Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Min-Hsien Wu | -- | 1954 | 2023-11-14 13:06:35 | | | |
| 2 | Fanny Huang | Meta information modification | 1954 | 2023-11-15 07:56:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nguyen, T.N.A.; Huang, P.; Chu, P.; Hsieh, C.; Wu, M. Characteristics of Circulating Tumor Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/51542 (accessed on 02 March 2026).
Nguyen TNA, Huang P, Chu P, Hsieh C, Wu M. Characteristics of Circulating Tumor Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/51542. Accessed March 02, 2026.
Nguyen, Thi Ngoc Anh, Po-Shuan Huang, Po-Yu Chu, Chia-Hsun Hsieh, Min-Hsien Wu. "Characteristics of Circulating Tumor Cells" Encyclopedia, https://encyclopedia.pub/entry/51542 (accessed March 02, 2026).
Nguyen, T.N.A., Huang, P., Chu, P., Hsieh, C., & Wu, M. (2023, November 14). Characteristics of Circulating Tumor Cells. In Encyclopedia. https://encyclopedia.pub/entry/51542
Nguyen, Thi Ngoc Anh, et al. "Characteristics of Circulating Tumor Cells." Encyclopedia. Web. 14 November, 2023.
Copy Citation
Circulating tumor cells (CTCs), as novel cancer biomarkers, were first described by Dr. Ashworth in 1869, and they were found to exist in the blood circulation of cancer patients with distant metastases. Analysis of circulating tumor cells (CTCs) holds promise to diagnose cancer or monitor its development.
circulating tumor cells
CTCs
CTC counts
Multiparameter
1. Introduction
Cancer has been a leading threat to global health in recent decades. According to World Health Organization (WHO) statistics, the number of people dying from cancer in 2018 was approximately 9.6 million [1]. In particular, 90% of cancer deaths are related to metastasis, highlighting the importance of early detection in the treatment of cancer [2]. Indeed, the survival rate for almost all cancers significantly improves when they are identified, diagnosed, and treated in their early stages [3][4]. The occurrence of cancer involves the imbalance of many complex molecular mechanisms and regulatory pathways. Based on these pathogenic mechanisms, the progress of biomarkers and target drugs in recent years has, indeed, brought progress to the diagnosis and treatment of cancer [5]. Simultaneously, researchers have also been actively searching for optimal cancer biomarkers or combinations that can be used for early cancer diagnosis and subsequent development monitoring.
Circulating tumor cells (CTCs), as novel cancer biomarkers, were first described by Dr. Ashworth in 1869 [6], and they were found to exist in the blood circulation of cancer patients with distant metastases. Detecting CTCs in blood samples is valuable due to the morphological resemblance between CTCs and primary tumor cells [6]. It holds significant promise in assessing and predicting the status of the primary tumor, offering substantial potential for a wide range of clinical cancer applications [7][8]. Previous research has shown that tumor cells can be transmitted even in the early stages of tumor development [9], which reveals that CTCs could be used for early cancer detection. In other aspects of clinical applications, counting CTC numbers in cancer patients’ peripheral blood samples was also reported to provide valuable insights into cancer prognosis [10][11]. For example, a higher CTC count has been demonstrated to correlate with more advanced disease stages, a poorer prognosis, and more sites of metastasis [12]. Meanwhile, monitoring changes in CTC numbers over time can help clinicians track the trajectory of cancer progression during treatment and adjust treatment programs in a real-time manner [13]. Taken together, counting and monitoring CTC numbers holds high potential for early cancer detection, prognosis evaluation, and therapeutic response monitoring for cancer patients.
Even though the clinical applications of CTCs are expected, precise counting of CTCs from surrounding blood cells remains technically challenging, mainly due to the rarity of CTCs (i.e., 1–10 CTCs in a 10 mL cancer patient’s blood sample) and the lack of unique CTC markers. To tackle the hurdles, the Cell Search platform, as the first and only FDA-approved system, was developed for the isolation, identification, and counting of CTCs based on multi-marker staining (i.e., EpCAM+, CK+, CD45−, and DAPI+) [14][15]. Its application was reported to predict the cancer progression or death of patients with various solid cancers (e.g., metastatic prostate cancer [16], breast cancer [17], colorectal cancer [18], head and neck cancer [15], and pancreatic cancer [19]. With advancements in cell analysis technologies, however, researchers also found that there is an issue of heterogeneity in CTCs in terms of genetic mutations, surface markers, and biological properties [20]. Moreover, certain specific subpopulations of CTCs (e.g., EpCAM− CTCs or CTC clusters) have been shown to play a critical role in cancer metastasis and the development of therapy resistance [21]. Their detection and counting are also clinically meaningful regarding cancer treatment or care. Therefore, based on the facts mentioned above, the conventional CTC marker-staining scheme (i.e., EpCAM+, CK+, CD45−, and cell nucleus+) might not be able to identify all CTC subpopulations. This highlights the possibility that current CTC detection methods might not be able to provide precise enough information for cancer care.
To overcome the bottleneck coming across in recent CTC detection, the combination of conventional CTC counting with other clinical parameters (e.g., CTC clusters, tumor blood markers, tumor imaging, personal physiological parameters, medical history, and cancer screening tests) for analysis could provide more precise detection results for clinical applications. For instance, in early cancer detection, CTC counts can be combined with some blood tumor markers (e.g., CEA, CA125, CYFRA21, and SCC) for analysis to improve the accuracy of lung cancer detection and even differentiate malignant pulmonary nodules (MPNs) from benign pulmonary nodules (BPNs) [22]. In addition to blood tumor markers, combining CTC counts with tumor imaging data has also shown its value in early cancer detection. Whether ultrasound (US) or mammogram (MMG), it has been confirmed that the combination of tumor imaging data with conventional CTC counts can help clinicians rapidly detect breast cancer at an early stage [23]. Similarly, the combination of CTC counts with the results of standard cancer screening tests, such as the immunochemical fecal occult blood test (iFOBT), also has a high potential to identify patients with colorectal cancer at an early stage [24]. Apart from early cancer detection, the integration of CTC counts, and other cancer-related parameters, has been reported to improve the prognostic assessment of cancer patients. For example, the information from the combined analysis of CTC counts and CTC clusters can be used to predict breast cancer patient outcomes (e.g., cancer progression or cancer death) [25] or the recurrence probability in colorectal cancer patients [26]. In terms of lung cancer, moreover, the combination of CTC counts with computed tomography (CT) can more accurately distinguish lung cancer types with different invasive capabilities and provide patients with more accurate follow-up treatment [27]. However, compared with the diagnosis and prognosis assessment of cancer, the studies relevant to the combination of CTC counts and other parameters for monitoring cancer treatment response are relatively few.
2. Characteristics of CTCs
CTCs are a population of cancer cells detached from the primary tumor which enter the bloodstream, and their existence is highly correlated with cancer metastasis [28]. In cancer metastasis, CTCs can spread through human blood circulation and potentially lead to new distant metastatic lesion formation when CTCs are trapped in the capillaries of organs or tissues [29]. Based on the abovementioned phenomenon, the detection of CTCs in blood samples from cancer patients was regarded as an indicator for monitoring the status of the primary tumor and evaluating its development. Although researchers first observed CTCs in the blood of metastatic cancer patients in 1869 [6], the significance and application of CTCs in clinical cancer diagnosis and treatment were not accepted until advances in cell enrichment and isolation techniques were made. In particular, CTCs existing in blood circulation could be easily sampled by a vein blood draw, offering a promising, minimally invasive “liquid biopsy” method for oncologists to monitor and evaluate the status of nonhematologic cancers as a feasible alternative to current highly invasive tissue biopsies [30][31][32] (Figure 1).
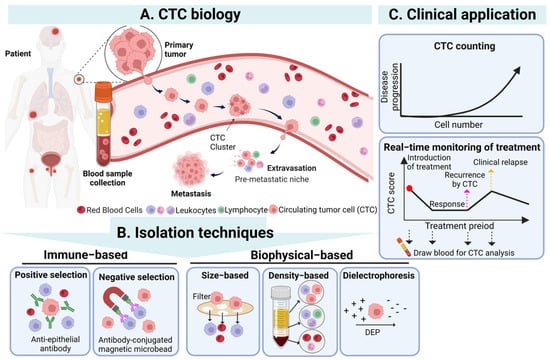
Figure 1. The overview of CTCs’ biology, isolation techniques, and clinical application of CTC. (A) CTCs detach from the primary tumor, enter the blood, and then undergo the stages of intravasation, circulation, and extravasation to reach the occurrence of distant metastasis of the tumor. (B) CTCs in the blood can be isolated through immunological and biophysical principles. Immunological methods include positive and negative screening, while biophysical methods include based-on-size, density, and dielectrophoresis. (C) The isolated CTCs can be used clinically as a biomarker and an effective tool for diagnosis, prognosis, and treatment monitoring. Created with Biorender. Abbreviations: DEP, dielectrophoresis.
In addition, in clinical cancer research, many studies have demonstrated that CTC numbers in the blood samples of cancer patients could be a biomarker for evaluating cancer progression. For example, in a lung cancer study, the results revealed that the CTC numbers in a blood sample of a patient would gradually increase along with the patient’s cancer stage) [12]. Specifically, counts below 3 CTCs/mL blood sample tended to correspond to stage I cancer, 3–20 CTCs/mL corresponded to stage II or III cancer, and exceeding 20 CTCs/mL exhibited a high risk of malignancy and distant metastasis, typically corresponding to stage IV cancer [33]. On average, a patient with metastatic carcinoma typically has a range of 5 to 50 CTCs/7.5 mL of blood [34]. The detection of higher CTC numbers might indicate a more aggressive disease state and a high recurrence rate for cancer patients [35][36]. In addition, as a clinical prognostic indicator, the detection of higher CTC numbers has been reported to be highly related to poorer survival rates of cancer patients in various solid cancers, such as breast, lung, prostate, and colorectal cancers [18][37][38][39]. For example, in metastatic breast cancer patients, it was found that the detection of more than 5 CTCs in 7.5 mL blood samples resulted in a shorter overall survival [17]. Moreover, according to clinical observations, the total amount of CTCs in the blood sample of cancer patients also decreased significantly after the administration of effective treatment, indicating that monitoring the change in CTC numbers can be an indicator for physicians to evaluate the cancer patient’s response to therapy [37]. Based on the abovementioned studies, counting and monitoring of CTC numbers in a specific amount of blood samples are commonly accepted as one of the feasible clinical CTC applications, especially the Cell Search system, whose mechanism of CTC counting has been FDA-approved for utilization in cancer patients’ prognosis evaluation [11].
In addition to the CTC counts, the analysis of CTCs’ characteristics also plays a pivotal role in a more comprehensive understanding of cancer biology, behavior, metastasis, and drug resistance, which can bring further applications for CTC detection [40]. Generally, owing to the heterogeneity of the primary tumor, CTCs, originating from the primary tumor, also exhibit remarkable cell heterogeneity in their genotypes, phenotypes, and morphologies [41]. For instance, Gasch et al. conducted a study that revealed that CTCs presenting heterogeneous PI3K mutations and HER2 expression were detected in metastatic breast cancer patients [42]. Furthermore, the dynamic interplay between CTCs and their microenvironments is crucial. A tumor can be viewed as an integrated ecosystem where the co-evolution of neoplastic cells within the tumor microenvironment results in a wide range of cancer cell phenotypes. This is closely related to the tumor heterogeneity of CTCs [43]. Therefore, in a study on CTC morphologies, researchers found that CTCs in blood samples had a diverse array of sizes (diameter: 12–30 μm) [44] and shapes (e.g., clusters), reflecting that cancer cells maintained their high phenotypic plasticity in the bloodstream [45]. On the other hand, detection of CTC heterogeneity also reflects cancer progression, metastasis, and adaptation to environmental changes (e.g., chemotherapeutic resistance) [46]. For example, many studies found that an aggregate consisting of two or more CTCs (called a CTC cluster) prolonged the cell survival status in the bloodstream, enhanced the probability of cancer metastasis, and led to poorer prognosis in cancer patients than a single CTC [47][48]. Moreover, epithelial-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET) serve as two critical mechanisms in cancer metastasis [49][50], also affecting the phenotypic changes of CTCs. In the EMT and MET process, the CTC phenotypes dynamically change among epithelial CTCs (E-CTCs), mesenchymal CTCs (M-CTCs), and mixed CTCs (E/M-CTCs) [51]. CTCs in early-stage cancer patients may retain the characteristics of primary tumors and tend to be more epithelial. However, in advanced-stage cancer patients, due to the cell metastasis mechanism of cells undergoing the EMT process, the pattern of CTCs becomes more mesenchymal type [52]. This phenotypic plasticity based on EMT and MET enables CTCs to adapt to different metastasis stages and increases CTCs’ drug resistance, viability, motility, and invasion abilities [49][50]. However, even though a deep understanding of the cell characteristics and heterogeneity of CTCs is essential for developing new cancer diagnosis and treatment approaches, these detection techniques are commonly costly, time consuming, and complicated to implement [53][54]. In terms of current clinical CTC applications, therefore, counting CTC numbers and monitoring their dynamic changes have become the current mainstream options for cancer early detection, prognosis evaluation, and therapeutic response monitoring owing to their relatively low cost and simple detection process [55][56].
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 2018, 68, 394–424.
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904.
- Bannister, N.; Broggio, J. Cancer survival by stage at diagnosis for England (experimental statistics): Adults diagnosed 2012, 2013 and 2014 and followed up to 2015. Prod. Collab. Public Health Engl. 2016, 1–30.
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040.
- Kumar, L.; Kumar, S.; Sandeep, K.; Patel, S.K. Therapeutic Approaches in Pancreatic Cancer: Recent Updates. Biomedicines 2023, 11, 1611.
- Ashworth, T.R. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust. Med. J. 1869, 14, 146.
- Thery, L.; Meddis, A.; Cabel, L.; Proudhon, C.; Latouche, A.; Pierga, J.-Y.; Bidard, F.-C. Circulating Tumor Cells in Early Breast Cancer. JNCI Cancer Spectr. 2019, 3, pkz026.
- Barrière, G.; Riouallon, A.; Renaudie, J.; Tartary, M.; Rigaud, M. Mesenchymal and stemness circulating tumor cells in early breast cancer diagnosis. BMC Cancer 2012, 12, 114.
- Hosseini, H.; Obradović, M.M.S.; Hoffmann, M.; Harper, K.L.; Sosa, M.S.; Werner-Klein, M.; Nanduri, L.K.; Werno, C.; Ehrl, C.; Maneck, M.; et al. Early dissemination seeds metastasis in breast cancer. Nature 2016, 540, 552–558.
- Budd, G.T.; Cristofanilli, M.; Ellis, M.J.; Stopeck, A.; Borden, E.; Miller, M.C.; Matera, J.; Repollet, M.; Doyle, G.V.; Terstappen, L.W.M.M. Circulating tumor cells versus imaging—predicting overall survival in metastatic breast cancer. Clin. Cancer Res. 2006, 12, 6403–6409.
- Janni, W.J.; Rack, B.; Terstappen, L.W.M.M.; Pierga, J.-Y.; Taran, F.-A.; Fehm, T.; Hall, C.; De Groot, M.R.; Bidard, F.-C.; Friedl, T.W.P. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin. Cancer Res. 2016, 22, 2583–2593.
- Wang, P.-P.; Liu, S.-H.; Chen, C.-T.; Lv, L.; Li, D.; Liu, Q.-Y.; Liu, G.-L.; Wu, Y. Circulating tumor cells as a new predictive and prognostic factor in patients with small cell lung cancer. J. Cancer 2020, 11, 2113–2122.
- Kazunori, O.; Hiroo, I.; Hiroshi, S.; Keigo, K.; Chikashi, I.; Hiroyuki, S. Practical Utility of Circulating Tumour Cells as Biomarkers in Cancer Chemotherapy for Advanced Colorectal Cancer. Anticancer Res. 2013, 33, 625.
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.J.; Uhr, J.W.; Terstappen, L.W.M.M. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904.
- Garrel, R.; Mazel, M.; Perriard, F.; Vinches, M.; Cayrefourcq, L.; Guigay, J.; Digue, L.; Aubry, K.; Alfonsi, M.; Delord, J.-P. Circulating tumor cells as a prognostic factor in recurrent or metastatic head and neck squamous cell carcinoma: The CIRCUTEC prospective study. Clin. Chem. 2019, 65, 1267–1275.
- Goldkorn, A.; Ely, B.; Quinn, D.I.; Tangen, C.M.; Fink, L.M.; Xu, T.; Twardowski, P.; Van Veldhuizen, P.J.; Agarwal, N.; Carducci, M.A. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: A phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014, 32, 1136.
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791.
- Abdalla, T.S.A.; Meiners, J.; Riethdorf, S.; König, A.; Melling, N.; Gorges, T.; Karstens, K.-F.; Izbicki, J.R.; Pantel, K.; Reeh, M. Prognostic value of preoperative circulating tumor cells counts in patients with UICC stage I-IV colorectal cancer. PLoS One 2021, 16, e0252897.
- Effenberger, K.E.; Schroeder, C.; Hanssen, A.; Wolter, S.; Eulenburg, C.; Tachezy, M.; Gebauer, F.; Izbicki, J.R.; Pantel, K.; Bockhorn, M. Improved risk stratification by circulating tumor cell counts in pancreatic cancer. Clin. Cancer Res. 2018, 24, 2844–2850.
- Xu, J.; Liao, K.; Yang, X.; Wu, C.; Wu, W. Using single-cell sequencing technology to detect circulating tumor cells in solid tumors. Mol. Cancer 2021, 20, 104.
- Hanssen, A.; Wagner, J.; Gorges, T.M.; Taenzer, A.; Uzunoglu, F.G.; Driemel, C.; Stoecklein, N.H.; Knoefel, W.T.; Angenendt, S.; Hauch, S.; et al. Characterization of different CTC subpopulations in non-small cell lung cancer. Sci. Rep. 2016, 6, 28010.
- Ma, G.; Yang, D.; Li, Y.; Li, M.; Li, J.; Fu, J.; Peng, Z. Combined measurement of circulating tumor cell counts and serum tumor marker levels enhances the screening efficiency for malignant versus benign pulmonary nodules. Thorac. Cancer 2022, 13, 3393–3401.
- Gao, Y.; Fan, W.-H.; Duan, C.; Zhao, W.; Zhang, J.; Kang, X. Enhancing the screening efficiency of breast cancer by combining conventional medical imaging examinations with circulating tumor cells. Front. Oncol. 2021, 11, 643003.
- Tsai, W.-S.; Hung, W.-S.; Wang, T.-M.; Liu, H.; Yang, C.-Y.; Wu, S.-M.; Hsu, H.-L.; Hsiao, Y.-C.; Tsai, H.-J.; Tseng, C.-P. Circulating tumor cell enumeration for improved screening and disease detection of patients with colorectal cancer. Biomed. J. 2021, 44, S190–S200.
- Costa, C.; Muinelo-Romay, L.; Cebey-López, V.; Pereira-Veiga, T.; Martínez-Pena, I.; Abreu, M.; Abalo, A.; Lago-Lestón, R.M.; Abuín, C.; Palacios, P.; et al. Analysis of a Real-World Cohort of Metastatic Breast Cancer Patients Shows Circulating Tumor Cell Clusters (CTC-clusters) as Predictors of Patient Outcomes. Cancers 2020, 12, 1111.
- Chu, H.-Y.; Yang, C.-Y.; Yeh, P.-H.; Hsu, C.-J.; Chang, L.-W.; Chan, W.-J.; Lin, C.-P.; Lyu, Y.-Y.; Wu, W.-C.; Lee, C.-W.; et al. Highly Correlated Recurrence Prognosis in Patients with Metastatic Colorectal Cancer by Synergistic Consideration of Circulating Tumor Cells/Microemboli and Tumor Markers CEA/CA19-9. Cells 2021, 10, 1149.
- Zhang, W.; Duan, X.; Zhang, Z.; Yang, Z.; Zhao, C.; Liang, C.; Liu, Z.; Cheng, S.; Zhang, K. Combination of CT and telomerase-positive circulating tumor cells improves diagnosis of small pulmonary nodules. JCI Insight 2021.
- Lara, O.; Tong, X.; Zborowski, M.; Chalmers, J.J. Enrichment of rare cancer cells through depletion of normal cells using density and flow-through, immunomagnetic cell separation. Exp. Hematol. 2004, 32, 891–904.
- Adams, A.A.; Okagbare, P.I.; Feng, J.; Hupert, M.L.; Patterson, D.; Göttert, J.; McCarley, R.L.; Nikitopoulos, D.; Murphy, M.C.; Soper, S.A. Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor. J. Am. Chem. Soc. 2008, 130, 8633–8641.
- Mocellin, S.; Hoon, D.; Ambrosi, A.; Nitti, D.; Rossi, C.R. The prognostic value of circulating tumor cells in patients with melanoma: A systematic review and meta-analysis. Clin. Cancer Res. 2006, 12, 4605–4613.
- Smerage, J.; Hayes, D. The measurement and therapeutic implications of circulating tumour cells in breast cancer. Br. J. Cancer 2006, 94, 8–12.
- Braun, S.; Marth, C. Circulating tumor cells in metastatic breast cancer—toward individualized treatment? N. Engl. J. Med. 2004, 351, 824–826.
- Ried, K.; Eng, P.; Sali, A. Screening for Circulating Tumour Cells Allows Early Detection of Cancer and Monitoring of Treatment Effectiveness: An Observational Study. Asian Pac. J. Cancer Prev. 2017, 18, 2275–2285.
- Gold, B.; Cankovic, M.; Furtado, L.V.; Meier, F.; Gocke, C.D. Do Circulating Tumor Cells, Exosomes, and Circulating Tumor Nucleic Acids Have Clinical Utility? J. Mol. Diagn. 2015, 17, 209–224.
- Li, Z.; Xu, K.; Tartarone, A.; Santarpia, M.; Zhu, Y.; Jiang, G. Circulating tumor cells can predict the prognosis of patients with non-small cell lung cancer after resection: A retrospective study. Transl. Lung Cancer Res. 2021, 10, 995–1006.
- Park, Y.; Jun, H.R.; Choi, H.W.; Hwang, D.W.; Lee, J.H.; Song, K.B.; Lee, W.; Kwon, J.; Ha, S.H.; Jun, E.; et al. Circulating tumour cells as an indicator of early and systemic recurrence after surgical resection in pancreatic ductal adenocarcinoma. Sci. Rep. 2021, 11, 1644.
- Deutsch, T.M.; Stefanovic, S.; Feisst, M.; Fischer, C.; Riedel, F.; Fremd, C.; Domschke, C.; Pantel, K.; Hartkopf, A.D.; Sutterlin, M.; et al. Cut-Off Analysis of CTC Change under Systemic Therapy for Defining Early Therapy Response in Metastatic Breast Cancer. Cancers 2020, 12, 1055.
- Kang, B.J.; Ra, S.W.; Lee, K.; Lim, S.; Son, S.H.; Ahn, J.-J.; Kim, B.C. Circulating Tumor Cell Number Is Associated with Primary Tumor Volume in Patients with Lung Adenocarcinoma. Tuberc. Respir. Dis. 2020, 83, 61.
- Goldkorn, A.; Tangen, C.; Plets, M.; Morrison, G.J.; Cunha, A.; Xu, T.; Pinski, J.K.; Ingles, S.A.; Triche, T.; Harzstark, A.L. Baseline circulating tumor cell count as a prognostic marker of PSA response and disease progression in metastatic castrate-sensitive prostate cancer (SWOG S1216). Clin. Cancer. Res. 2021, 27, 1967–1973.
- Lozar, T.; Gersak, K.; Cemazar, M.; Kuhar, C.G.; Jesenko, T. The biology and clinical potential of circulating tumor cells. Radiol. Oncol. 2019, 53, 131–147.
- Bulfoni, M.; Turetta, M.; Del Ben, F.; Di Loreto, C.; Beltrami, A.P.; Cesselli, D. Dissecting the heterogeneity of circulating tumor cells in metastatic breast cancer: Going far beyond the needle in the haystack. Int. J. Mol. Sci. 2016, 17, 1775.
- Gasch, C.; Oldopp, T.; Mauermann, O.; Gorges, T.M.; Andreas, A.; Coith, C.; Müller, V.; Fehm, T.; Janni, W.; Pantel, K.; et al. Frequent detection of PIK3CA mutations in single circulating tumor cells of patients suffering from HER2-negative metastatic breast cancer. Mol. Oncol. 2016, 10, 1330–1343.
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics 2023, 13, 1607–1631.
- Rostami, P.; Kashaninejad, N.; Moshksayan, K.; Saidi, M.S.; Firoozabadi, B.; Nguyen, N.-T. Novel approaches in cancer management with circulating tumor cell clusters. J. Sci. Adv. Mater. Devices 2019, 4, 1–18.
- Harouaka, R.A.; Nisic, M.; Zheng, S.-Y. Circulating tumor cell enrichment based on physical properties. J. Lab. Autom. 2013, 18, 455–468.
- Hodgkinson, C.L.; Morrow, C.J.; Li, Y.; Metcalf, R.L.; Rothwell, D.G.; Trapani, F.; Polanski, R.; Burt, D.J.; Simpson, K.L.; Morris, K. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 2014, 20, 897–903.
- Liu, X.; Taftaf, R.; Kawaguchi, M.; Chang, Y.-F.; Chen, W.; Entenberg, D.; Zhang, Y.; Gerratana, L.; Huang, S.; Patel, D.B. Homophilic CD44 Interactions Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer ModelsHomophilic CD44 Interactions Mediate Tumor Cell Aggregation. Cancer Discov. 2019, 9, 96–113.
- Beans, C. Targeting metastasis to halt cancer’s spread. Proc. Natl. Acad. Sci. USA 2018, 115, 12539–12543.
- Pore, A.A.; Bithi, S.S.; Zeinali, M.; Navaid, H.B.; Nagrath, S.; Layeequr Rahman, R.; Vanapalli, S.A. Phenotyping of rare circulating cells in the blood of non-metastatic breast cancer patients using microfluidic Labyrinth technology. Biomicrofluidics 2022, 16, 064107.
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306.
- Semaan, A.; Bernard, V.; Kim, D.U.; Lee, J.J.; Huang, J.; Kamyabi, N.; Stephens, B.M.; Qiao, W.; Varadhachary, G.R.; Katz, M.H.; et al. Characterisation of circulating tumour cell phenotypes identifies a partial-EMT sub-population for clinical stratification of pancreatic cancer. Br. J. Cancer 2021, 124, 1970–1977.
- Kallergi, G.; Papadaki, M.A.; Politaki, E.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011, 13, R59.
- Zhong, Y.; Ma, T.; Qiao, T.; Hu, H.; Li, Z.; Luo, K.; Wang, Y.; Tang, Q.; Wang, G.; Huang, R.; et al. Role of Phenotypes of Circulating Tumor Cells in the Diagnosis and Treatment of Colorectal Cancer. Cancer Manag. Res. 2021, 13, 7077–7085.
- Saxena, K.; Subbalakshmi, A.R.; Jolly, M.K. Phenotypic heterogeneity in circulating tumor cells and its prognostic value in metastasis and overall survival. EBioMedicine 2019, 46, 4–5.
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013, 339, 580–584.
- Tamminga, M.; de Wit, S.; Hiltermann, T.J.N.; Timens, W.; Schuuring, E.; Terstappen, L.W.M.M.; Groen, H.J.M. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 173.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
572
Revisions:
2 times
(View History)
Update Date:
15 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





