Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Polina Galitskaya | -- | 3670 | 2023-11-15 10:00:59 | | | |
| 2 | Lindsay Dong | Meta information modification | 3670 | 2023-11-17 01:56:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kuryntseva, P.; Karamova, K.; Galitskaya, P.; Selivanovskaya, S.; Evtugyn, G. Influence factor of Biochar Functions in Soil. Encyclopedia. Available online: https://encyclopedia.pub/entry/51594 (accessed on 08 February 2026).
Kuryntseva P, Karamova K, Galitskaya P, Selivanovskaya S, Evtugyn G. Influence factor of Biochar Functions in Soil. Encyclopedia. Available at: https://encyclopedia.pub/entry/51594. Accessed February 08, 2026.
Kuryntseva, Polina, Kamalya Karamova, Polina Galitskaya, Svetlana Selivanovskaya, Gennady Evtugyn. "Influence factor of Biochar Functions in Soil" Encyclopedia, https://encyclopedia.pub/entry/51594 (accessed February 08, 2026).
Kuryntseva, P., Karamova, K., Galitskaya, P., Selivanovskaya, S., & Evtugyn, G. (2023, November 15). Influence factor of Biochar Functions in Soil. In Encyclopedia. https://encyclopedia.pub/entry/51594
Kuryntseva, Polina, et al. "Influence factor of Biochar Functions in Soil." Encyclopedia. Web. 15 November, 2023.
Copy Citation
Biochar effects are strongly dependent on its properties. Biochar improves physical soil properties by decreasing bulk density and increasing medium and large aggregates, leading to faster and deeper water infiltration and root growth. Improvement of the chemical properties of soil is connected with pH neutralization of acidic soils, increase of cation exchange capacity and base saturation, providing a larger surface for sorption of toxicants and exchange of cations. Biochar increases the stocks of macro- and micronutrients in soil and remains sufficient for decades.
pyrogenic carbon
land use
soil fertility and productivity
1. Introduction
In accordance with the International Biochar Initiative (IBI), biochar is defined as a solid material obtained by thermochemical carbonization in oxygen-limited conditions [1]. It is mostly produced during the pyrolysis process from organic matter at temperatures 300–700 °C at low access to oxygen [2]. Other pyrolysis products are liquid fuel and pyrolysis gas [3]. During the last decade, biochar has attracted attention in agroindustry and environmental sciences due to the prospects of its application as a soil conditioner and fertilizer, fodder additive, wastewater cleaning agent, renewable energy source and inexpensive sorbent used to remove heavy metals and organic pollutants. Biochar improves soil structure and fertility, promotes water holding capacity and is an important source of microelements and nutrients for plants, in stock and chicken farming [4]. Biochar application decreases methane and N2O emission [5][6], stimulates the activity of soil microorganisms and plant germination. It is important that biochar shows advantages by amendments in almost all agricultural conditions, including soil type and climate [7]. Contrary to many organic fertilizers, biochar carbon (C) is quite stable and is mineralized very slowly with the release of CO2 [8][9][10]. This makes biochar important in green sustainability policy, C sequestration, yield improvements and decrease of greenhouse gas emissions.
Biochar properties and hence the consequences of its application in agriculture depend on the biomass used for its production and the pyrolysis conditions [11]. Organic wastes including manure, plant residues, food wastes, sewage sludge, etc. have been used for soil fertilization since ancient times. In natural processes of biomass decomposition, about 90 to 95% of carbon is returned in the atmosphere within a few years (or earlier), whereas pyrolysis of bioresidues results in C sequestration and about half decrease CO2 emissions [12]. Therefore, biomass pyrolysis dramatically affects the C cycle (Figure 1). A slower rate of biochar mineralization prolongs its effect on soil fertility and decreases demands in other amendments. Biochar can also affect nitrogen cycling by mitigating N2O, another greenhouse gas also involved in atmospheric ozone decomposition, and by stimulating nitrification via indirect influence on the soil microbial community [13].
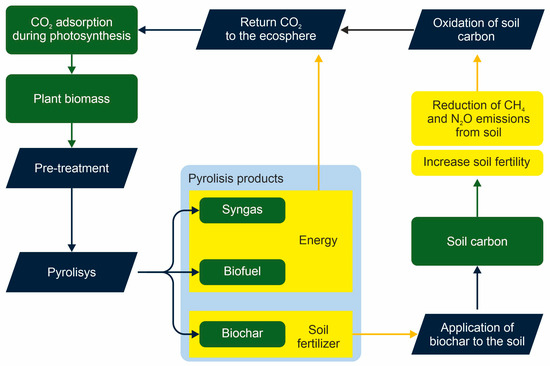
Figure 1. Carbon turnover in the production and application of biochar to soils.
2. Biochar Production, Main Sources and Properties
2.1. Feedstock Materials
Biochar is commonly produced by pyrolysis of plant biomass, animal and food residues, forestry wastes, crop residues (nut shells, fruit pits, bagasse, straw [14][15][16][17]), algae [18][19], manures [11][20][21][22], biosolids and sewage sludge formed in wastewater treatment, etc. [23][24][25][26]. In the latter case, the content of heavy metals should be limited to avoid possible contamination of the soil. The elemental content of biochar (mainly, C, N, K, P, Ca and Mg) depends on the feedstock source and pyrolysis parameters.
The classification of biochar feedstocks mostly uses moisture content as one of the key features. Freshly cropped biomass, including agricultural plant and forest residues, sewage sludge, algae, animal manure, etc., typically contains more than 30% of water. They are referred to as wet biomass. Agricultural wastes with a water content below 30% are classified as dry feedstock. Wet biomass can be pre-dried using technologies developed for drying other biomaterials. However, in most cases, such technologies are labor consuming and decrease the total economic effect of biochar application [27]. Biomass is classified on that specially harvested as bioenergy crops (bamboo, sorghum, willows, Miscanthus, switchgrass [28][29] and agricultural wastes [30]. The use of energy crops provides a high yield of the target product and minimal preliminary treatment is required. Thus, no pre-drying is commonly needed. The ash content of such a biomass can significantly vary depending on the harvesting time and planting density complicates the selection of optimal pyrolysis parameters and feedstock unification. Biomass from various wastes is more variable regarding its content. This type of feedstock involves wastes formed in agriculture, forestry, food production, sewage sludge from water treatment plants, animal manure, etc. Their conversion to biochar is always beneficial because it reduces expenses for their environmentally safe storage and/or processing. On the other hand, parts of such wastes that do not contain toxic metals and organic contaminants can be applied on land as soil amendments and organic fertilizers with no or minimal treatment. Thus, full processing of the plant biomass into biochar seems exhausting. It should also be taken into account that crop residue removal negatively affects the soil organic carbon pool, which should be reimbursed by agrochemical measures [31]. Preliminary treatment of biomass is one of the key factors affecting both the pyrolysis products and the repeatability of the technological parameters of biochar production. Moisture content, preliminary crushing or, vice versa, pressing of the feedstock are most frequently utilized on the preliminary stage of pyrolysis [32]. Appropriate protocols influence both the economy and preferable directions of biochar application.
2.2. Pyrolysis Conditions
The technological process of biomass pyrolysis starts with supplementary drying of the substrate if its moisture is higher than optimal. The following heating removes the natural volatile components present in the raw material. After that, decomposition of the biopolymers present in biomass results in consecutive increases in the C content and release of permanent gases (CO2 and nitrogen oxides, ammonia and methane) and volatile organic compounds of decomposition. The latter can be condensed and then used as liquid fuel. The solid residue is biochar. The reaction pathways of the formation of gaseous and liquid pyrolysis products partially compete with each other and differ depending on the pyrolysis parameters (residence time, heating rate, pyrolysis temperature) and substrate properties. Thus, fast heating increases the yield of pyrolysis gases and fuel, whereas slow and rather long pyrolysis is more appropriate for predominant carbonization and maximum biochar yield.
Thermal treatment of biomass can be performed using several regimes, which are chosen depending on the type of feedstock, its pre-treatment, desired properties and future application of biochar. They are classified as slow, fast, flash and intermediate pyrolysis, dry torrefaction and hydrothermal carbonization, which are performed in the absence or limited access of oxygen. Pyrolysis can also be accelerated by microwave treatment.
Long pyrolysis is a traditional technology of biochar production with heating at 300–700 °C by the rate of 5 to 20 °C/min for more than one hour. In traditional biochar production, carbonization can take place for more than one week. It produces a maximum yield of biochar against other technologies mentioned above (more than 50% of dry weight). Hard wood can be heated up to 1000 °C with the final carbonization level. Meanwhile, biomass from agricultural waste and other sources with rather mild temperature of ash melting is normally heated at not more than 700 °C [33][34].
Fast pyrolysis is mostly used for the production of liquid fuel: volatile products are condensed by rapid cooling to avoid polymerization of by-products. The biomass is heated for several seconds to pyrolysis temperatures. Gases contain various quantities of carbon oxides, methane and hydrogen [35]. Fast pyrolysis may result in incomplete conversion of biomass so that up to 9% of hydrocarbons (mostly in the form of cellulosic and hemicellulosic fractions) remain in the product.
Flash pyrolysis [36] is performed by ignition of the press biomass at elevated pressure (1–2 MPa). The reactions provide heating of the feedstock up to 300 to 600 °C for about 30 min. The yield of biochar increases with temperature and pressure [37].
Dry torrefaction is a process in which biomass is heated in an inert atmosphere at 200 to 300 °C for the period of 30 min to 2–3 h. Up to 30% of biomass is lost, including a 10% decrease in energy content. Polysaccharides are depolymerized to solid residues with a low O/C ratio. Torrefaction assumes slow heating and can be categorized as mild pyrolysis. Torrefaction is commonly used for pre-treatment of biomass prior to its combustion or gasification. The product of dry torrefaction still contains significant amounts of volatile organic matter from the feedstock.
Gasification is partial combustion of the biomass at a high temperature (600 to 1200 °C) for several seconds [38]. As follows from the name of the process, gas consisting mostly of methane and hydrogen is formed. Up to 10% of the dry weight of the biomass is converted into biochar. Unlike other biochar sources, it can be contaminated with polyaromatic hydrocarbons and heavy metals so that it cannot be recommended for application as a soil amendment. Nevertheless, its application improves the physical properties of the soil and positively affects microbial activity [39].
Hydrothermal carbonization is performed at 180 to 260 °C with biomass dispersed in water under elevated pressure (2–6 MPa) for 5 to 240 min [40][41]. The char produced has a higher carbon content than the products of dry pyrolysis [42]. Its characteristics depend on the reaction temperature, pressure, residence time and biomass/water ratio. An increase in the temperature results in a higher yield of liquid products. At 250 °C, up to 70% (dry weight) of biochar are obtained.
Chemical reactions during biomass pyrolysis depend on substrate composition and heating conditions. Cellulose, hemicellulose and lignin comprising biomass undergo various conversion paths, including depolymerization, cross-linking and decomposition of the fragments. Hemicellulose decomposes at 200 to 260 °C, cellulose breaks at 240 to 350 °C and lignin starts decomposing at 280 to 500 °C [43]. Slow pyrolysis at rather low temperatures results in decomposition and carbonization of cellulose, whereas fast pyrolysis promotes volatilization and formation of levoglucosan [44], which is later decomposed by C-O, C-C scission and dehydration to low-molecular compounds [39][45].
2.3. Improving Biochar Properties by Modification Approaches
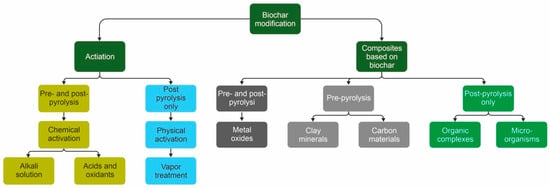
Biochar application often assumes pre-treatment aimed at introduction of additional functional groups, increase of porosity and specific surface area [46]. Such a treatment is also desirable for the application of biochar for catalyst production because it increases the adsorptive capacity of potential catalytic sites, such as transient metal cations. Meanwhile, biochar application may have unexpected and undesired effects, e.g., formation and accumulation of toxic species (polyaromatic hydrocarbons). The general classification of biochar pre-treatment approaches is presented in Figure 2.

Figure 2. Classification of biochar modification approaches.
The introduction of additional functional groups starts from partial oxidation of biochar. Hydrogen peroxide [46], ozone [47], potassium permanganate [48] (and nitric acid [49] are used for this purpose. Oxidation products contain carboxyl, phenolic, peroxide fragments and lactones. Oxidation increases the hydrophilicity of the char surface. Similar results can be obtained by heating biochar in an inert atmosphere with low amounts of oxygen. The appropriate changes depend on the treatment temperature. Thus, post-treatment of chars obtained from carbohydrates and activated by heating to 300 °C in the presence of oxygen was investigated by FT-IR spectroscopy and X-ray fluorescence analysis [50]. Treatment below 450 °C promotes opening inner space without mesopore widening and results in preferable surface adsorption of inorganic components. Treatment temperature increase to 900 °C removes most oxygen-containing functional groups and stimulates the formation of more common aromatic structures.
Oxidation within the range of 450 to 900 °C makes mesopores wider and deeper. The contribution of the adsorption of inorganic species on initial organic matter decreases. Metal cations remain attached in pores and on the surface due to electrostatic interactions with aromatic π-electron systems. Meanwhile, full pore volume, surface area and capacity toward anions (phosphates) can decrease during high temperature pyrolysis [39][45]. High temperature post-treatment decreases the biochar mass by about 10% per each 100 °C. In turn, this increases the yield of mineral residue and the content of metal oxides and carbonates [51].
In addition to oxidation in the presence of small amounts of oxygen, micropore widening is achieved by controlled biochar gasification. For this purpose, it is treated with water vapor or carbon dioxide. This increases the internal volume of micropores, but the number of mesopores increases insignificantly. Thus, post-activation of biochar from coconut shells has been described with various rates of vapor and carbon dioxide supply with simultaneous microwave treatment [52].
Some catalysts, i.e., zinc chloride and phosphoric acid, make for porosity increase and are added to raw biomass prior to pyrolysis. Being absorbed by biomass, they prevent gumming in the first heating stages. Phosphoric acid hydrolysis glycoside bonds in hemicellulose and cellulose and breaks ether bonds in lignin. The reactions mentioned are often followed by degradation and dehydration of the cellulose structure and condensation of oligomeric by-products. In such a conversion, carbon oxides and methane are released. Phosphoric acid can also form esteric bonds with hydroxide groups of biomasses if temperatures do not exceed 200 °C. This promotes the cross-binding of polymeric chains [53]. As a result, carbon retention, sorption capacity and pore formation are synchronously improved [54]. Unlike phosphoric acid, zinc chloride acts as a dehydration agent. It decreases carbonization temperature for cellulose, hemicellulose and lignin and changes paths of their following chemical conversion in favor of gumming suppression and open pore formation. ZnCl2 can then stimulate wood swelling due to its implementation in the biomass and depolymerization processes. Zinc chloride remains in melted conditions up to the end of pyrolysis (melting point below 700 °C) and hence inhibits C rearrangement in the structures to be obtained.
Biochar amination is the second most widespread method of biochar functionalization. It simplifies CO2 capture and toxicant sorption [55][56]. Amination starts with preliminary biochar oxidation, followed by its ammonia addition [57][58]. Polyethyleneimine [59][60] is involved in similar reactions. In addition, amino groups are formed by nitration of biochar followed by reduction of nitro groups introduced with dithionite [34]. Aminated biochar finds applications in binding metal cations. In addition, amino groups change the hydrophilicity of the surface and improve biochar wettability, which is important for its field application. It should be mentioned that in addition to chemical modification, increased nitrogen content is achieved by an environmentally safer approach based on pyrolysis of biomass enriched with nitrogen-containing materials, e.g., chitin [61] and chitosan [62].
Biochar sulfurization (introduction of sulfonate groups) can be achieved by post-treatment of biochar with sulfuric acid, filtration and centrifugation of target products [63]. They are used for sorptional removal of toxic metals and as solid acids in soil remediation and wastewater treatment [64][65].
Most of the modified biochar particles have negative surface charges associated with a few carboxylic, carbonyl and sulfonate groups. Therefore, they can adsorb metal cations that are additionally bonded by complexation with amino (amide) groups. High ash content in the substrate increases the accumulation of metals, whereas higher pyrolysis temperatures affect this parameter in the opposite way due to partial destruction of acidic groups. Instead, the products of high temperature pyrolysis adsorb increased quantities of organic species, e.g., catechols or humic acids [53]. This is referred to as the general increase of the pore volume [66]. However, they do not effectively bind anions, e.g., nitrates, arsenates or phosphates. To avoid this limitation, metal oxides can be introduced into the biochar structure. The feedstock is soaked in a solution of metal nitrates or chlorides. Their following heating in the presence of atmospheric oxygen to about 300 °C releases nitrogen oxides (chlorine) so that metals are fixed in the biochar material in oxide form. Meanwhile, impregnation of metal oxides regularly decreases the biochar surface area due to partial filling of the pores [67]. Treatment of the biochar obtained from the corn comb by slow pyrolysis at 500 °C with Fe(III) nitrate resulted in 20 times higher accumulation of As [68]. Hybrid material obtained by pyrolysis of the mixture of naturally occurring hematite (γ-Al2O3) and pinewood biomass showed a much stronger ability to remove arsenic from wastewaters than biochar with no impregnation [69].
Biochars were also mixed with carbonaceous nanomaterials, e.g., graphene oxide [70] and carbon nanotubes [71]. They increase the porosity of the product, specific surface area and surface concentration of oxygenated functional groups. As a result, they accumulated metal cations better than the biochars themselves. Hybrid materials described have been utilized for the removal of Cr(VI) and Hg(II) from the wastes of sugar production.
2.4. Biochar Properties
2.4.1. Physical Properties
The physical properties of biochar are pre-determined by the processes taking place during carbonization. Biochar obtained in low temperature pyrolysis consists of graphitic layers. The distance between the layers, as well as their specific surface area, increases with the carbonization temperature [72]. Aromatic carbon atoms dominate the biochar structure obtained at 350 °C with rather small contribution of alkyl oxygen and C atoms. An increase in the pyrolysis temperature to 500 °C converts alkyl fragments into aryl groups with a low H/C ratio. Hydrothermal conversion results in the formation of roundish particles. The model study showed that they resulted from the preliminary formation of lignin-like structures with a high number of oxygen-containing functional groups (ether, quinone and pyron groups) distributed within hydrophobic internal layers covered by hydrophilic coats [32]. The nature and distribution of the surface functional groups were established using FTIR spectroscopy [73] and XRD analysis [74]. Biochar obtained at low temperatures contains a shell formed by furan-like pentatonic rings (60% of carbon atoms) bridged with sp2- (sp3-) C atoms [75]. At higher temperatures, such layers are converted into condensed aromatic rings to form graphitic particles. Based on solid-phase 13C NMR, FT-IR spectroscopy and GC-MS, the conversion starts at 270 °C and is accompanied by the evolution of volatile furan derivatives [76].
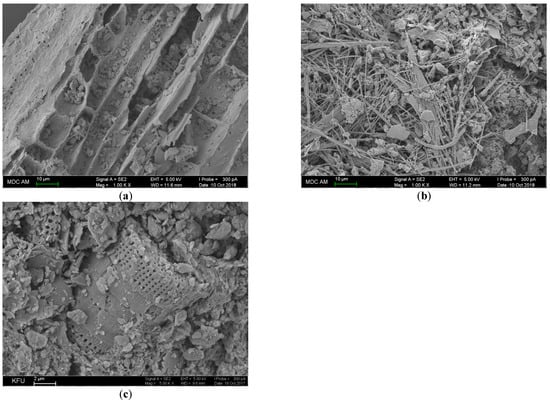
The biochar particle morphology results from the chemical reactions during pyrolysis. At low temperature (at about 300 °C) free radicals appear [77]. The following heating destroys cellulose with the formation of anhydrosugars that are less reactive than radical products of the bond cleavage. Being volatile, such intermediates are condensed in the pores of biochar and then in secondary carbonization form a typical crystal lamellar structure [78]. The surface groups of biochar depend on the pyrolysis rate. Oxygen containing hydroxide and carboxylic groups are mostly present on the materials obtained in fast pyrolysis, whereas aromatic C-H bonds are mostly found on the surface of slow pyrolysis products [79]. Heating changes ratio of main components and structural properties (Figure 3).

Figure 3. SEM images from biochar prepared (a)—from plant residues, (b)—from chicken manure, and (c)—from sewage sludge.
The structure of the biochar particles changes slightly with the heating rate but is sensitive to the final pyrolysis temperature. With its increase, crystal fragments become bigger and their internal structure—more regular [80]. The variety of the structure fragments is higher in fast pyrolysis. As gases are released from the solid matrix, bulk density decreases. This results in a lower bulk density that also takes into account free space between the biochar particles. Thus, pyrolysis of woody biomass decreases density twofold against feedstock at the pyrolysis temperature of 350 °C, which is considered a lower limit of fastest changes of biochar porosity [81].
It should be mentioned that the lower density of biochar against substrate is related to bulk density. The so-called true density describing solid phase disregarding pore space increases with residence time and pyrolysis temperature. Shrinking biomass during pyrolysis can also result in incomplete removal of volatiles, especially in low temperature pyrolysis [82].
Changes in the specific surface area during pyrolysis follow porosity increase. Thus, it starts raising at 400–500 °C until 900 °C from about 10 to 100–500 m2/g depending on the feedstock and the pyrolysis conditions [83][84]. The following heating above 900 °C leaves surface area constant or slowly decreasing. This was attributed to changes in the particle structure resulting in pore widening or collapse of some pore walls. In addition, decreased area can be influenced by ash melting and fusing [85]. Similar consequences are expected from secondary reactions between char and volatiles remaining entrapped in the inner pore volume [86] and from crystallization of amorphous carbon into graphite [87]. Thus, the average pore size of the biochar particles obtained from sewage sludge increased from 4.7 nm (feedstock) to 8 and 28 nm at 500 and 600 °C and then decreased to 16 nm at 700 °C [88].
2.4.2. Chemical Properties
The chemical composition of biochar is mostly determined by carbonization resulting from the loss of hydrogen and oxygen containing groups. The progress of carbonization is reflected by changes in the atomic rations. Regarding three main elements, i.e., carbon, oxygen and hydrogen, such changes are expressed by a diagram presented first by D. van Kleveren [89]. In natural carbonization there are various natural ways of carbonization. However, the release of oxygen is twofold faster than that of hydrogen until coal is formed. After that, the decrease in the H/C ratio becomes constant if the oxygen content is maintained at a low level. In technical conditions, the relative decrease of the O/C and H/C molar ratios can vary, but contrary to natural conditions, the appropriate rates do not change within the pyrolysis duration.
Carbon, hydrogen, oxygen and minor quantities of nitrogen are the main components of biochar. Elemental composition follows changes in the atomic ratios corresponding to the appropriate temperature of pyrolysis and heating rate. In addition, moisture, silicon, phosphorus and metal residues should be considered. The particular content of biochar highly depends on the substrate used. Carbon content varies commonly from 45 to 60 wt.%, hydrogen from 2 to 5 wt.% and oxygen at the level of 10 to 20% [90]. Inorganic components (minerals) are present in a higher amount in plant resides and algae, and in a lower amount in woody biomass. Biochar obtained from plants contains more carbon than that from manure.
In most cases, carbonization of biomass promotes increase of the pH of the product to final pH = 8–10. The higher the pyrolysis temperature, the more the pH shift. In a similar manner, pH correlates with ash content. Thus, the use of woody biomass results in the formation of the product with a pH lower than that of manure carbonization products rich in mineral components. The consideration of the pH is complicated by the changes in acidity observed within the time after implementation of biochar in soil. As an example, the pH of the biochar obtained from oak chips decreased within a year of application to soil, whereas the coals from corn wastes increased from 6.7 to 8.1 [91]. Such relationships are related to the destruction of acidic functional groups at high temperatures of pyrolysis. Another factor influencing the chemical composition of biochar is particle size. A number of authors have found that different particle sizes of raw materials can lead to different heating during pyrolysis, so a smaller fraction could be more carbonated than a larger fraction and contain more C, as well as Ca, Mg and K ions [92][93].
References
- IBI. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil; International Biochar Initiative: Bowdoinham, ME, USA, 2015; Available online: http://www.biochar-international.org/characterizationstandard (accessed on 7 August 2023).
- Xu, G.; Lv, Y.; Sun, J.; Shao, H.; Wei, L. Recent Advances in Biochar Applications in Agricultural Soils: Benefits and Environmental Implications. CLEAN—Soil Air Water 2012, 40, 1093–1098.
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal Responses to Biochar in Soil—Oncepts and Mechanisms; Springer: Berlin/Heidelberg, Germany, 2007; Volume 300, pp. 9–20.
- An, N.; Zhang, L.; Liu, Y.; Shen, S.; Li, N.; Wu, Z.; Yang, J.; Han, W.; Han, X. Biochar application with reduced chemical fertilizers improves soil pore structure and rice productivity. Chemosphere 2022, 298, 134304.
- Huang, Y.; Wang, C.; Lin, C.; Zhang, Y.; Chen, X.; Tang, L.; Liu, C.; Chen, Q.; Onwuka, M.I.; Song, T. Methane and Nitrous Oxide Flux after Biochar Application in Subtropical Acidic Paddy Soils under Tobacco-Rice Rotation. Sci. Rep. 2019, 9, 17277.
- Tang, Z.; Liu, X.; Li, G.; Liu, X. Mechanism of biochar on nitrification and denitrification to N2O emissions based on isotope characteristic values. Environ. Res. 2022, 212, 113219.
- Bruun, E.W.; Hauggaard-Nielsen, H.; Ibrahim, N.; Egsgaard, H.; Ambus, P.; Jensen, P.A.; Dam-Johansen, K. Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass. Bioenergy 2011, 35, 1182–1189.
- Verheijen, F.G.A.; Graber, E.R.; Ameloot, N.; Bastos, A.C.; Sohi, S.; Knicker, H. Biochars in soils: New insights and emerging research needs. Eur. J. Soil Sci. 2014, 65, 22–27.
- Awad, Y.M.; Blagodatskaya, E.; Ok, Y.S.; Kuzyakov, Y. Effects of polyacrylamide, biopolymer and biochar on the decomposition of 14C-labelled maize residues and on their stabilization in soil aggregates. Eur. J. Soil Biol. 2013, 64, 488–499.
- Yin, J.; Zhao, L.; Xu, X.; Li, D.; Qiu, H.; Cao, X. Evaluation of long-term carbon sequestration of biochar in soil with biogeochemical field model. Sci. Total Environ. 2022, 822, 153576.
- Kuryntseva, P.; Galitskaya, P.; Selivanovskaya, S. Optimization of pyrolysis regime for chicken manure treatment and biochar production. Water Environ. J. 2022, 36, 270–281.
- Ennis, C.J.; Evans, A.G.; Islam, M.; Ralebitso-Senior, T.K.; Senior, E. Biochar: Carbon Sequestration, Land Remediation, and Impacts on Soil Icrobiology. Environ. Sustain. 2012, 42, 2311–2364.
- Clough, T.J.; Condron, L.M. Biochar and the Nitrogen Cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223.
- Junna, S.; Bingchen, W.; Gang, X.; Hongbo, S. Effects of wheat straw biochar on carbon mineralization and guidance for large-scale soil quality improvement in the coastal wetland. Ecol. Eng. 2014, 62, 43–47.
- Barrow, C.J. Biochar: Potential for countering land degradation and for improving agriculture. Appl. Geogr. 2012, 34, 21–28.
- Haris, M.; Hamid, Y.; Usman, M.; Wang, L.; Saleem, A.; Su, F.; Guo, J.; Li, Y. Crop-residues derived biochar: Synthesis, properties, characterization and application for the removal of trace elements in soils. J. Hazard. Mater. 2021, 416, 126212.
- Gao, J.; Zhao, Y.; Zhang, W.; Sui, Y.; Jin, D.; Xin, W.; Yi, J.; He, D. Biochar prepared at different pyrolysis temperatures affects urea-nitrogen immobilization and N2O emissions in paddy fields. PeerJ 2019, 2019, e7027.
- Yu, K.L.; Lau, B.F.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.H.; Ng, E.P.; Chang, J.S. Recent developments on algal biochar production and characterization. Bioresour. Technol. 2017, 246, 2–11.
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115.
- Qi, L.; Pokharel, P.; Chang, S.X.; Zhou, P.; Niu, H.; He, X.; Wang, Z.; Gao, M. Biochar application increased methane emission, soil carbon storage and net ecosystem carbon budget in a 2-year vegetable–rice rotation. Agric. Ecosyst. Environ. 2020, 292, 106831.
- Tsai, W.T.; Liu, S.C.; Chen, H.R.; Chang, Y.M.; Tsai, Y.L. Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 2012, 89, 198–203.
- Cely, P.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. Agronomic properties of biochars from different manure wastes. J. Anal. Appl. Pyrolysis 2015, 111, 173–182.
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285.
- Xue, Y.; Wang, C.; Hu, Z.; Zhou, Y.; Xiao, Y.; Wang, T. Pyrolysis of sewage sludge by electromagnetic induction: Biochar properties and application in adsorption removal of Pb(II), Cd(II) from aqueous solution. Waste Manag. 2019, 89, 48–56.
- Xu, G.; Zhang, Z.; Deng, L. Adsorption behaviors and removal efficiencies of inorganic, polymeric and organic phosphates from aqueous solution on biochar derived from sewage sludge of chemically enhanced primary treatment process. Water 2018, 10, 869.
- Paz-Ferreiro, J.; Nieto, A.; Méndez, A.; Askeland, M.P.J.; Gascó, G. Biochar from biosolids pyrolysis: A review. Int. J. Environ. Res. Public Health 2018, 15, 956.
- Mani, S.; Sokhansanj, S.; Bi, X.; Turhollow, A. Economics of producing fuel pellets from biomass. Appl. Eng. Agric. 2006, 22, 421–426.
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69.
- Posom, J.; Saechua, W.; Sirisomboon, P. Evaluation of pyrolysis characteristics of milled bamboo using near-infrared spectroscopy. Renew. Energy 2017, 103, 653–665.
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716.
- Blanco-Canqui, H. Crop Residue Removal for Bioenergy Reduces Soil Carbon Pools: How Can We Offset Carbon Losses? Bioenergy Res. 2013, 6, 358–371.
- Fuertes, A.B.; Arbestain, M.C.; Sevilla, M.; MacIá-Agulló, J.A.; Fiol, S.; López, R.; Smernik, R.J.; Aitkenhead, W.P.; Arce, F.; MacIas, F. Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Aust. J. Soil Res. 2010, 48, 618–626.
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145.
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in Preparation and Application of Modified Biochar for Improving Heavy Metal Ion Removal From Wastewater. J. Bioresour. Bioprod. 2019, 4, 31–42.
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrological properties of biochars produced at different temperatures. Biomass Bioenergy 2012, 41, 34–43.
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212.
- Mašek, O.; Budarin, V.; Gronnow, M.; Crombie, K.; Brownsort, P.; Fitzpatrick, E.; Hurst, P. Microwave and slow pyrolysis biochar—Comparison of physical and functional properties. J. Anal. Appl. Pyrolysis 2013, 100, 41–48.
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46.
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9.
- Mumme, J.; Eckervogt, L.; Pielert, J.; Diakité, M.; Rupp, F.; Kern, J. Hydrothermal carbonization of anaerobically digested maize silage. Bioresour. Technol. 2011, 102, 9255–9260.
- Hoekman, S.K.; Broch, A.; Felix, L.; Farthing, W. Hydrothermal carbonization (HTC) of loblolly pine using a continuous, reactive twin-screw extruder. Energy Convers. Manag. 2017, 134, 247–259.
- Kim, K.H.; Eom, I.Y.; Lee, S.M.; Choi, D.; Yeo, H.; Choi, I.-G.; Choi, J.W. Investigation of physicochemical properties of biooils produced from yellow poplar wood (Liriodendron tulipifera) at various temperatures and residence times. J. Anal. Appl. Pyrolysis 2011, 92, 2–9.
- Weber, K.; Quicker, P. Properties of Biochar; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 217, pp. 240–261.
- Bruun, E.W.; Ambus, P.; Egsgaard, H.; Hauggaard-Nielsen, H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol. Biochem. 2012, 46, 73–79.
- Zhao, L.; Cao, X.; Wang, Q.; Yang, F.; Xu, S. Mineral Constituents Profile of Biochar Derived from Diversified Waste Biomasses: Implications for Agricultural Applications. J. Environ. Qual. 2013, 42, 545–552.
- Sabio, E.; Álvarez-Murillo, A.; Román, S.; Ledesma, B. Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: Influence of the processing variables. Waste Manag. 2016, 47, 122–132.
- Lynam, J.G.; Coronella, C.J.; Yan, W.; Reza, M.T.; Vasquez, V.R. Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 6192–6199.
- Lynam, J.G.; Toufiq Reza, M.; Vasquez, V.R.; Coronella, C.J. Effect of salt addition on hydrothermal carbonization of lignocellulosic biomass. Fuel 2012, 99, 271–273.
- Babu, B.V. Biomass pyrolysis: A state-of-the-art review. Biofuels Bioprod. Biorefining 2008, 2, 393–414.
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504.
- Patwardhan, P.R.; Brown, R.C.; Shanks, B.H. Product distribution from the fast pyrolysis of hemicellulose. ChemSusChem 2011, 4, 636–643.
- Mu, W.; Ben, H.; Ragauskas, A.; Deng, Y. Lignin Pyrolysis Components and Upgrading-Technology Review. Bioenergy Res. 2013, 6, 1183–1204.
- Jagtoyen, M.; Derbyshire, F. Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 1998, 36, 1085–1097.
- Zhao, L.; Zheng, W.; Mašek, O.; Chen, X.; Gu, B.; Sharma, B.K.; Cao, X. Roles of Phosphoric Acid in Biochar Formation: Synchronously Improving Carbon Retention and Sorption Capacity. J. Environ. Qual. 2017, 46, 393–401.
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671.
- Liu, N.; Zhou, J.; Han, L.; Ma, S.; Sun, X.; Huang, G. Role and multi-scale characterization of bamboo biochar during poultry manure aerobic composting. Bioresour. Technol. 2017, 241, 190–199.
- Jansen, R.J.J.; van Bekkum, H. Amination and ammoxidation of activated carbons. Carbon 1994, 32, 1507–1516.
- Shafeeyan, M.S.; Wan Daud, W.M.A.; Houshmand, A.; Arami-Niya, A. The application of response surface methodology to optimize the amination of activated carbon for the preparation of carbon dioxide adsorbents. Fuel 2012, 94, 465–472.
- Anfruns, A.; García-Suárez, E.J.; Montes-Morán, M.A.; Gonzalez-Olmos, R.; Martin, M.J. New insights into the influence of activated carbon surface oxygen groups on H2O2 decomposition and oxidation of pre-adsorbed volatile organic compounds. Carbon 2014, 77, 89–98.
- Wu, X.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Responses of soil microbial community to different concentration of fomesafen. J. Hazard. Mater. 2014, 273, 155–164.
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359.
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576.
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204.
- Xin-hui, D.; Srinivasakannan, C.; Jin-hui, P.; Li-bo, Z.; Zheng-yong, Z. Comparison of activated carbon prepared from Jatropha hull by conventional heating and microwave heating. Biomass Bioenergy 2011, 35, 3920–3926.
- Yang, K.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Xia, H.; Duan, X. Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour. Technol. 2010, 101, 6163–6169.
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293.
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.W.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291.
- Frišták, V.; Micháleková-Richveisová, B.; Víglašová, E.; Ďuriška, L.; Galamboš, M.; Moreno-Jimenéz, E.; Pipíška, M.; Soja, G. Sorption separation of Eu and As from single-component systems by Fe-modified biochar: Kinetic and equilibrium study. J. Iran. Chem. Soc. 2017, 14, 521–530.
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395.
- Shang, M.R.; Liu, Y.G.; Liu, S.B.; Zeng, G.M.; Tan, X.F.; Jiang, L.H.; Huang, X.X.; Ding, Y.; Guo, Y.M.; Wang, S.F. A novel graphene oxide coated biochar composite: Synthesis, characterization and application for Cr(VI) removal. RSC Adv. 2016, 6, 85202–85212.
- Inyang, M.; Gao, B.; Zimmerman, A.; Zhou, Y.; Cao, X. Sorption and cosorption of lead and sulfapyridine on carbon nanotube-modified biochars. Environ. Sci. Pollut. Res. 2015, 22, 1868–1876.
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. In Biochar for Environmental Management: Science and Technology; Routledge: Abingdon, UK, 2012; ISBN 9781849770552.
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG-FTIR and Py-GC-FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206.
- Kappler, A.; Wuestner, M.L.; Ruecker, A.; Harter, J.; Halama, M.; Behrens, S. Biochar as an Electron Shuttle between Bacteria and Fe(III) Minerals. Environ. Sci. Technol. Lett. 2014, 1, 339–344.
- Baccile, N.; Laurent, G.; Babonneau, F.; Fayon, F.; Titirici, M.M.; Antonietti, M. Structural characterization of hydrothermal carbon spheres by advanced solid-state MAS 13C NMR investigations. J. Phys. Chem. C 2009, 113, 9644–9654.
- Pastorova, I.; Botto, R.E.; Arisz, P.W.; Boon, J.J. Cellulose char structure: A combined analytical Py-GC-MS, FTIR, and NMR study. Carbohydr. Res. 1994, 262, 27–47.
- Shafizadeh, F. Introduction to pyrolysis of biomass. J. Anal. Appl. Pyrolysis 1982, 3, 283–305.
- Wornat, M.J.; Hurt, R.H.; Yang, N.Y.C.; Headley, T.J. Structural and compositional transformations of biomass chars during combustion. Combust. Flame 1995, 100, 131–143.
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396.
- Lua, A.C.; Yang, T.; Guo, J. Effects of pyrolysis conditions on the properties of activated carbons prepared from pistachio-nut shells. J. Anal. Appl. Pyrolysis 2004, 72, 279–287.
- Plötze, M.; Niemz, P. Porosity and pore size distribution of different wood types as determined by mercury intrusion porosimetry. Eur. J. Wood Wood Prod. 2011, 69, 649–657.
- Jimenez-Cordero, D.; Heras, F.; Alonso-Morales, N.; Gilarranz, M.A.; Rodriguez, J.J. Porous structure and morphology of granular chars from flash and conventional pyrolysis of grape seeds. Biomass Bioenergy 2013, 54, 123–132.
- Cetin, E.; Gupta, R.; Moghtaderi, B. Effect of pyrolysis pressure and heating rate on radiata pine char structure and apparent gasification reactivity. Fuel 2005, 84, 1328–1334.
- Wafiq, A.; Reichel, D.; Hanafy, M. Pressure influence on pyrolysis product properties of raw and torrefied Miscanthus: Role of particle structure. Fuel 2016, 179, 156–167.
- Fu, P.; Hu, S.; Xiang, J.; Sun, L.; Li, P.; Zhang, J.; Zheng, C. Pyrolysis of maize stalk on the characterization of chars formed under different devolatilization conditions. Energy Fuels 2009, 23, 4605–4611.
- Burhenne, L.; Damiani, M.; Aicher, T. Effect of feedstock water content and pyrolysis temperature on the structure and reactivity of spruce wood char produced in fixed bed pyrolysis. Fuel 2013, 107, 836–847.
- Chen, Y.; Yang, H.; Wang, X.; Zhang, S.; Chen, H. Biomass-based pyrolytic polygeneration system on cotton stalk pyrolysis: Influence of temperature. Bioresour. Technol. 2012, 107, 411–418.
- Yuan, H.; Lu, T.; Huang, H.; Zhao, D.; Kobayashi, N.; Chen, Y. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289.
- Van Krevelen, D. Graphical statistical method for the study of structure and reaction processes of coal. Fuel 1950, 29, 269–284.
- Mullen, C.A.; Boateng, A.A.; Goldberg, N.M.; Lima, I.M.; Laird, D.A.; Hicks, K.B. Bio-oil and bio-char production from corn cobs and stover by fast pyrolysis. Biomass Bioenergy 2010, 34, 67–74.
- Cheng, C.-H.H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488.
- He, P.; Liu, Y.; Shao, L.; Zhang, H.; Lü, F. Particle size dependence of the physicochemical properties of biochar. Chemosphere 2018, 212, 385–392.
- Prasad, M.; Chrysargyris, A.; McDaniel, N.; Kavanagh, A.; Gruda, N.S.; Tzortzakis, N. Plant nutrient availability and pH of biochars and their fractions, with the possible use as a component in a growing media. Agronomy 2020, 10, 10.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
493
Revisions:
2 times
(View History)
Update Date:
17 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




