Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nianqiao Gong | -- | 4269 | 2023-11-13 16:48:16 | | | |
| 2 | Camila Xu | Meta information modification | 4269 | 2023-11-14 02:23:44 | | | | |
| 3 | Camila Xu | -81 word(s) | 4188 | 2023-11-14 02:24:00 | | | | |
| 4 | Camila Xu | -31 word(s) | 4157 | 2023-11-15 09:45:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, J.; Zhao, Y.; Gong, N. XBP1 Modulates the Aging Cardiorenal System. Encyclopedia. Available online: https://encyclopedia.pub/entry/51490 (accessed on 07 February 2026).
Zhang J, Zhao Y, Gong N. XBP1 Modulates the Aging Cardiorenal System. Encyclopedia. Available at: https://encyclopedia.pub/entry/51490. Accessed February 07, 2026.
Zhang, Ji, Yuanyuan Zhao, Nianqiao Gong. "XBP1 Modulates the Aging Cardiorenal System" Encyclopedia, https://encyclopedia.pub/entry/51490 (accessed February 07, 2026).
Zhang, J., Zhao, Y., & Gong, N. (2023, November 13). XBP1 Modulates the Aging Cardiorenal System. In Encyclopedia. https://encyclopedia.pub/entry/51490
Zhang, Ji, et al. "XBP1 Modulates the Aging Cardiorenal System." Encyclopedia. Web. 13 November, 2023.
Copy Citation
X-box binding protein 1 (XBP1) is a unique basic-region leucine zipper (bZIP) transcription factor. Over recent years, the powerful biological functions of XBP1 in oxidative stress have been gradually revealed. When the redox balance remains undisturbed, oxidative stress plays a role in physiological adaptations and signal transduction. However, during the aging process, increased cellular senescence and reduced levels of endogenous antioxidants cause an oxidative imbalance in the cardiorenal system.
XBP1
aging
cardiorenal system
oxidative stress
1. Introduction
As research efforts have intensified, a complex hemodynamic and neurohumoral connection between the heart and the kidneys has become highly evident [1][2]. Even in the absence of damage, the glomerular filtration rate (GFR) is known to decrease by approximately 8 mL/min/1.73 m2 per decade after the age of 40 years [3]. Furthermore, research has shown that the incidence of acute kidney injury (AKI) is higher in older adults [4]. The progression of kidney damage leads to an expansion of blood volume, along with an upregulation of the sympathetic nervous system (SNS) and the renin–angiotensin–aldosterone system (RAAS); these changes exert numerous maladaptive systemic effects on the heart, vasculature, and kidneys [5][6][7][8]. Compared to young hearts, aged hearts are more susceptible to both acute and chronic damage [9]. Aged hearts have little protection against such injury [10]; thus, heart failure is a typical disease related to the elderly [11]. Consequent venous congestion, inadequate renal blood flow, and poor renal perfusion result in renal dysfunction attributed to a decline in renal blood flow and glomerular filtration rate (GFR), along with reduced urinary output [12][13][14]. In addition, these changes elevate the activity of SNS and the release of renin from the juxtamedullary apparatus, thus resulting in sodium retention and enhanced vascular congestion, further exacerbating heart failure [8][14]. In a previous study, Claudio et al. defined five types of cardiorenal syndromes based on pathophysiology, time frame, and the presence or absence of cardiac and renal dysfunction. Types 1 and 2 are caused by acute heart injury and chronic heart failure, respectively, while types 3 and 4 are caused by AKI and chronic kidney disease (CKD), respectively. Type 5 is caused by other systemic diseases such as sepsis and diabetes [15].
Although a variety of factors can cause senescence-related cardiorenal interactions, a strong body of evidence indicates that oxidative stress plays a central role [14][16] via age-induced metabolic, hemodynamic, neurohormonal, and inflammatory mechanisms, and atherosclerotic degeneration [17][18][19]. Aging is highly correlated to the process of mitochondrial dysfunction and causes the accumulation of oxidative stress in cardiomyocytes and nephrocytes [17][20][21]. Furthermore, oxidative stress participates in the generation of biomarkers that can be used to detect cardiorenal aging and functional deterioration, including brain natriuretic peptide (BNP)/N-terminal (NT)-pro hormone BNP (NT-proBNP), interleukin-1β (IL-1β), nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX), NLR family pyrin domain containing 3 (NLRP3) and neutrophil gelatinase associated lipocalin (NGAL) [7][22]. In addition to stimulating SNS and RAAS, oxidative stress weakens cardiorenal function by attenuating the restoration of mitochondrial health, alleviating mitochondrial biogenesis, enhancing proinflammatory and profibrotic pathways, and damaging the integrity and viability of cells and organs [6][7][8] (Figure 1).
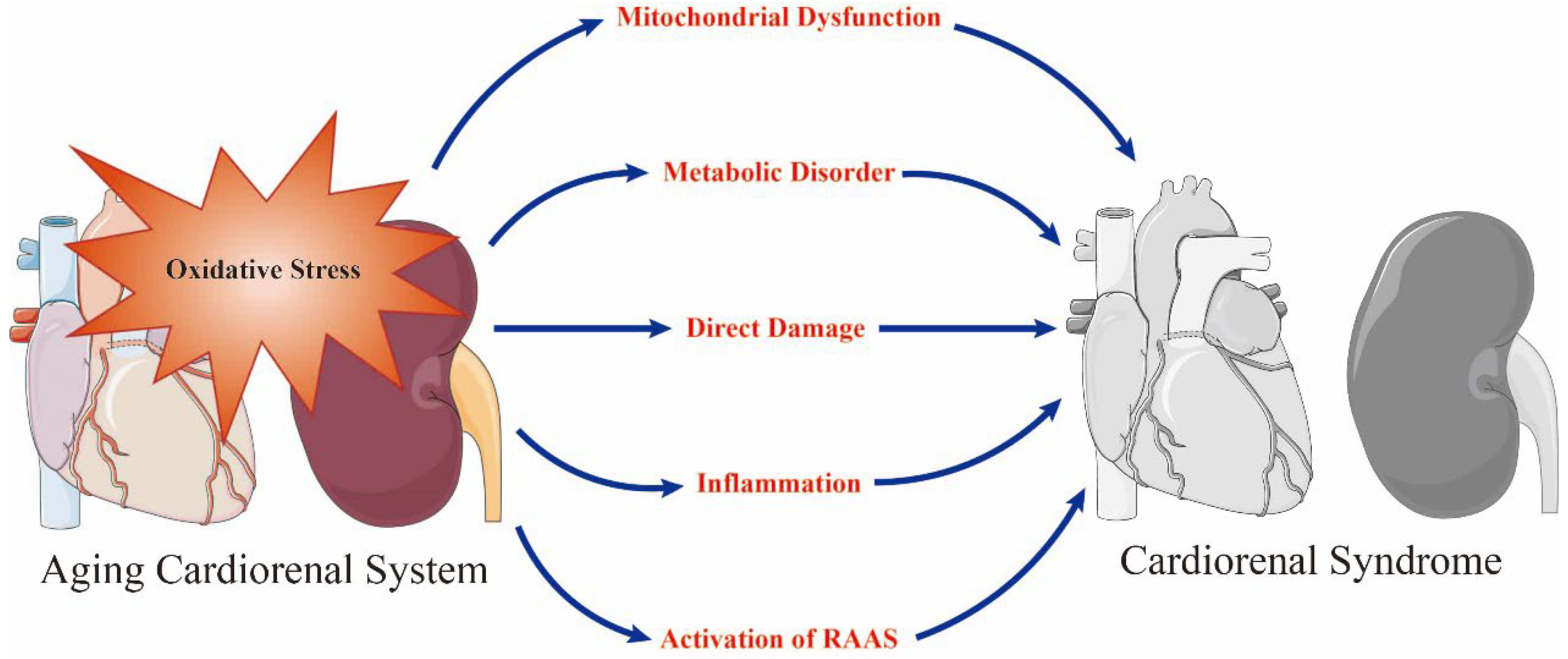
Figure 1. Oxidative stress is a prominent initiator of mitochondrial dysfunction, metabolic disorder, direct damage, inflammation and the activation of renin–angiotensin–aldosterone system (RAAS) in the aging cardiorenal system and plays a key role in the occurrence of cardiorenal syndrome. Adobe Illustrator (version 2021 25.0) was used for the production of this figure.
Cardiorenal abnormalities contribute to the accumulation of misfolded or unfolded proteins in the endoplasmic reticulum (ER), thereby exerting significant load on the ER protein-folding mechanism; this can overwhelm the capacity of the ER, a disease state known as ER stress [23][24]. Cells have evolved an adaptive signal transduction pathway that transmits signals from the ER to the nucleus, known as the unfolded protein response (UPR); this pathway attempts to restore ER homeostasis and improve cellular functional recovery against ER stress [25]. X-box binding protein 1 (XBP1), a member of the cAMP response element binding protein (CREB)/activating transcription factor (ATF) basic region-leucine zipper family of transcription factors, is one of the highly conserved effector molecules of UPR [26]. Upon ER stress, inositol requiring enzyme 1α (IRE1α) splices XBP1 pre-mRNA to XBP1 mRNA, which is then translated into an active transcriptional factor (XBP1s; the spliced form of XBP1), thus regulating the transcription of UPR genes encoding ER chaperones that are closely related to physiological and pathological activities such as cell death, regeneration, and metabolism [25] (Figure 2). XBP1 has been demonstrated to be expressed ubiquitously, from yeast to humans [27][28][29][30][31][32][33]. The complete knockout of XBP1 was previously shown to result in embryonic lethality [34][35][36][37].
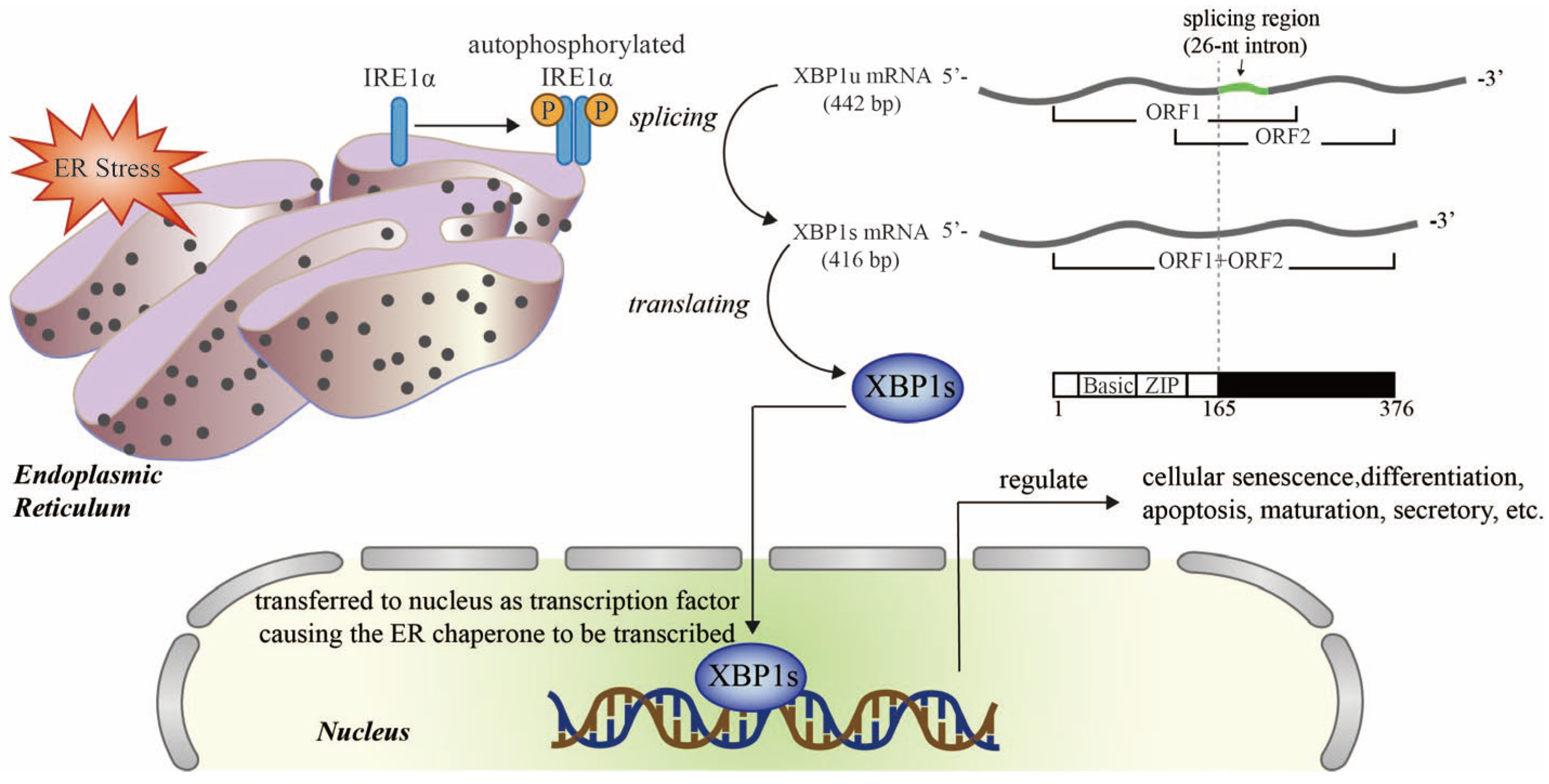
Figure 2. Schematic diagram of the XBP1 signaling pathway in endoplasmic reticulum stress (ER stress). Upon ER stress, IRE1α is auto-phosphorylated and transformed into an active dimer which splices XBP1u mRNA into XBP1s mRNA, which codes for an active transcription factor, XBP1s. The translocation of XBP1s to the nucleus promotes the transcription of target genes that regulate senescence, survival, metabolism, and the immune system. Ⓟ Refers to phosphoric acid. Adobe Illustrator (version 2021 25.0) was used for the production of this figure.
2. Mitochondrial Maintenance
Mitochondria generate more than 90% of adenosine triphosphate (ATP); this represents approximately one-third of the high energy demand of cardiomyocytes; consequently, the human heart contains the highest concentration of mitochondria in the entire body [38]. Owing to the high energy demands of solute reabsorption, the kidneys, particularly cells in the proximal tubules and the medullary thick ascending limb, possess an abundance of mitochondria [39][40]. Mitochondria are bioenergetic and biosynthetic signaling organelles that provide a critical stress-sensing function that helps cells to adapt to their environment [41]. Over the past few decades, researchers have firmly concluded that the mitochondrion is a central contributor to oxidative stress in the cardiorenal system [22][42][43][44][45]. XBP1 is often implicated in many cardiorenal diseases as consequences of failed mitochondrial maintenance [22][26].
In both healthy kidneys and hearts, physiological levels of mitochondrial reactive oxygen species (ROS) can activate survival pathways [46]. When the heart experiences biological stressors, such as myocardial ischemia-reperfusion injury (IRI), there is a marked reduction of mitochondrial membrane potential (ΔΨm) and ATP stores become exhausted; these changes are accompanied by acidosis that is secondary to lactate accumulation, and an increase in the intracellular calcium (Ca2+) concentration. Furthermore, the outer mitochondrial membrane (OMM) remains intact and the mitochondrial permeability transition pore (mPTP) remains closed. With subsequent reperfusion, the reintroduction of oxygen leads to the rapid normalization of pH and a rapid restoration of ΔΨm, thus precipitating a range of adverse sequelae including the production of mitochondrial ROS, the exacerbation of Ca2+ overload, OMM destruction, and mPTP formation [47].
It is well established that acute kidney injury (AKI) perturbs the usual vectorial pumping of protons across the inner mitochondrial membrane by enzymatic complexes within the mitochondrial electron transport chain (ETC) [48]. The subsequent loss of ΔΨm disrupts selective permeability. As a consequence, the mitochondria expand [49]. Mitochondrial biogenesis refers to the cellular process by which new mitochondrial mass and mitochondrial DNA (mtDNA) replication are produced [50], enhancing mitochondrial oxidative phosphorylation (OXPHOS) capacity and the repair of mitochondrial dysfunction after AKI. Mitochondrial fusion joins two mitochondria at the outer and inner membrane interfaces via several membrane GTPases, Mitofusin 1 (MFN1), Mitofusin 2 (MFN2), and Optic atrophy protein 1(OPA1) [51][52]. Mitochondrial fission is a multistep process that allows a mitochondrion to split in two separate mitochondrial organelles [53]. The network formed by mitochondrial fusion–fission is mitochondrial dynamics. Mitochondrial dynamics is deployed to sequester damage and efficiently eliminate damaged mitochondria through mitophagy, which is a mechanism that selectively degrades excess and defective mitochondria [54][55]. It is worth noting that changes in the mitochondrial structure have been observed in the ischemic human kidney prior to the occurrence of the clinical symptoms of AKI, thus suggesting that mitochondrial perturbation may not be a mere epiphenomenon following injury. Rather, mitochondrial dysfunction could potentially be a factor that contributes to injury [56].
In addition, heart failure and CKD also induce mitochondrial biosynthesis, mitochondrial dynamics, mitophagy, and mitochondrial proteostasis; collectively, these processes facilitate mitochondrial quality control (MQC).
It is thought that appropriate MQC is a compensatory molecular mechanism that removes anomalous mitochondrial proteins or completely damaged mitochondria, and restores mitochondrial both function and homeostasis, thus favoring cardiorenal protection [57][58]. In contrast, the disruption of MQC accelerates lethal damage [59]. Mitochondrial dysfunction and imbalances in MQC are hallmarks of the aging cardiorenal system [26][60]. These mitochondrial events driven by oxidative stress contribute to increased susceptibility of the aging kidneys and hearts to acute and chronic injuries [61]. Intriguingly, the specific ablation of mitochondria from senescent cells was previously shown to be sufficient to reverse many features of the senescent phenotype [62] (Figure 3).
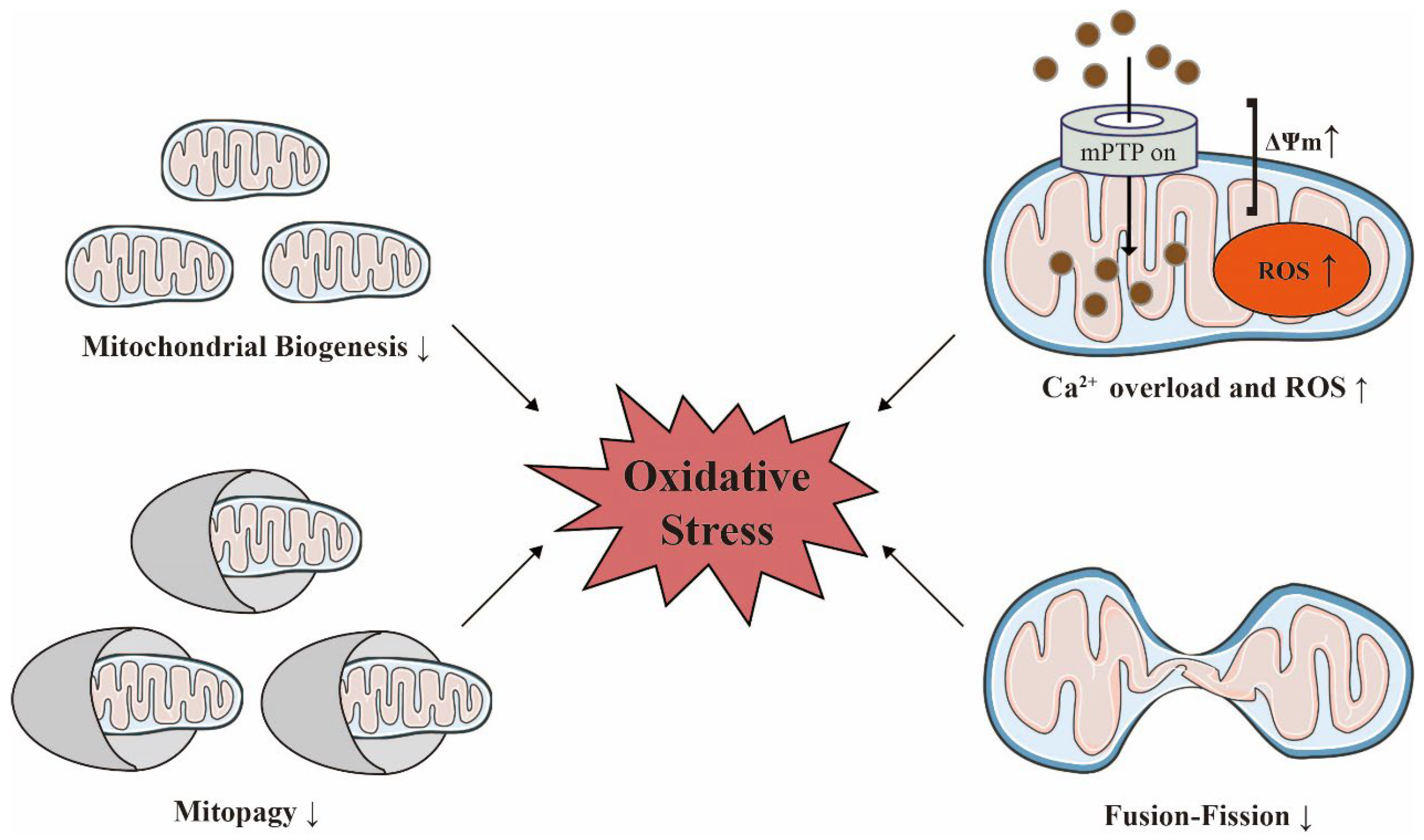
Figure 3. Mitochondrial homeostasis is decisive for maintaining oxidative stress with age. Acute injuries can trigger an increase in mitochondrial membrane potential (ΔΨm), calcium overload, and the overproduction of reactive oxygen species (ROS) in damaged mitochondria. The reduced functionality of biosynthesis, dynamics, and quality control in heart failure and chronic kidney disease can also exacerbate oxidative stress. The ↑ indicates an increase; the ↓ indicates a decrease. Adobe Illustrator (version 2021 25.0) was used for the production of this figure.
2.1. Nuclear Factor Erythroid 2-Related Factor 2 (NRF2)
NRF2, encoded by nuclear factor, erythroid 2 like 2 (NFE2L2) gene, is a master regulator of anti-aging and antioxidant defense and protects against various insult-induced organ damage [8][22][42][63][64] by regulating responses to mitochondrial-derived ROS via its pleiotropic effects on controlling antioxidant and detoxification genes, including NADPH-quinone oxidoreductase (NQO1), heme oxygenase-1 (HO-1), and superoxide dismutase 1 (SOD1) [63][64][65], preventing the progression of AKI to CKD transition and deeply participating in CKD development. AKI activates the expression of the NRF2 in the kidneys, thereby enhancing antioxidant target gene transcription that protects the kidney from oxidative damage [42]. Compared to healthy kidney tissue, NRF2 and its downstream molecules were found mainly upregulated in earlier human CKD samples, like glomeruli of diabetic nephropathy (DN) patients, and kidney tissue from patients with lupus nephritis [66][67]. Because of the imbalance of antioxidant mechanism, decreased expression of the NRF2 system is often detected in patients with advanced CKD [68]. The transcription of NRF2 can also increase mitochondrial biogenesis through the expression of genes that are essential for mitochondrial biogenesis, including nuclear respiratory factor 1 (NRF-1) and peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) [69][70][71].
It has been well documented that the Kelch-like ECH-associated protein 1 (Keap1)/NRF2 and the glycogen synthase kinase-3β (GSK-3β)/NRF2 signaling pathways represent redox-sensitive regulator axes through which NRF2 dissociates from Keap1 or GSK-3β under oxidative stress prior to the induction of various antioxidant genes [22][72][73]. Ferroptosis-associated epithelial and endothelial to mesenchymal transition (EMT) is the principal pathological basis underlying the progression of DN to end-stage renal disease (ESRD), which often involves the accumulation of ROS and iron overload in renal tubular epithelial cells. This is probably caused by activation of the XBP1-HRD1-NRF2 pathway by high levels of glucose, at least in part [74]. In line with this, investigations from another laboratory reported that the cardiac expression of XBP1s could effectively rescue the expression of HRD1 and mediate the ubiquitination and degradation of NRF2 [8].
2.2. O-Linked GlcNAc Modification (O-GlcNAcylation)
O-GlcNAcylation, a pro-survival pathway that counterbalances age-related decline in a self-healing capacity [75], can modulate protein stability and function, and has been implicated in various cardiovascular diseases. In cardiac IRI, XBP1s couples ER stress with the hexosamine biosynthetic pathway (HBP) by triggering activation of the major HBP enzymes: glutamine fructose-6-phosphate aminotransferase 1 (GFAT1), glucosamine-phosphate N-acetyltransferase 1 (GNPNAT1), phosphoglucomutase 3 (PGM3), and uridine diphosphate-glucose 4-epimease (GalE) [76]. The XBP1s-HBP axis promotes the synthesis of uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), an obligate substrate for the O-GlcNAcylation of cardiac proteins during IRI [76][77]. O-GlcNAcylation has also been implicated in various IRI-related cardiovascular diseases. A body of evidence suggests that increasing O-GlcNAcylation during IRI may represent a unique and endogenously recruitable mechanism of cardioprotection that acts directly via O-GlcNAcylating voltage-dependent anion channel 1 (VDAC1) in the mitochondria [78][79][80]. Because VDAC is a putative form of mPTP, the O-GlcNAcylation of VDAC prevents the formation of mPTP, alters sensitivity to the loss of ΔΨm, relieves Ca2+ overload-induced mitochondrial swelling, and hence maintains mitochondrial stability [78].
2.3. BNP and NT-proBNP
Under pathological conditions, XBP1s, which is induced in response to ER stress, is an essential regulator of BNP transcription in cardiomyocytes, and binds to the proximal activator protein 1 (AP-1)/cAMP response element (CRE)-like element in the BNP promoter and increases the activity of its promoter [7][81]. The transcribed unstable BNP mRNA can rapidly synthesize a 134 amino acid BNP precursor (pre-proBNP) and remove the N-terminal 26 amino acid signal peptide to form a 108 amino acid BNP (proBNP) [82]. Subsequently, proBNP is cleaved into inactive NT proBNP (comprised of 76 amino acids) and active BNP (comprised of 32 amino acids) by proBNP convertases (corin or furin) [82][83][84]. Both BNP and NT-proBNP status are widely used as diagnostic biomarkers for heart failure, hypertension, and cardiac hypertrophy [82][85]. The significant interaction between changes in NT-proBNP and the reduction of GFR values indicate that NT-proBNP may precede the deterioration of renal function in patients with heart failure [86].
As a compensatory protection mechanism in the early stages of disease progression, BNP is a novel mitochondrial fusion activator, in addition to inducing natriuresis and dieresis, and reducing RAAS and SNS activity [87][88][89]. BNP binds to its receptor, the natriuretic peptide receptor-A (NPRA), activates protein kinase G (PKG), and then stimulates signal transducer and activator of transcription 3 (STAT3) by phosphorylation [89]. Phosphorylated STAT3 binds to the optic atrophy 1 (OPA1) promoter and promotes OPA1-mediated mitochondrial fusion [89]; this process protects against the cardiac dysfunction associated with mitochondrial depolarization and ROS production [87][89].
3. Inflammation
It is now well established that oxidative stress links XBP1 to inflammation [1][2][26]. The overexpression of proinflammatory cytokines may be linked to the pathogenesis of anemia in patients with heart failure. Inflammation can impact the function of endothelial kidney cells by causing exposure to a proinflammatory and prothrombotic profile, vasoconstriction, and capillary obstruction, thus resulting in AKI. Moreover, proinflammatory cytokines may reduce the production of red blood cells in the bone marrow by damaging red blood cell precursors and by limiting the expression of erythropoietin receptors; this process may result in anemia. The reduction of renal oxygen delivery due to the hypoperfusion of nephrons and low hemoglobin levels may affect aerobic metabolism in cells, thus resulting in cellular death [90][91]. Hence, oxidative stress can cause cardiorenal tissue inflammation, a process characterized by the activation of inflammatory cells and high circulating levels of inflammatory molecules; this process has been proposed as an oxidative stressor in acute and chronic cardiorenal impairment with advancing age [92][93][94][95]. In addition, proinflammatory and anti-inflammatory cytokines, along with chemokines, released by the kidneys can reach the circulation, thus leading to the dysfunction of the cardiovascular system via the main circulation [96].
3.1. NLRP3
Aging-related cardiovascular diseases and renal diseases are often associated with inflammasomes; these cytosolic multi-protein complexes, consisting of apoptosis-associated speck-like protein containing a caspase-associated recruitment domains (ASC), procaspase-1, inflammasome nucleators such as NLRs, AIM2, and pyrin, are responsible for innate immunity and involved in almost all cardiorenal diseases [97]. There are many types of inflammasomes; NLRP3 is the most well-characterized of these inflammasomes and acts as a receptor for cardiorenal damage, metabolic stress and ROS surveillance [98][99]. In addition, NLRP3 triggers the induction of cleaved caspase-1 which plays a role in pyroptosis and the release of cytokines belonging to the IL-1β family, primarily IL-1β and IL-18 [26][99]. In the clinical data of AKI and early-stage CKD, it is evident that the expression of NLRP3 component increases, indicating that NLRP3 guides the occurrence and prognosis of these diseases [100][101][102]. On the contrary, low levels of NLRP3 or caspase-1 were measured in some populations of lupus nephritis, urate nephropathy (UAN), and ESRD patients [103]. The changes in NLRP3 activation differs also between acute and chronic cardiac injury. Acute cardiac injury, such as cardiac IRI, is associated with remarkably increasing NLRP3 inflammasomes [104][105], while low basal activation of NLRP3 inflammosomes contributes to chronic cardiac diseases progression, including atherosclerosis, hypertensive heart disease, diabetic cardiomyopathy, and heart failure [105].
3.2. NOX and Nuclear Factor Kappa-B (NF-κB)
The NOX family of proteins represents the chief source of controlled ROS formation and includes seven isoforms with a broad tissue distribution and activation mechanism, including NOX1, NOX2, NOX3, NOX4, NOX5, and the dual oxidases (Duox1 and Duox2) [106]. NOX functionality and redox-based signaling play critical physiological and pathophysiological roles in aging [107]. NF-κB is an important nuclear transcription factor that plays a key role in all diseases characterized by inflammatory processes.
Recent studies have shown that hearts suffering from pressure overload exhibit increased NOX4 expression and ROS generation, thus resulting in the splicing of XBP1 and the activation of receptor interacting protein kinase 1 (RIPK1)-related NF-κB signaling downstream of XBP1s, ultimately leading to cardiomyocyte hypertrophy [7][108]. Correspondingly, XBP1s can also exacerbate lipopolysaccharide (LPS)-associated cardiomyocyte injury by downregulating the X-linked inhibitor of apoptosis protein (XIAP) and SOD by activating the NF-κB signaling pathway [109]. In human mesangial cells (HMCs), the silencing of XBP1 expression amplifies low-density lipoprotein (LDL)-induced inflammation via feedback based on the increased activity of the IRE1α/IκB kinase (IKK)/NF-κB pathway [94]. In the vasculature of spontaneously hypertensive rats (SHR), NOX4-related ROS provoked IRE1α oxidation which then accelerated the activation of XBP1s; this process improved the survival and proliferation of vascular smooth muscle cells (VSMCs) and improved the hypertension causing vascular dysfunction [110].
Macrophage-mediated innate and adaptive immune responses have been postulated as a notable mechanism during the pathogenesis of cardiorenal syndrome. Toll-like receptors (TLR) 2 and 4 are highly expressed by macrophages and respond to bioactive molecules secreted into the circulation due to cardiorenal tissue damage, such as the soluble form of biglycan (sBGN) [111][112]. Activated TLRs specifically upregulate XBP1s via NOX, thus facilitating the activation of NF-κB to elicit the release of proinflammatory cytokines, predominantly in macrophages. Interestingly, a recent study showed that TLR/NOX2-induced ROS was able to impair the expression and maturation of IL-1β [113]; furthermore, TLR/NOX1/4-induced ROS favors the expression and maturation of IL-1β in macrophages [114][115].
3.3. Transforming Growth Factor-β (TGF-β) 1
TGF-β1 has been identified as an essential regulator of the cardiorenal fibrotic process in the aging population [116][117][118]. After extensive or sustained inflammation, resident macrophages, myocardial cells, and renal parenchymal cells secrete TGF-β1 [119][120][121]; this process reduces the production of antioxidants and enhances oxidative stress, thereupon accentuating fibrosis by stimulating the production of extracellular matrix (ECM), activating resident populations of fibroblasts, and inducing EMT [122]. Furthermore, TGF-β1-induced fibrosis is known to primarily depend on TGF-β1/Smad signaling [121][123][124]. Tumor necrosis factor (TNF) receptor-associated protein 1 (TRAP1, also known as heat shock protein (HSP75), a member of the HSP90 chaperone family that resides principally in the mitochondria [125][126], is associated with the TGF-β/Smad signal transduction pathway [127]. XBP1s acts directly upstream of TRAP1; furthermore, the XBP1s-TRAP1 axis can inhibit the production of TGF-β1, prolong G2/M cell cycle arrest, reduce the expression of profibrotic factors, and ameliorate the progression of fibrosis [128].
3.4. Vascular Endothelial Growth Factor (VEGF) A
VEGFA is produced by most cells in the body, but is known to be upregulated in hypoxia. Furthermore, research has shown that VEGFA plays an important role in regenerative capacity via vasculogenesis and angiogenesis [129][130][131]; this requires ROS derived from NOX (especially NOX2 and NOX4) and gradually declines with age [132][133].
As a transcription factor, XBP1s binds to two regions on the VEGFA promoter and induces the upregulation of VEGFA in the heart and kidneys [134]. The XBP1s/VEGFA axis has been proven to be a novel regulatory pathway for vasculogenesis and angiogenesis [135] and can contribute to the progression of adaptive hypertrophy during heart failure. In addition, this axis can contribute to chronic inflammation and oxidative stress to induce diabetic cardiomyopathy [134]. During IRI, there is a reduction in renal VEGFA levels; however, research has shown that VEGFA is upregulated in both glomeruli (podocytes, endothelial cells, and mesangial cells) and in the tubular compartment during the early stages of diabetic kidney disease (DKD); in addition, VEGFA promotes subsequent alterations in vascular remodeling, inflammatory processes, glomerulosclerosis, and tubulointerstitial fibrosis [134][136][137][138].
4. SNS and RAAS
SNS influences intrarenal hemodynamics and stimulates RAAS via the juxtaglomerular apparatus of the kidney. Activation of the RAAS cascade typically commences with the secretion of prorenin and renin; this process induces pro-fibrotic effects in the kidneys by binding to the prorenin–renin receptor (PRR) [6][8]. The hypoperfusion of peripheral tissue in heart failure induces over-activity of SNS, thus resulting in the increased release of renin from the juxtamedullary apparatus. The synthesis of renin is also stimulated by the reduction of hydrostatic pressure in the glomerular afferent arterioles and a reduction in the amount of sodium delivered to the macula densa [7][139]. Subsequently, angiotensinogen (AGT) is converted into the decapeptide angiotensin I (AT1) by renin or the chymase enzyme. Then, AT1 is cleaved by angiotensin converting enzyme (ACE) to generate angiotensin II (AT2); this can lead to renal efferent arteriolar vasoconstriction and the formation of aldosterone, which increases tubular sodium reabsorption in the kidney and the effective circulating plasma volume by binding to either angiotensin II type 1 receptor (AT1R) or angiotensin II type 2 receptor (AT2R) [14]. ACE2, a homologue of ACE, acts on AT1 and AT2 to produce a 9-amino acid peptide known as angiotensin 1-9 (Ang1-9) and a 7-amino acid peptide known as angiotensin 1-7 (Ang1-7), which can protect hearts and kidneys against damage, fibrosis and remodeling by binding to angiotensin receptor type 2 or the endogenous orphan Mas receptor (MasR) [140]. Aged kidneys possess a lower abundance of cells of renin lineage and reduced responsiveness to RAAS inhibition [141]. One of the consequences of the RAAS system is an increase in the generation of ROS via NOX and increased protein aggregation, thus assisting the development of cardiorenal syndrome [8][14][142].
The XBP1s-HRD1-NRF2 axis exerts functional dichotomy in the cardiorenal system. When the ubiquitination/degradation functionality of the XBP1s-HRD1 axis is inhibited, NRF2 is aberrantly expressed and retained in the nucleus, thus resulting in the dysfunctional expression of RAAS genes, including the upregulation of AGT/ACE genes and the downregulation of ACE2/MasR genes; these effects can aggravate cardiorenal diseases [8][143]. XBP1s can also directly manipulate the expression of some genes that encode components of the RAAS. In human umbilical vein endothelial cells (HUVECs) treated with arsenite, XBP1s was responsible for the accumulation of hypoxia-inducible factor 1α (HIF1α) and the assembly of an XBP1s/HIF1α transcriptional complex, which occupies the responsive elements of the ACE/AT2/AT1R axis within the promoter region of the RAAS gene. This process attracts RNA polymerase II and drives transcription, thus causing the occurrence of oxidative stress and proinflammatory response by the activation of RAAS in several cardiovascular diseases, including hypertension, atherosclerosis, and microvascular abnormalities [6][7].
5. The Akt Pathway
Akt, also known as protein kinase B (PKB), is a crucial signaling protein that is strongly activated by cellular damage and governs cellular survival, proliferation, and senescence [24][144][145][146][147]. Many studies have demonstrated that Akt plays a significant role in protecting the aging cardiorenal system from oxidative stress [24][144][145][146]. The 78 kDa glucose-regulated protein (GRP78, also known as BiP/HSPA5), is a master protein chaperone located in the ER and modulates homeostasis in the ER [148]. As myocardial cells experience IRI, the XBP1s signaling branch and its downstream target GRP78 are activated in a highly robust manner. Subsequently, a fraction of GRP78 is translocated to the cell surface to stimulate the Akt-dependent suppression of ROS accumulation by interacting with phosphatidylinositol 3-kinase (PI3K) [144]. Previous DN research discovered that the overexpression of XBP1 in glomerular mesangial cells (MCs) exposed to high glucose (HG) could activate the phosphatase and tensin homolog deleted on chromosome ten (PTEN)/Akt signaling pathway, thus mitigating oxidative stress caused by HG or MCs apoptosis [24].
6. Myo-Inositol Oxygenase (MIOX)
MIOX, a cytosolic enzyme expressed predominantly in the renal proximal kidney tubules, catalyzes the conversion of myo-inositol to d-glucuronic acid and activates the glucuronate-xylulose pathway, which increases the ROS generation and can impose oxidative stress. It has been noted to be associated with tubulopathy in the context of DKD and AKI [23][149]. Sharma et al. reported that an increased nuclear translocation of XBP1s could bind MIOX promoter in the tubular compartment of diabetic mice, prompting the excessiveness of ROS via MIOX-mediated increased oxidant stress, which eventuated deleterious tubulointerstitial effects [23].
7. XBP1u
XBP1u, translated from XBP1, is an unspliced mRNA isoform and acts as a regulator with the same N-terminal and internal DNA binding domains as XBP1s. XBP1u consists of a basic region and a leucine zipper region [150]; in addition, the C-terminus of XBP1u contains a nuclear exclusion signal and lacks the transcription activation domain that is evident in XBP1s [151].
Most cardiorenal studies of XBP1u have focused on blood vessels [152][153][154]. In the aging cardiorenal system, pathological states involving disturbed flow can cause ligand-independent activation of the kinase insert domain receptor (KDR), which then upregulates XBP1u and histone deacetylase 3 (HDAC3) in endothelial cells [154]. Furthermore, because XBP1u can counteract XBP1s by sequestering XBP1s for proteasomal degradation, the overexpression of XBP1u has been shown to protect HDAC3 from the transcriptional repression caused by XBP1s [152][155]. XBP1u and HDAC3 promote the formation of the mammalian target of rapamycin complex 2 (mTORC2)-Akt1-XBP1u-HDAC3 complex and increases mTORC2-dependent Akt1 phosphorylation, which subsequently enhances the stability of NRF2 and NRF2-mediated HO-1 expression and reduces oxidative stress in endothelial cells during atherosclerosis and the formation of neointima post-injury [152][154].
References
- Pastori, S.; Virzì, G.M.; Brocca, A.; de Cal, M.; Clementi, A.; Vescovo, G.; Ronco, C. Cardiorenal syndrome type 1: A defective regulation of monocyte apoptosis induced by proinflammatory and proapoptotic factors. Cardiorenal Med. 2015, 5, 105–115.
- Virzì, G.M.; Clementi, A.; de Cal, M.; Brocca, A.; Day, S.; Pastori, S.; Bolin, C.; Vescovo, G.; Ronco, C. Oxidative stress: Dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Oxidative Med. Cell. Longev. 2015, 2015, 391790.
- Hoy, W.E.; Douglas-Denton, R.N.; Hughson, M.D.; Cass, A.; Johnson, K.; Bertram, J.F. A stereological study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int. Suppl. 2003, 83, S31–S37.
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nature reviews. Nephrology 2017, 13, 241–257.
- Di Lullo, L.; Reeves, P.B.; Bellasi, A.; Ronco, C. Cardiorenal Syndrome in Acute Kidney Injury. Semin. Nephrol. 2019, 39, 31–40.
- Xu, X.; Liu, S.; Aodengqimuge; Wang, H.; Hu, M.; Xing, C.; Song, L. Arsenite Induces Vascular Endothelial Cell Dysfunction by Activating IRE1α/XBP1s/HIF1α-Dependent ANGII Signaling. Toxicol. Sci. 2017, 160, 315–328.
- Liu, S.; Ding, H.; Li, Y.; Zhang, X. Molecular Mechanism Underlying Role of the XBP1s in Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2022, 9, 459.
- Binder, P.; Nguyen, B.; Collins, L.; Zi, M.; Liu, W.; Christou, F.; Luo, X.; Hille, S.S.; Frey, N.; Cartwright, E.J.; et al. Pak2 Regulation of Nrf2 Serves as a Novel Signaling Nexus Linking ER Stress Response and Oxidative Stress in the Heart. Front. Cardiovasc. Med. 2022, 9, 851419.
- Zhang, J.; Ren, D.; Fedorova, J.; He, Z.; Li, J. SIRT1/SIRT3 Modulates Redox Homeostasis during Ischemia/Reperfusion in the Aging Heart. Antioxidants 2020, 9, 858.
- van den Munckhof, I.; Riksen, N.; Seeger, J.P.; Schreuder, T.H.; Borm, G.F.; Eijsvogels, T.M.; Hopman, M.T.; Rongen, G.A.; Thijssen, D.H. Aging attenuates the protective effect of ischemic preconditioning against endothelial ischemia-reperfusion injury in humans. American journal of physiology. Heart Circ. Physiol. 2013, 304, H1727–H1732.
- McMurray, J.J.; Petrie, M.C.; Murdoch, D.R.; Davie, A.P. Clinical epidemiology of heart failure: Public and private health burden. Eur. Heart J. 1998, 19, P9–P16.
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol. 2009, 53, 582–588.
- Chen, C.Y.; Zhou, Y.; Wang, P.; Qi, E.Y.; Gu, W.J. Elevated central venous pressure is associated with increased mortality and acute kidney injury in critically ill patients: A meta-analysis. Crit. Care 2020, 24, 80.
- Gembillo, G.; Visconti, L.; Giusti, M.A.; Siligato, R.; Gallo, A.; Santoro, D.; Mattina, A. Cardiorenal Syndrome: New Pathways and Novel Biomarkers. Biomolecules 2021, 11, 1581.
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal Syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539.
- Daneshgar, N.; Dai, D.F. ROS, Klotho and mTOR in cardiorenal aging. Aging 2020, 12, 19830–19831.
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Di Martino, A.; Albanese, G.; Di Salvo, J.; Epifani, R.; Marfella, R.; Docimo, G.; et al. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 3651.
- Theofilis, P.; Vordoni, A.; Kalaitzidis, R.G. Oxidative Stress Management in Cardiorenal Diseases: Focus on Novel Antidiabetic Agents, Finerenone, and Melatonin. Life 2022, 12, 1663.
- Barrera-Chimal, J.; Bonnard, B.; Jaisser, F. Roles of Mineralocorticoid Receptors in Cardiovascular and Cardiorenal Diseases. Annu. Rev. Physiol. 2022, 84, 585–610.
- Campbell, M.D.; Duan, J.; Samuelson, A.T.; Gaffrey, M.J.; Merrihew, G.E.; Egertson, J.D.; Wang, L.; Bammler, T.K.; Moore, R.J.; White, C.C.; et al. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic. Biol. Med. 2019, 134, 268–281.
- Salehpour, F.; Farajdokht, F.; Mahmoudi, J.; Erfani, M.; Farhoudi, M.; Karimi, P.; Rasta, S.H.; Sadigh-Eteghad, S.; Hamblin, M.R.; Gjedde, A. Photobiomodulation and Coenzyme Q(10) Treatments Attenuate Cognitive Impairment Associated With Model of Transient Global Brain Ischemia in Artificially Aged Mice. Front. Cell. Neurosci. 2019, 13, 74.
- Zhang, J.; Zhang, J.; Ni, H.; Wang, Y.; Katwal, G.; Zhao, Y.; Sun, K.; Wang, M.; Li, Q.; Chen, G.; et al. Downregulation of XBP1 protects kidney against ischemia-reperfusion injury via suppressing HRD1-mediated NRF2 ubiquitylation. Cell Death Discov. 2021, 7, 44.
- Sharma, I.; Deng, F.; Liao, Y.; Kanwar, Y.S. Myo-inositol Oxygenase (MIOX) Overexpression Drives the Progression of Renal Tubulointerstitial Injury in Diabetes. Diabetes 2020, 69, 1248–1263.
- Wang, Y.; He, Z.; Yang, Q.; Zhou, G. XBP1 inhibits mesangial cell apoptosis in response to oxidative stress via the PTEN/AKT pathway in diabetic nephropathy. FEBS Open Bio 2019, 9, 1249–1258.
- Sun, K.; Fan, C.; Zhang, J.; Ni, H.; Wang, M.; Zhang, J.; Li, Q.; Kuang, B.; Wang, Z.; Hou, S.; et al. Prevention of alloimmune rejection using XBP1-deleted bone marrow-derived dendritic cells in heart transplantation. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2022, 41, 1660–1671.
- Ni, H.; Ou, Z.; Wang, Y.; Liu, Y.; Sun, K.; Zhang, J.; Zhang, J.; Deng, W.; Zeng, W.; Xia, R.; et al. XBP1 modulates endoplasmic reticulum and mitochondria crosstalk via regulating NLRP3 in renal ischemia/reperfusion injury. Cell Death Discov. 2023, 9, 69.
- Tam, A.B.; Koong, A.C.; Niwa, M. Ire1 has distinct catalytic mechanisms for XBP1/HAC1 splicing and RIDD. Cell Rep. 2014, 9, 850–858.
- Kaser, A.; Blumberg, R.S. Survive an innate immune response through XBP1. Cell Res. 2010, 20, 506–507.
- Cox, J.S.; Walter, P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 1996, 87, 391–404.
- Mori, K.; Kawahara, T.; Yoshida, H.; Yanagi, H.; Yura, T. Signalling from endoplasmic reticulum to nucleus: Transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells Devoted Mol. Cell. Mech. 1996, 1, 803–817.
- Shen, X.; Ellis, R.E.; Lee, K.; Liu, C.Y.; Yang, K.; Solomon, A.; Yoshida, H.; Morimoto, R.; Kurnit, D.M.; Mori, K.; et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 2001, 107, 893–903.
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891.
- Calfon, M.; Zeng, H.; Urano, F.; Till, J.H.; Hubbard, S.R.; Harding, H.P.; Clark, S.G.; Ron, D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002, 415, 92–96.
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461.
- Iwakoshi, N.N.; Lee, A.H.; Glimcher, L.H. The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol. Rev. 2003, 194, 29–38.
- Acosta-Alvear, D.; Zhou, Y.; Blais, A.; Tsikitis, M.; Lents, N.H.; Arias, C.; Lennon, C.J.; Kluger, Y.; Dynlacht, B.D. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 2007, 27, 53–66.
- Reimold, A.M.; Etkin, A.; Clauss, I.; Perkins, A.; Friend, D.S.; Zhang, J.; Horton, H.F.; Scott, A.; Orkin, S.H.; Byrne, M.C.; et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000, 14, 152–157.
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724.
- Brooks, C.; Wei, Q.; Cho, S.G.; Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Investig. 2009, 119, 1275–1285.
- Takemura, K.; Nishi, H.; Inagi, R. Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia. Front. Physiol. 2020, 11, 565023.
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566.
- Kuang, B.-C.; Wang, Z.-H.; Hou, S.-H.; Zhang, J.; Wang, M.-Q.; Zhang, J.-S.; Sun, K.-L.; Ni, H.-Q.; Gong, N.-Q. Methyl eugenol protects the kidney from oxidative damage in mice by blocking the Nrf2 nuclear export signal through activation of the AMPK/GSK3β axis. Acta Pharmacol. Sin. 2023, 44, 367–380.
- Bugger, H.; Pfeil, K. Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim. Biophys. Acta. Mol. Basis Dis. 2020, 1866, 165768.
- Wang, M.; Zhang, J.; Zhang, J.; Sun, K.; Li, Q.; Kuang, B.; Wang, M.M.Z.; Hou, S.; Gong, N. Methyl eugenol attenuates liver ischemia reperfusion injury via activating PI3K/Akt signaling. Int. Immunopharmacol. 2021, 99, 108023.
- Poznyak, A.V.; Sukhorukov, V.N.; Popov, M.A.; Chegodaev, Y.S.; Postnov, A.Y.; Orekhov, A.N.J.G.E. Mitochondrial Mutations Affect the Cardiovascular System during Aging and Oxidative Stress. Gene Expr. 2023, 22, 232–239.
- Birk, A.V.; Chao, W.M.; Bracken, C.; Warren, J.D.; Szeto, H.H. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br. J. Pharmacol. 2014, 171, 2017–2028.
- Kulek, A.R.; Anzell, A.; Wider, J.M.; Sanderson, T.H.; Przyklenk, K. Mitochondrial Quality Control: Role in Cardiac Models of Lethal Ischemia-Reperfusion Injury. Cells 2020, 9, 214.
- Funk, J.A.; Schnellmann, R.G. Persistent disruption of mitochondrial homeostasis after acute kidney injury. American journal of physiology. Ren. Physiol. 2012, 302, F853–F864.
- Clark, A.J.; Parikh, S.M. Mitochondrial Metabolism in Acute Kidney Injury. Semin. Nephrol. 2020, 40, 101–113.
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84.
- Gao, S.; Hu, J. Mitochondrial Fusion: The Machineries In and Out. Trends Cell Biol. 2021, 31, 62–74.
- van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072.
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259.
- Doke, T.; Susztak, K. The multifaceted role of kidney tubule mitochondrial dysfunction in kidney disease development. Trends Cell Biol. 2022, 32, 841–853.
- Tang, W.X.; Wu, W.H.; Qiu, H.Y.; Bo, H.; Huang, S.M. Amelioration of rhabdomyolysis-induced renal mitochondrial injury and apoptosis through suppression of Drp-1 translocation. J. Nephrol. 2013, 26, 1073–1082.
- Parekh, D.J.; Weinberg, J.M.; Ercole, B.; Torkko, K.C.; Hilton, W.; Bennett, M.; Devarajan, P.; Venkatachalam, M.A. Tolerance of the human kidney to isolated controlled ischemia. J. Am. Soc. Nephrol. JASN 2013, 24, 506–517.
- Wang, J.; Zhou, H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury. Acta Pharm. Sinica. B 2020, 10, 1866–1879.
- Qiu, Z.; Wei, Y.; Song, Q.; Du, B.; Wang, H.; Chu, Y.; Hu, Y. The Role of Myocardial Mitochondrial Quality Control in Heart Failure. Front. Pharmacol. 2019, 10, 1404.
- Zhu, H.; Tan, Y.; Du, W.; Li, Y.; Toan, S.; Mui, D.; Tian, F.; Zhou, H. Phosphoglycerate mutase 5 exacerbates cardiac ischemia-reperfusion injury through disrupting mitochondrial quality control. Redox Biol. 2021, 38, 101777.
- Chen, Q.; Thompson, J.; Hu, Y.; Lesnefsky, E.J. Chronic metformin treatment decreases cardiac injury during ischemia-reperfusion by attenuating endoplasmic reticulum stress with improved mitochondrial function. Aging 2021, 13, 7828–7845.
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447.
- Correia-Melo, C.; Marques, F.D.; Anderson, R.; Hewitt, G.; Hewitt, R.; Cole, J.; Carroll, B.M.; Miwa, S.; Birch, J.; Merz, A.; et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016, 35, 724–742.
- Cominacini, L.; Mozzini, C.; Garbin, U.; Pasini, A.; Stranieri, C.; Solani, E.; Vallerio, P.; Tinelli, I.A.; Fratta Pasini, A. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic. Biol. Med. 2015, 88, 233–242.
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genom. 2018, 50, 77–97.
- Zhang, Y.; Zhang, J.J.; Liu, X.H.; Wang, L. CBX7 suppression prevents ischemia-reperfusion injury-induced endoplasmic reticulum stress through the Nrf-2/HO-1 pathway. American journal of physiology. Ren. Physiol. 2020, 318, F1531–F1538.
- Jiang, T.; Huang, Z.; Lin, Y.; Zhang, Z.; Fang, D.; Zhang, D.D. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 2010, 59, 850–860.
- Jiang, T.; Tian, F.; Zheng, H.; Whitman, S.A.; Lin, Y.; Zhang, Z.; Zhang, N.; Zhang, D.D. Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB-mediated inflammatory response. Kidney Int. 2014, 85, 333–343.
- Juul-Nielsen, C.; Shen, J.; Stenvinkel, P.; Scholze, A. Systematic review of the nuclear factor erythroid 2-related factor 2 (NRF2) system in human chronic kidney disease: Alterations, interventions and relation to morbidity. Nephrol. Dial. Transplant. 2022, 37, 904–916.
- Athale, J.; Ulrich, A.; MacGarvey, N.C.; Bartz, R.R.; Welty-Wolf, K.E.; Suliman, H.B.; Piantadosi, C.A. Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice. Free Radic. Biol. Med. 2012, 53, 1584–1594.
- Chen, Q.M. Nrf2 for cardiac protection: Pharmacological options against oxidative stress. Trends Pharmacol. Sci. 2021, 42, 729–744.
- Li, C.; Han, S.; Zhu, J.; Cheng, F. MiR-132-3p activation aggravates renal ischemia-reperfusion injury by targeting Sirt1/PGC1alpha axis. Cell. Signal. 2023, 110, 110801.
- Liang, H.; Xu, P.; Xu, G.; Zhang, L.; Huang, D.; Ren, M.; Zhang, L. Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides). Antioxidants 2022, 11, 2399.
- Chen, Y.; Shao, X.; Cao, J.; Zhu, H.; Yang, B.; He, Q.; Ying, M. Phosphorylation regulates cullin-based ubiquitination in tumorigenesis. Acta Pharm. Sinica. B 2021, 11, 309–321.
- Liu, Z.; Nan, P.; Gong, Y.; Tian, L.; Zheng, Y.; Wu, Z. Endoplasmic reticulum stress-triggered ferroptosis via the XBP1-Hrd1-Nrf2 pathway induces EMT progression in diabetic nephropathy. Biomed. Pharmacother. 2023, 164, 114897.
- Jiang, M.; Yu, S.; Yu, Z.; Sheng, H.; Li, Y.; Liu, S.; Warner, D.S.; Paschen, W.; Yang, W. XBP1 (X-Box-Binding Protein-1)-Dependent O-GlcNAcylation Is Neuroprotective in Ischemic Stroke in Young Mice and Its Impairment in Aged Mice Is Rescued by Thiamet-G. Stroke 2017, 48, 1646–1654.
- Wang, Z.V.; Deng, Y.; Gao, N.; Pedrozo, Z.; Li, D.L.; Morales, C.R.; Criollo, A.; Luo, X.; Tan, W.; Jiang, N.; et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 2014, 156, 1179–1192.
- Li, R.; Shen, Y.; Li, X.; Lu, L.; Wang, Z.; Sheng, H.; Hoffmann, U.; Yang, W. Activation of the XBP1s/O-GlcNAcylation Pathway Improves Functional Outcome After Cardiac Arrest and Resuscitation in Young and Aged Mice. SHOCK 2021, 56, 755–761.
- Ngoh, G.A.; Watson, L.J.; Facundo, H.T.; Dillmann, W.; Jones, S.P. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J. Mol. Cell. Cardiol. 2008, 45, 313–325.
- Jones, S.P.; Zachara, N.E.; Ngoh, G.A.; Hill, B.G.; Teshima, Y.; Bhatnagar, A.; Hart, G.W.; Marbán, E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 2008, 117, 1172–1182.
- Ngoh, G.A.; Facundo, H.T.; Hamid, T.; Dillmann, W.; Zachara, N.E.; Jones, S.P. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ. Res. 2009, 104, 41–49.
- Sawada, T.; Minamino, T.; Fu, H.Y.; Asai, M.; Okuda, K.; Isomura, T.; Yamazaki, S.; Asano, Y.; Okada, K.; Tsukamoto, O.; et al. X-box binding protein 1 regulates brain natriuretic peptide through a novel AP1/CRE-like element in cardiomyocytes. J. Mol. Cell. Cardiol. 2010, 48, 1280–1289.
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820.
- de Lemos, J.A.; McGuire, D.K.; Drazner, M.H. B-type natriuretic peptide in cardiovascular disease. Lancet 2003, 362, 316–322.
- Kerkelä, R.; Ulvila, J.; Magga, J. Natriuretic Peptides in the Regulation of Cardiovascular Physiology and Metabolic Events. J. Am. Heart Assoc. 2015, 4, e002423.
- Kociol, R.D.; Horton, J.R.; Fonarow, G.C.; Reyes, E.M.; Shaw, L.K.; O’Connor, C.M.; Felker, G.M.; Hernandez, A.F. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: Data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circulation. Heart Fail. 2011, 4, 628–636.
- Takahama, H.; Nishikimi, T.; Takashio, S.; Hayashi, T.; Nagai-Okatani, C.; Asada, T.; Fujiwara, A.; Nakagawa, Y.; Amano, M.; Hamatani, Y.; et al. Change in the NT-proBNP/Mature BNP Molar Ratio Precedes Worsening Renal Function in Patients With Acute Heart Failure: A Novel Predictor Candidate for Cardiorenal Syndrome. J. Am. Heart Assoc. 2019, 8, e011468.
- Bhandari, P.; Song, M.; Dorn, G.W., 2nd. Dissociation of mitochondrial from sarcoplasmic reticular stress in Drosophila cardiomyopathy induced by molecularly distinct mitochondrial fusion defects. J. Mol. Cell. Cardiol. 2015, 80, 71–80.
- Okamoto, R.; Ali, Y.; Hashizume, R.; Suzuki, N.; Ito, M. BNP as a Major Player in the Heart-Kidney Connection. Int. J. Mol. Sci. 2019, 20, 3581.
- Chang, P.; Zhang, X.; Zhang, J.; Wang, J.; Wang, X.; Li, M.; Wang, R.; Yu, J.; Fu, F. BNP protects against diabetic cardiomyopathy by promoting Opa1-mediated mitochondrial fusion via activating the PKG-STAT3 pathway. Redox Biol. 2023, 62, 102702.
- Virzì, G.M.; Breglia, A.; Brocca, A.; de Cal, M.; Bolin, C.; Vescovo, G.; Ronco, C. Levels of Proinflammatory Cytokines, Oxidative Stress, and Tissue Damage Markers in Patients with Acute Heart Failure with and without Cardiorenal Syndrome Type 1. Cardiorenal Med. 2018, 8, 321–331.
- Clementi, A.; Virzì, G.M.; Battaglia, G.G.; Ronco, C. Neurohormonal, Endocrine, and Immune Dysregulation and Inflammation in Cardiorenal Syndrome. Cardiorenal Med. 2019, 9, 265–273.
- Li, X.N.; Zuo, Y.Z.; Qin, L.; Liu, W.; Li, Y.H.; Li, J.L. Atrazine-xenobiotic nuclear receptor interactions induce cardiac inflammation and endoplasmic reticulum stress in quail (Coturnix coturnix coturnix). Chemosphere 2018, 206, 549–559.
- Zha, X.; Yue, Y.; Dong, N.; Xiong, S. Endoplasmic Reticulum Stress Aggravates Viral Myocarditis by Raising Inflammation Through the IRE1-Associated NF-κB Pathway. Can. J. Cardiol. 2015, 31, 1032–1040.
- Yu, Y.; Zhang, L.; Liu, Q.; Tang, L.; Sun, H.; Guo, H. Endoplasmic reticulum stress preconditioning antagonizes low-density lipoprotein-induced inflammation in human mesangial cells through upregulation of XBP1 and suppression of the IRE1α/IKK/NF-κB pathway. Mol. Med. Rep. 2015, 11, 2048–2054.
- Ronco, C.; Bellasi, A.; Di Lullo, L. Cardiorenal Syndrome: An Overview. Adv. Chronic Kidney Dis. 2018, 25, 382–390.
- Rosner, M.H.; Ronco, C.; Okusa, M.D. The role of inflammation in the cardio-renal syndrome: A focus on cytokines and inflammatory mediators. Semin. Nephrol. 2012, 32, 70–78.
- Li, Y.; Li, J.; Li, S.; Li, Y.; Wang, X.; Liu, B.; Fu, Q.; Ma, S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015, 286, 53–63.
- Hasanzadeh, S.; Read, M.I.; Bland, A.R.; Majeed, M.; Jamialahmadi, T.; Sahebkar, A. Curcumin: An inflammasome silencer. Pharmacol. Res. 2020, 159, 104921.
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489.
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21, e49799.
- Zhuang, Y.; Ding, G.; Zhao, M.; Bai, M.; Yang, L.; Ni, J.; Wang, R.; Jia, Z.; Huang, S.; Zhang, A. NLRP3 inflammasome mediates albumin-induced renal tubular injury through impaired mitochondrial function. J. Biol. Chem. 2014, 289, 25101–25111.
- Lucafò, M.; Granata, S.; Bonten, E.J.; McCorkle, R.; Stocco, G.; Caletti, C.; Selvestrel, D.; Cozzarolo, A.; Zou, C.; Cuzzoni, E.; et al. Hypomethylation of NLRP3 gene promoter discriminates glucocorticoid-resistant from glucocorticoid-sensitive idiopathic nephrotic syndrome patients. Clin. Transl. Sci. 2021, 14, 964–975.
- Aranda-Rivera, A.K.; Srivastava, A.; Cruz-Gregorio, A.; Pedraza-Chaverri, J.; Mulay, S.R.; Scholze, A. Involvement of Inflammasome Components in Kidney Disease. Antioxidants 2022, 11, 246.
- Toldo, S.; Mezzaroma, E.; Mauro, A.G.; Salloum, F.; Van Tassell, B.W.; Abbate, A. The inflammasome in myocardial injury and cardiac remodeling. Antioxid. Redox Signal. 2015, 22, 1146–1161.
- Toldo, S.; Mezzaroma, E.; Buckley, L.F.; Potere, N.; Di Nisio, M.; Biondi-Zoccai, G.; Van Tassell, B.W.; Abbate, A. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol. Ther. 2022, 236, 108053.
- Pecchillo Cimmino, T.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOX Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086.
- Schiffers, C.; Reynaert, N.L.; Wouters, E.F.M.; van der Vliet, A. Redox Dysregulation in Aging and COPD: Role of NOX Enzymes and Implications for Antioxidant Strategies. Antioxidants 2021, 10, 1799.
- Chen, L.; Zhao, M.; Li, J.; Wang, Y.; Bao, Q.; Wu, S.; Deng, X.; Tang, X.; Wu, W.; Liu, X. Critical role of X-box binding protein 1 in NADPH oxidase 4-triggered cardiac hypertrophy is mediated by receptor interacting protein kinase 1. Cell Cycle 2017, 16, 348–359.
- Zhang, C.; Chen, X.; Wang, C.; Ran, Y.; Sheng, K. Inhibition of XBP1 Alleviates LPS-Induced Cardiomyocytes Injury by Upregulating XIAP through Suppressing the NF-κB Signaling Pathway. Inflammation 2021, 44, 974–984.
- Camargo, L.L.; Harvey, A.P.; Rios, F.J.; Tsiropoulou, S.; Da Silva, R.N.O.; Cao, Z.; Graham, D.; McMaster, C.; Burchmore, R.J.; Hartley, R.C.; et al. Vascular Nox (NADPH Oxidase) Compartmentalization, Protein Hyperoxidation, and Endoplasmic Reticulum Stress Response in Hypertension. Hypertension 2018, 72, 235–246.
- Hsieh, L.T.; Nastase, M.V.; Zeng-Brouwers, J.; Iozzo, R.V.; Schaefer, L. Soluble biglycan as a biomarker of inflammatory renal diseases. Int. J. Biochem. Cell Biol. 2014, 54, 223–235.
- Moreth, K.; Frey, H.; Hubo, M.; Zeng-Brouwers, J.; Nastase, M.V.; Hsieh, L.T.; Haceni, R.; Pfeilschifter, J.; Iozzo, R.V.; Schaefer, L. Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 35, 143–151.
- Kim, S.Y.; Jeong, J.M.; Kim, S.J.; Seo, W.; Kim, M.H.; Choi, W.M.; Yoo, W.; Lee, J.H.; Shim, Y.R.; Yi, H.S.; et al. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex. Nat. Commun. 2017, 8, 2247.
- Miller, F.J., Jr.; Filali, M.; Huss, G.J.; Stanic, B.; Chamseddine, A.; Barna, T.J.; Lamb, F.S. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ. Res. 2007, 101, 663–671.
- van Bruggen, R.; Köker, M.Y.; Jansen, M.; van Houdt, M.; Roos, D.; Kuijpers, T.W.; van den Berg, T.K. Human NLRP3 inflammasome activation is Nox1-4 independent. Blood 2010, 115, 5398–5400.
- Boor, P.; Ostendorf, T.; Floege, J. Renal fibrosis: Novel insights into mechanisms and therapeutic targets. Nat. Rev. Nephrol. 2010, 6, 643–656.
- Wang, Q.; Usinger, W.; Nichols, B.; Gray, J.; Xu, L.; Seeley, T.W.; Brenner, M.; Guo, G.; Zhang, W.; Oliver, N.; et al. Cooperative interaction of CTGF and TGF-β in animal models of fibrotic disease. Fibrogenesis Tissue Repair 2011, 4, 4.
- Yeh, Y.C.; Wei, W.C.; Wang, Y.K.; Lin, S.C.; Sung, J.M.; Tang, M.J. Transforming growth factor-1 induces Smad3-dependent 1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am. J. Pathol. 2010, 177, 1743–1754.
- Guan, Q.; Nguan, C.Y.; Du, C. Expression of transforming growth factor-beta1 limits renal ischemia-reperfusion injury. Transplantation 2010, 89, 1320–1327.
- Huen, S.C.; Moeckel, G.W.; Cantley, L.G. Macrophage-specific deletion of transforming growth factor-β1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. American journal of physiology. Ren. Physiol. 2013, 305, F477–F484.
- Wang, M.; Weng, X.; Guo, J.; Chen, Z.; Jiang, G.; Liu, X. Metformin alleviated EMT and fibrosis after renal ischemia-reperfusion injury in rats. Ren. Fail. 2016, 38, 614–621.
- Lan, H.Y. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067.
- Zhou, C.; Liu, J.; Ge, Y.; Zhu, Y.; Zhou, L.; Xu, L.; Xu, Z.; Wu, R.; Jia, R. Remote Ischemic Preconditioning Ameliorates Renal Fibrosis After Ischemia-Reperfusion Injury via Transforming Growth Factor beta1 (TGF-β1) Signalling Pathway in Rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e919185.
- Gifford, C.C.; Tang, J.; Costello, A.; Khakoo, N.S.; Nguyen, T.Q.; Goldschmeding, R.; Higgins, P.J.; Samarakoon, R. Negative regulators of TGF-β1 signaling in renal fibrosis; pathological mechanisms and novel therapeutic opportunities. Clin. Sci. 2021, 135, 275–303.
- Felts, S.J.; Owen, B.A.; Nguyen, P.; Trepel, J.; Donner, D.B.; Toft, D.O. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J. Biol. Chem. 2000, 275, 3305–3312.
- Cechetto, J.D.; Gupta, R.S. Immunoelectron microscopy provides evidence that tumor necrosis factor receptor-associated protein 1 (TRAP-1) is a mitochondrial protein which also localizes at specific extramitochondrial sites. Exp. Cell Res. 2000, 260, 30–39.
- Song, C.; Xu, X.; Wu, Y.; Ji, B.; Zhou, X.; Qin, L. Study of the mechanism underlying hsa-miR338-3p downregulation to promote fibrosis of the synovial tissue in osteoarthritis patients. Mol. Biol. Rep. 2019, 46, 627–637.
- Chen, J.H.; Wu, C.H.; Jheng, J.R.; Chao, C.T.; Huang, J.W.; Hung, K.Y.; Liu, S.H.; Chiang, C.K. The down-regulation of XBP1, an unfolded protein response effector, promotes acute kidney injury to chronic kidney disease transition. J. Biomed. Sci. 2022, 29, 46.
- Xue, J.; Zhu, K.; Cao, P.; Long, C.; Deng, Y.; Liu, T.; Yin, G.; Li, X.; Wang, Z. Ischemic preconditioning-induced protective effect for promoting angiogenesis in renal ischemia-reperfusion injury by regulating miR-376c-3p/HIF-1α/VEGF axis in male rats. Life Sci. 2022, 299, 120357.
- Maulik, N.; Das, D.K. Redox signaling in vascular angiogenesis. Free Radic. Biol. Med. 2002, 33, 1047–1060.
- Faleo, G.; Neto, J.S.; Kohmoto, J.; Tomiyama, K.; Shimizu, H.; Takahashi, T.; Wang, Y.; Sugimoto, R.; Choi, A.M.; Stolz, D.B.; et al. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation 2008, 85, 1833–1840.
- Ribatti, D.; d’Amati, A. Bone angiocrine factors. Front. Cell Dev. Biol. 2023, 11, 1244372.
- Hepp, M.; Werion, A.; De Greef, A.; de Ville de Goyet, C.; de Bournonville, M.; Behets, C.; Lengelé, B.; Daumerie, C.; Mourad, M.; Ludgate, M.; et al. Oxidative Stress-Induced Sirtuin1 Downregulation Correlates to HIF-1α, GLUT-1, and VEGF-A Upregulation in Th1 Autoimmune Hashimoto’s Thyroiditis. Int. J. Mol. Sci. 2021, 22, 3806.
- Ricciardi, C.A.; Gnudi, L. Vascular growth factors as potential new treatment in cardiorenal syndrome in diabetes. Eur. J. Clin. Investig. 2021, 51, e13579.
- Ricciardi, C.A.; Gnudi, L. The endoplasmic reticulum stress and the unfolded protein response in kidney disease: Implications for vascular growth factors. J. Cell. Mol. Med. 2020, 24, 12910–12919.
- Lemos, F.B.; Ijzermans, J.N.; Zondervan, P.E.; Peeters, A.M.; van den Engel, S.; Mol, W.M.; Weimar, W.; Baan, C.C. Differential expression of heme oxygenase-1 and vascular endothelial growth factor in cadaveric and living donor kidneys after ischemia-reperfusion. J. Am. Soc. Nephrol. 2003, 14, 3278–3287.
- Kanellis, J.; Mudge, S.J.; Fraser, S.; Katerelos, M.; Power, D.A. Redistribution of cytoplasmic VEGF to the basolateral aspect of renal tubular cells in ischemia-reperfusion injury. Kidney Int. 2000, 57, 2445–2456.
- Basile, D.P.; Fredrich, K.; Chelladurai, B.; Leonard, E.C.; Parrish, A.R. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am. J. Physiol. Ren. Physiol. 2008, 294, F928–F936.
- Dutta, A.; Saha, S.; Bahl, A.; Mittal, A.; Basak, T. A comprehensive review of acute cardio-renal syndrome: Need for novel biomarkers. Front. Pharmacol. 2023, 14, 1152055.
- Larouche-Lebel, É.; Loughran, K.A.; Oyama, M.A.; Solter, P.F.; Laughlin, D.S.; Sánchez, M.D.; Assenmacher, C.A.; Fox, P.R.; Fries, R.C. Plasma and tissue angiotensin-converting enzyme 2 activity and plasma equilibrium concentrations of angiotensin peptides in dogs with heart disease. J. Vet. Intern. Med. 2019, 33, 1571–1584.
- Hamatani, H.; Eng, D.G.; Kaverina, N.V.; Gross, K.W.; Freedman, B.S.; Pippin, J.W.; Shankland, S.J. Lineage tracing aged mouse kidneys shows lower number of cells of renin lineage and reduced responsiveness to RAAS inhibition. Am. J. Physiol. Ren. Physiol. 2018, 315, F97–F109.
- Alshahrani, S. Aliskiren—A promising antioxidant agent beyond hypertension reduction. Chem.-Biol. Interact. 2020, 326, 109145.
- Zhao, S.; Ghosh, A.; Lo, C.S.; Chenier, I.; Scholey, J.W.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S.D. Nrf2 Deficiency Upregulates Intrarenal Angiotensin-Converting Enzyme-2 and Angiotensin 1-7 Receptor Expression and Attenuates Hypertension and Nephropathy in Diabetic Mice. Endocrinology 2018, 159, 836–852.
- Bi, X.; Zhang, G.; Wang, X.; Nguyen, C.; May, H.I.; Li, X.; Al-Hashimi, A.A.; Austin, R.C.; Gillette, T.G.; Fu, G.; et al. Endoplasmic Reticulum Chaperone GRP78 Protects Heart From Ischemia/Reperfusion Injury Through Akt Activation. Circ. Res. 2018, 122, 1545–1554.
- Yang, J.; Xu, J.; Danniel, M.; Wang, X.; Wang, W.; Zeng, L.; Shen, L. The interaction between XBP1 and eNOS contributes to endothelial cell migration. Exp. Cell Res. 2018, 363, 262–270.
- Khoi, C.S.; Xiao, C.Q.; Hung, K.Y.; Lin, T.Y.; Chiang, C.K. Oxidative Stress-Induced Growth Inhibitor (OSGIN1), a Target of X-Box-Binding Protein 1, Protects Palmitic Acid-Induced Vascular Lipotoxicity through Maintaining Autophagy. Biomedicines 2022, 10, 992.
- Fayard, E.; Tintignac, L.A.; Baudry, A.; Hemmings, B.A. Protein kinase B/Akt at a glance. J. Cell Sci. 2005, 118, 5675–5678.
- Borok, Z.; Horie, M.; Flodby, P.; Wang, H.; Liu, Y.; Ganesh, S.; Firth, A.L.; Minoo, P.; Li, C.; Beers, M.F.; et al. Grp78 Loss in Epithelial Progenitors Reveals an Age-linked Role for Endoplasmic Reticulum Stress in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 198–211.
- Deng, F.; Sharma, I.; Dai, Y.; Yang, M.; Kanwar, Y.S. Myo-inositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule. J. Clin. Investig. 2019, 129, 5033–5049.
- Yoshida, H. Unconventional splicing of XBP-1 mRNA in the unfolded protein response. Antioxid. Redox Signal. 2007, 9, 2323–2333.
- Yücel, S.S.; Stelzer, W.; Lorenzoni, A.; Wozny, M.; Langosch, D.; Lemberg, M.K. The Metastable XBP1u Transmembrane Domain Defines Determinants for Intramembrane Proteolysis by Signal Peptide Peptidase. Cell Rep. 2019, 26, 3087–3099.e3011.
- Nowak, W.N.; Deng, J.; Ruan, X.Z.; Xu, Q. Reactive Oxygen Species Generation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e41–e52.
- Zeng, L.; Zampetaki, A.; Margariti, A.; Pepe, A.E.; Alam, S.; Martin, D.; Xiao, Q.; Wang, W.; Jin, Z.G.; Cockerill, G.; et al. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc. Natl. Acad. Sci. USA 2009, 106, 8326–8331.
- Martin, D.; Li, Y.; Yang, J.; Wang, G.; Margariti, A.; Jiang, Z.; Yu, H.; Zampetaki, A.; Hu, Y.; Xu, Q.; et al. Unspliced X-box-binding protein 1 (XBP1) protects endothelial cells from oxidative stress through interaction with histone deacetylase 3. J. Biol. Chem. 2014, 289, 30625–30634.
- Yang, L.; Dai, R.; Wu, H.; Cai, Z.; Xie, N.; Zhang, X.; Shen, Y.; Gong, Z.; Jia, Y.; Yu, F.; et al. Unspliced XBP1 Counteracts β-Catenin to Inhibit Vascular Calcification. Circ. Res. 2022, 130, 213–229.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
808
Revisions:
4 times
(View History)
Update Date:
15 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




