Rowley, W.S. AtrR is an essential determinant of azole resistance in Aspergillus fumigatus. MBio 2019, 10, e02563-18.
- Ukai, Y.; Kuroiwa, M.; Kurihara, N.; Naruse, H.; Homma, T.; Maki, H.; Naito, A. Contributions of yap1 mutation and subsequent atrF upregulation to voriconazole resistance in Aspergillus flavus. Antimicrob. Agents Chemother. 2018, 62, e01216-18.
- Wei, X.; Zhang, Y.; Lu, L. The molecular mechanism of azole resistance in Aspergillus fumigatus: From bedside to bench and back. J. Microbiol. 2015, 53, 91–99.
- Law, C.J.; Maloney, P.C.; Wang, D.N. Ins and outs of major facilitator superfamily antiporters. Ann. Rev. Microbiol. 2008, 62, 289–305.
- Fraczek, M.G.; Bromley, M.; Buied, A.; Moore, C.B.; Rajendran, R.; Rautemaa, R.; Rautemaa, R.; Ramage, G.; Denning, D.W.; Bowyer, P. The cdr1B efflux transporter is associated with non-cyp51a -mediated itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 2013, 68, 1486–1496.
- Paul, S.; Diekema, D.; Moye-Rowley, W.S. Contributions of both ATP-binding cassette transporter and Cyp51A proteins are essential for azole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2017, 61, e02748-16.
- Meneau, I.; Coste, A.T.; Sanglard, D. Identification of Aspergillus fumigatus multidrug transporter genes and their potential involvement in antifungal resistance. Med. Mycol. 2016, 54, 616–627.
- Arastehfar, A.; Lass-Flörl, C.; Garcia-Rubio, R.; Daneshnia, F.; Ilkit, M.; Boekhout, T.; Gabaldon, T.; Perlin, D.S. The quiet and underappreciated rise of drug-resistant invasive fungal pathogens. J. Fungi 2020, 6, 138.
- Jimenez-Ortigosa, C.; Moore, C.; Denning, D.W.; Perlin, D.S. Emergence of echinocandin resistance due to a point mutation in the fks1 gene of Aspergillus fumigatus in a patient with chronic pulmonary aspergillosis. Antimicrob. Agents Chemother. 2017, 61, e01277.
- Satish, S.; Jimenez-Ortigosa, C.; Zhao, Y.; Lee, M.H.; Dolgov, E.; Krüger, T.; Park, S.; Denning, D.W.; Kniemeyer, O.; Brakhage, A.A.; et al. Stress-induced changes in the lipid microenvironment of β-(1,3)-d-glucan synthase cause clinically important echinocandin resistance in Aspergillus fumigatus. MBio 2019, 10, e00779–e00819.
- Gardiner, R.E.; Souteropoulos, P.; Park, S.; Perlin, D.S. Characterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofungin. Med. Mycol. 2005, 43, 299–305.
- Silva, A.P.; Miranda, I.M.; Branco, J.; Oliveira, P.; Faria-Ramos, I.; Silva, R.M.; Rodrigues, A.G.; Costa-de-Oliveira, S. FKS1 mutation associated with decreased echinocandin susceptibility of Aspergillus fumigatus following anidulafungin exposure. Sci. Rep. 2020, 10, 11976.
- Yassin, Z.; Loftali, E.; Khourgami, M.R.; Omidi, N.; Fattahi, A.; Nasrollahi, S.A.; Ghasemi, R. Caspofungin resistance in clinical Aspergillus flavus isolates. J. Mycol. Med. 2021, 31, 1011166.
More
Information
Subjects:
Primary Health Care
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
786
Revisions:
2 times
(View History)
Update Date:
17 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




