
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karsten Königstein | -- | 2511 | 2023-11-13 09:27:17 | | | |
| 2 | Lindsay Dong | Meta information modification | 2511 | 2023-11-14 01:13:21 | | |
Video Upload Options
Accelerated biological vascular ageing is still a major driver of the increasing burden of cardiovascular disease and mortality. Exercise training delays this process, known as early vascular ageing, but often lacks effectiveness due to a lack of understanding of molecular and clinical adaptations to specific stimuli.
1. Background
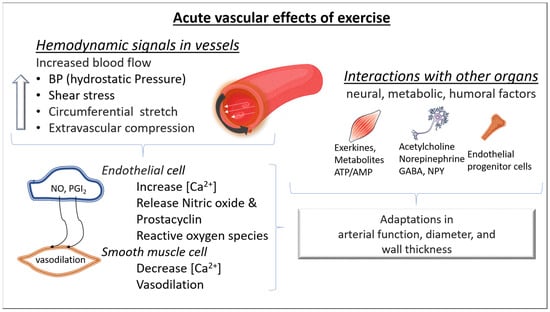
2. Chronic Adaptations of Blood Vessels to Exercise
2.1. Long-Term Structural and Functional Vascular Adaptations in Response to Regular Exercise Training
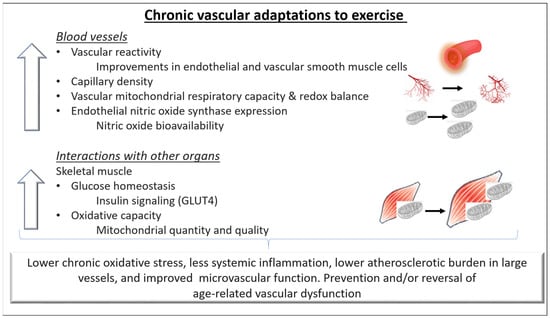
2.2. The Role of Mitochondria in Exercise-Induced Vascular Benefits
3. Clinical Vascular Effects of Exercise
3.1. Effects of Exercise on Vascular Health
3.2. Clinical Vascular Effects of Short-Versus Long-Term Exercise
3.2.1. Influence of Age
3.2.2. Influence of Cardiovascular Risk Factors and Diseases
3.2.3. Influence of Training Status and Physical Fitness Level
4. Exercise Training to Improve Vascular Fitness
4.1. General Aspects of Exercise Training to Improve Vascular Health
4.2. F-I-T-T Principle: F(requency)
4.3. F-I-T-T Principle: I(ntensity)
Aerobic exercise training: One study conducted a 12-week aerobic, ergometer-based training intervention at mild, moderate, and high exercise intensity [56]. Interestingly, only moderate but not high intensity led to improvements of nitric oxide-dependent endothelial function and less oxidative stress. The authors concluded that high intensities of aerobic exercise might induce massive acute oxidative and inflammatory stress, potentially attenuating favorable effects of elevated shear stress. Consistently, the short-term decrease in vascular function immediately after an exercise bout becomes larger with increasing exercise intensities [57].
4.4. F-I-T-T Principle: T(ime)
4.5. F-I-T-T Principle: T(ype)
4.6. Individualization
References
- Widlansky, M.E.; Gokce, N.; Keaney, J.F.; Vita, J.A., Jr. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160.
- Danninger, K.; Hafez, A.; Binder, R.K.; Aichberger, M.; Hametner, B.; Wassertheurer, S.; Weber, T. High prevalence of hypertension and early vascular aging: A screening program in pharmacies in Upper Austria. J. Hum. Hypertens. 2020, 34, 326–334.
- Nilsson, P.M.; Boutouyrie, P.; Cunha, P.; Kotsis, V.; Narkiewicz, K.; Parati, G.; Rietzschel, E.; Scuteri, A.; Laurent, S. Early vascular ageing in translation: From laboratory investigations to clinical applications in cardiovascular prevention. J. Hypertens. 2013, 31, 1517–1526.
- Vaitkevicius, P.V.; Fleg, J.L.; Engel, J.H.; O’Connor, F.C.; Wright, J.G.; Lakatta, L.E.; Yin, F.C.; Lakatta, E.G. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 1993, 88, 1456–1462.
- Konigstein, K.; Wagner, J.; Infanger, D.; Knaier, R.; Nève, G.; Klenk, C.; Carrard, J.; Hinrichs, T.; Schmidt-Trucksäss, A. Cardiorespiratory Fitness and Endothelial Function in Aging Healthy Subjects and Patients With Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 9, 870847.
- Pinto, R.; Melo, X.; Angarten, V.; Pires, M.L.; Borges, M.; Santos, V.; Abreu, A.; Santa-Clara, H. The effects of 12-months supervised periodized training on health-related physical fitness in coronary artery disease: A randomized controlled trial. J. Sports Sci. 2021, 39, 1893–1902.
- Königstein, K.; Meier, J.; Angst, T.; Maurer, D.J.; Kröpfl, J.M.; Carrard, J.; Infanger, D.; Baumann, S.; Bischofsberger, I.; Harder, M.; et al. VascuFit: Vascular Effects of Non-linear Periodized Exercise Training in Sedentary Adults with Elevated Cardiovascular Risk—Protocol for a Randomized Controlled Trial. BMC Cardiovasc. Disord. 2022, 27, 449.
- Kojda, G.; Hambrecht, R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc. Res. 2005, 67, 187–197.
- Harrison, D.G.; Cai, H. Endothelial control of vasomotion and nitric oxide production. Cardiol. Clin. 2003, 21, 289–302.
- Vasiliadis, A.V.; Zafeiridis, A.; Dipla, K.; Galanis, N.; Chatzidimitriou, D.; Kyparos, A.; Nikolaidis, M.G.; Vrabas, I.S. Circulating angiogenic biomolecules at rest and in response to upper-limb exercise in individuals with spinal cord injury. J. Spinal Cord. Med. 2014, 37, 226–232.
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684.
- Tomanek, R.J.; Schatteman, G.C. Angiogenesis: New insights and therapeutic potential. Anat. Rec. 2000, 261, 126–135.
- Brodal, P.; Ingjer, F.; Hermansen, L. Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am. J. Physiol. 1977, 232, H705–H712.
- Gute, D.; Laughlin, M.H.; Amann, J.F. Regional changes in capillary supply in skeletal muscle of interval-sprint and low-intensity, endurance-trained rats. Microcirculation 1994, 1, 183–193.
- Taylor, J.L.; Keating, S.E.; Holland, D.J.; Green, D.J.; Coombes, J.S.; Bailey, T.G. Comparison of high intensity interval training with standard cardiac rehabilitation on vascular function. Scand. J. Med. Sci. Sports 2022, 32, 512–520.
- Thomas, H.J.; Marsh, C.E.; Naylor, L.H.; Ainslie, P.N.; Smith, K.J.; Carter, H.H.; Green, D.J. Resistance, but not endurance exercise training, induces changes in cerebrovascular function in healthy young subjects. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H881–H892.
- Hood, D.A.; Uguccioni, G.; Vainshtein, A.; D’Souza, D. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle: Implications for health and disease. Compr. Physiol. 2011, 1, 1119–1134.
- Zhang, Y.; Oliveira, A.N.; Hood, D.A. The intersection of exercise and aging on mitochondrial protein quality control. Exp. Gerontol. 2020, 131, 110824.
- Guan, Y.; Drake, J.C.; Yan, Z. Exercise-Induced Mitophagy in Skeletal Muscle and Heart. Exerc. Sport. Sci. Rev. 2019, 47, 151–156.
- Lundby, C.; Jacobs, R.A. Adaptations of skeletal muscle mitochondria to exercise training. Exp. Physiol. 2016, 101, 17–22.
- Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L. Autophagy and Mitophagy in Cardiovascular Disease. Circ. Res. 2017, 120, 1812–1824.
- Lee, D.C.; Sui, X.; Artero, E.G.; Lee, I.-M.; Church, T.S.; McAuley, P.A.; Stanford, F.C.; Kohl, H.W.; Blair, S.N. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: The Aerobics Center Longitudinal Study. Circulation 2011, 124, 2483–2490.
- Paffenbarger, R.S.; Kampert, J.B., Jr.; Lee, I.M.; Hyde, R.T.; Leung, R.W.; Wing, A.L. Changes in physical activity and other lifeway patterns influencing longevity. Med. Sci. Sports Exerc. 1994, 26, 857–865.
- Wagner, J.; Knaier, R.; Konigstein, K.; Klenk, C.; Carrard, J.; Lichtenstein, E.; Scharnagl, H.; März, W.; Hanssen, H.; Hinrichs, T.; et al. Composite Measures of Physical Fitness to Discriminate Between Healthy Aging and Heart Failure: The COmPLETE Study. Front. Physiol. 2020, 11, 596240.
- Green, D.J.; O’Driscoll, G.; Joyner, M.J.; Cable, N.T. Exercise and cardiovascular risk reduction: Time to update the rationale for exercise? J. Appl. Physiol. (1985) 2008, 105, 766–768.
- Miyachi, M.; Tanaka, H.; Yamamoto, K.; Yoshioka, A.; Takahashi, K.; Onodera, S. Effects of one-legged endurance training on fem oral arterial and venous size in healthy humans. J. Appl. Physiol. 2001, 90, 2439–2444.
- Montero, D. The association of cardiorespiratory fitness with endothelial or smooth muscle vasodilator function. Eur. J. Prev. Cardiol. 2015, 22, 1200–1211.
- Braun, G.; Hafner, B.; Konigstein, K.; Infanger, D.; Klenk, C.; Rossmeissl, A.; Schmidt-Trucksäss, A.; Hanssen, H. Association of cardiorespiratory fitness with retinal vessel diameters as a biomarker of cardiovascular risk. Microvasc. Res. 2018, 120, 36–40.
- Germano-Soares, A.H.; Andrade-Lima, A.; Meneses, A.L.; Correia, M.A.; Parmenter, B.J.; Tassitano, R.M.; Cucato, G.G.; Ritti-Dias, R.M. Association of time spent in physical activities and sedentary behaviors with carotid-femoral pulse wave velocity: A systematic review and meta-analysis. Atheroscler 2018, 269, 211–218.
- Garcia-Hermoso, A.; Gonzalez-Ruiz, K.; Triana-Reina, H.R.; Olloquequi, J.; Ramirez-Velez, R. Effects of Exercise on Carotid Arterial Wall Thickness in Obese Pediatric Populations: A Meta-Analysis of Randomized Controlled Trials. Child. Obes. 2017, 13, 138–145.
- Dawson, E.A.; Cable, N.T.; Green, D.J.; Thijssen, D.H.J. Do acute effects of exercise on vascular function predict adaptation to training? Eur. J. Appl. Physiol. 2018, 118, 523–530.
- Oudegeest-Sander, M.H.; Olde Rikkert, M.G.; Smits, P.; Thijssen, D.H.; van Dijk, A.P.; Levine, B.D.; Hopman, M.T. The effect of an advanced glycation end-product crosslink breaker and exercise training on vascular function in older individuals: A randomized factorial design trial. Exp. Gerontol. 2013, 48, 1509–1517.
- Rauramaa, R.; Halonen, P.; Vaisanen, S.B.; Lakka, T.A.; Schmidt-Trucksäss, A.; Berg, A.; Penttilä, I.M.; Rankinen, T.; Bouchard, C. Effects of aerobic physical exercise on inflammation and atherosclerosis in men: The DNASCO Study: A six-year randomized, controlled trial. Ann. Intern. Med. 2004, 140, 1007–1014.
- Cayres, S.U.; Agostinete, R.R.; de Moura Mello Antunes, B.; Lira, F.S.; Fernandes, R.A. Impact of physical exercise/activity on vascular structure and inflammation in pediatric populations: A literature review. J. Spec. Pediatr. Nurs. 2016, 21, 99–108.
- Ried-Larsen, M.; Grontved, A.; Kristensen, P.L.; Froberg, K.; Andersen, L.B. Moderate-and-vigorous physical activity from adolescence to adulthood and subclinical atherosclerosis in adulthood: Prospective observations from the European Youth Heart Study. Br. J. Sports Med. 2015, 49, 107–112.
- Konigstein, K.; Buschges, J.C.; Sarganas, G.; Krug, S.; Neuhauser, H.; Schmidt-Trucksass, A. Exercise and Carotid Properties in the Young-The KiGGS-2 Study. Front. Cardiovasc. Med. 2021, 8, 767025.
- Spinelli, A.; Buoncristiano, M.; Nardone, P.; Starc, G.; Hejgaard, T.; Júlíusson, P.B.; Fismen, A.; Weghuber, D.; Milanović, S.M.; García-Solano, M.; et al. Thinness, overweight, and obesity in 6- to 9-year-old children from 36 countries: The World Health Organization European Childhood Obesity Surveillance Initiative-COSI 2015–2017. Obes. Rev. 2021, 22 (Suppl. S6), e13214.
- Collier, S.R.; Kanaley, J.A.; Carhart, R., Jr.; Frechette, V.; Tobin, M.M.; Hall, A.K.; Luckenbaugh, A.N.; Fernhall, B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J. Hum. Hypertens. 2008, 22, 678–686.
- Pearson, M.J.; Smart, N.A. Effect of exercise training on endothelial function in heart failure patients: A systematic review meta-analysis. Int. J. Cardiol. 2017, 231, 234–243.
- Zhang, X.; Cheng, R.; Rowe, D.; Sethu, P.; Daugherty, A.; Yu, G.; Shin, H.Y. Shear-sensitive regulation of neutrophil flow behavior and its potential impact on microvascular blood flow dysregulation in hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 587–593.
- Sun, D.; Huang, A.; Yan, E.H.; Wu, Z.; Yan, C.; Kaminski, P.M.; Oury, T.D.; Wolin, M.S.; Kaley, G.; Csiszar, A.; et al. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2249–H2256.
- Kasikcioglu, E.; Oflaz, H.; Kasikcioglu, H.A.; Kayserilioglu, A.; Umman, S.; Meric, M. Endothelial flow-mediated dilatation and exercise capacity in highly trained endurance athletes. Tohoku J. Exp. Med. 2005, 205, 45–51.
- Tanriverdi, H.; Evrengul, H.; Tanriverdi, S.; Turgut, S.; Akdag, B.; Kaftan, H.A.; Semiz, E. Improved endothelium dependent vasodilation in endurance athletes and its relation with ACE I/D polymorphism. Circ. J. 2005, 69, 1105–1110.
- Walther, G.; Nottin, S.; Karpoff, L.; Perez-Martin, A.; Dauzat, M.; Obert, P. Flow-mediated dilation and exercise-induced hyperaemia in highly trained athletes: Comparison of the upper and lower limb vasculature. Acta Physiol. 2008, 193, 139–150.
- Rognmo, O.; Bjornstad, T.H.; Kahrs, C.; Tjønna, A.E.; Bye, A.; Haram, P.M.; Stølen, T.; Slørdahl, S.A.; Wisløff, U. Endothelial function in highly endurance-trained men: Effects of acute exercise. J. Strength. Cond. Res. 2008, 22, 535–542.
- Petersen, S.E.; Wiesmann, F.; Hudsmith, L.E.; Robson, M.D.; Francis, J.M.; Selvanayagam, J.B.; Neubauer, S.; Channon, K.M. Functional and structural vascular remodeling in elite rowers assessed by cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2006, 48, 790–797.
- Green, D.J.; Rowley, N.; Spence, A.; Carter, H.; Whyte, G.; George, K.; Naylor, L.H.; Cable, N.T.; Dawson, E.A.; Thijssen, D.H. Why isn’t flow-mediated dilation enhanced in athletes? Med. Sci. Sports Exerc. 2013, 45, 75–82.
- WHO. WHO Global Recommendations on Physical Activity for Health. 2010. WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2010.
- Williams, P.T.; Thompson, P.D. Walking versus running for hypertension, cholesterol, and diabetes mellitus risk reduction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1085–1091.
- Campbell, A.; Grace, F.; Ritchie, L.; Beaumont, A.; Sculthorpe, N. Long-Term Aerobic Exercise Improves Vascular Function into Old Age: A Systematic Review, Meta-Analysis and Meta Regression of Observational and Interventional Studies. Front. Physiol. 2019, 10, 31.
- Montero, D.; Roberts, C.K.; Vinet, A. Effect of aerobic exercise training on arterial stiffness in obese populations: A systematic review and meta-analysis. Sports Med. 2014, 44, 833–843.
- Konigstein, K.; Infanger, D.; Klenk, C.; Carrard, J.; Hinrichs, T.; Schmidt-Trucksass, A. Physical activity is favorably associated with arterial stiffness in patients with obesity and elevated metabolic risk. Int. J. Clin. Pract. 2020, 74, e13563.
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Oggioni, C.; Jakovljevic, D.G.; Mathers, J.C. Exercise modalities and endothelial function: A systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med. 2015, 45, 279–296.
- Pyke, K.E.; Tschakovsky, M.E. Peak vs. total reactive hyperemia: Which determines the magnitude of flow-mediated dilation? J. Appl. Physiol. (1985) 2007, 102, 1510–1519.
- Zhang, J.; Friedman, M.H. Adaptive response of vascular endothelial cells to an acute increase in shear stress frequency. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H894–H902.
- Goto, C.; Higashi, Y.; Kimura, M.; Noma, K.; Hara, K.; Nakagawa, K.; Kawamura, M.; Chayama, K.; Yoshizumi, M.; Nara, I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: Role of endothelium-dependent nitric oxide and oxidative stress. Circulation 2003, 108, 530–535.
- Dawson, E.A.; Green, D.J.; Cable, N.T.; Thijssen, D.H. Effects of acute exercise on flow-mediated dilatation in healthy humans. J. Appl. Physiol. (1985) 2013, 115, 1589–1598.
- Early, K.S.; Stewart, A.; Johannsen, N.; Lavie, C.J.; Thomas, J.R.; Welsch, M. The Effects of Exercise Training on Brachial Artery Flow-Mediated Dilation: A Meta-analysis. J. Cardiopulm. Rehabil. Prev. 2017, 37, 77–89.
- Rowley, N.J.; Dawson, E.A.; Birk, G.K.; Cable, N.T.; George, K.; Whyte, G.; Thijssen, D.H.; Green, D.J. Exercise and arterial adaptation in humans: Uncoupling localized and systemic effects. J. Appl. Physiol. 2011, 110, 1190–1195.
- Kozakova, M.; Palombo, C. Vascular Ageing and Aerobic Exercise. Int. J. Environ. Res. Public Health 2021, 18, 10666.
- Conraads, V.M.; Deaton, C.; Piotrowicz, E.; Santaularia, N.; Tierney, S.; Piepoli, M.F.; Pieske, B.; Schmid, J.-P.; Dickstein, K.; Ponikowski, P.; et al. Adherence of heart failure patients to exercise: Barriers and possible solutions: A position statement of the Study Group on Exercise Training in Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2012, 14, 451–458.
- Sallis, R.; Franklin, B.; Joy, L.; Ross, R.; Sabgir, D.; Stone, J. Strategies for promoting physical activity in clinical practice. Prog. Cardiovasc. Dis. 2015, 57, 375–386.
- Sjosten, N.M.; Salonoja, M.; Piirtola, M.; Vahlberg, T.J.; Isoaho, R.; Hyttinen, H.K.; Aarnio, P.T.; Kivelä, S.-L. A multifactorial fall prevention programme in the community-dwelling aged: Predictors of adherence. Eur. J. Public Health 2007, 17, 464–470.
- Santos-Parker, J.R.; Strahler, T.R.; Vorwald, V.M.; Pierce, G.L.; Seals, D.R. Habitual aerobic exercise does not protect against micro- or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. J. Appl. Physiol. 2017, 122, 11–19.
- Seals, D.R.; Nagy, E.E.; Moreau, K.L. Aerobic exercise training and vascular function with ageing in healthy men and women. J. Physiol. 2019, 597, 4901–4914.




