
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alyssa Francavilla | -- | 3031 | 2023-11-11 21:16:00 | | | |
| 2 | Jason Zhu | Meta information modification | 3031 | 2023-11-13 07:00:33 | | |
Video Upload Options
Bigels have been mainly applied in the pharmaceutical sector for the controlled release of drugs or therapeutics. However, these systems, with their intricate structures, hold great promise for wider application in food products. Besides their classical role as carrier and target delivery vehicles for molecules of interest, bigels may also be valuable tools for building complex food structures. In the context of reducing or even eliminating undesirable (but often highly functional) food components, current strategies often critically affect food structure and palatability. The production of solid fat systems that are trans-fat-free and have high levels of unsaturated fatty acids is one of the challenges the food industry currently faces.
1. Introduction
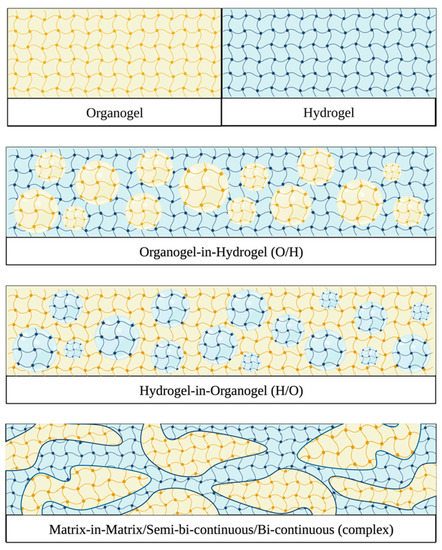
2. Composition and Production Methods

3. Characterization

3.1. Microstructural Analysis
3.2. Rheological and Mechanical Testing
3.3. Other Characterization Methods
References
- Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bonferoni, M.C.; Tamayo, A.; Veiga, M.D. Bigels as Drug Delivery Systems: From Their Components to Their Applications. Drug Discov. Today 2022, 27, 1008–1026.
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide Hydrogels for Modified Release Formulations. J. Control. Release 2007, 119, 5–24.
- Padma Ishwarya, S.; Sandhya, R.; Nisha, P. Advances and Prospects in the Food Applications of Pectin Hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 4393–4417.
- de Lima, C.S.A.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970.
- Alsaab, H.; Bonam, S.P.; Bahl, D.; Chowdhury, P.; Alexander, K.; Boddu, S.H. Organogels in Drug Delivery: A Special Emphasis on Pluronic Lecithin Organogels. J. Pharm. Pharm. Sci. 2016, 19, 252–273.
- Shakeel, A.; Farooq, U.; Gabriele, D.; Marangoni, A.G.; Lupi, F.R. Bigels and Multi-Component Organogels: An Overview from Rheological Perspective. Food Hydrocoll. 2021, 111, 106190.
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, Promising Drug Delivery Systems: An Update of State-of-the-Art and Recent Applications. J. Control. Release 2018, 271, 1–20.
- Frolova, Y.; Sarkisyan, V.; Sobolev, R.; Kochetkova, A. Ultrasonic Treatment of Food Colloidal Systems Containing Oleogels: A Review. Gels 2022, 8, 801.
- Singh, A.; Auzanneau, F.I.; Rogers, M.A. Advances in Edible Oleogel Technologies—A Decade in Review. Food Res. Int. 2017, 97, 307–317.
- Quilaqueo, M.; Iturra, N.; Contardo, I.; Millao, S.; Morales, E.; Rubilar, M. Food-Grade Bigels with Potential to Replace Saturated and Trans Fats in Cookies. Gels 2022, 8, 445.
- Shakeel, A.; Lupi, F.R.; Gabriele, D.; Baldino, N.; De Cindio, B. Bigels: A Unique Class of Materials for Drug Delivery Applications. Soft Mater. 2018, 16, 77–93.
- Almeida, I.F.; Fernandes, A.R.; Fernandes, L.; Pena Ferreira, M.R.; Costa, P.C.; Bahia, M.F. Moisturizing Effect of Oleogel/Hydrogel Mixtures. Pharm. Dev. Technol. 2008, 13, 487–494.
- Shakeel, A.; Farooq, U.; Iqbal, T.; Yasin, S.; Lupi, F.R.; Gabriele, D. Key Characteristics and Modelling of Bigels Systems: A Review. Mater. Sci. Eng. C 2019, 97, 932–953.
- Lupi, F.R.; Shakeel, A.; Greco, V.; Oliviero Rossi, C.; Baldino, N.; Gabriele, D. A Rheological and Microstructural Characterisation of Bigels for Cosmetic and Pharmaceutical Uses. Mater. Sci. Eng. C 2016, 69, 358–365.
- Chen, Z.; Bian, F.; Cao, X.; Shi, Z.; Meng, Z. Novel Bigels Constructed from Oleogels and Hydrogels with Contrary Thermal Characteristics: Phase Inversion and 3D Printing Applications. Food Hydrocoll. 2023, 134, 108063.
- Jiang, Q.; Wang, Y.; Du, L.; Li, S.; Liu, Y.; Meng, Z. Catastrophic Phase Inversion of Bigels Characterized by Fluorescence Intensity-Based 3D Modeling and the Formability for Decorating and 3D Printing. Food Hydrocoll. 2022, 126, 107461.
- Lan, Y.; Corradini, M.G.; Weiss, R.G.; Raghavan, S.R.; Rogers, M.A. To Gel or Not to Gel: Correlating Molecular Gelation with Solvent Parameters. Chem. Soc. Rev. 2015, 44, 6035–6058.
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053.
- Tanislav, A.E.; Pușcaș, A.; Mureșan, V.; Mudura, E. The Oxidative Quality of Bi-, Oleo- and Emulgels and Their Bioactives Molecules Delivery. Crit. Rev. Food Sci. Nutr. 2023, 1–27.
- Golodnizky, D.; Davidovich-Pinhas, M. The Effect of the HLB Value of Sucrose Ester on Physiochemical Properties of Bigel Systems. Foods 2020, 9, 1857.
- Saffold, A.C.; Acevedo, N.C. The Effect of Mono-Diglycerides on the Mechanical Properties, Microstructure, and Physical Stability of an Edible Rice Bran Wax–Gelatin Biphasic Gel System. JAOCS J. Am. Oil Chem. Soc. 2022, 99, 1033–1043.
- Gallegos-Infante, J.A.; Galindo-Galindo, M.d.P.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; González-Laredo, R.F. Effect of Aqueous Extracts of Quercus Resinosa on the Mechanical Behavior of Bigels. Sci. Pharm. 2022, 90, 73.
- Martinez, R.M.; Filho, P.L.O.; Gerbelli, B.B.; Magalhães, W.V.; Velasco, M.V.R.; Lannes, S.C.d.S.; de Oliveira, C.L.P.; Rosado, C.; Baby, A.R. Influence of the Mixtures of Vegetable Oil and Vitamin E over the Microstructure and Rheology of Organogels. Gels 2022, 8, 36.
- Vershkov, B.; Davidovich-Pinhas, M. The Effect of Preparation Temperature and Composition on Bigel Performance as Fat Replacers. Food Funct. 2023, 14, 3838–3848.
- Satapathy, S.; Singh, V.K.; Sagiri, S.S.; Agarwal, T.; Banerjee, I.; Bhattacharya, M.K.; Kumar, N.; Pal, K. Development and Characterization of Gelatin-Based Hydrogels, Emulsion Hydrogels, and Bigels: A Comparative Study. J. Appl. Polym. Sci. 2015, 132, 41502.
- Habibi, A.; Kasapis, S.; Truong, T. Effect of Hydrogel Particle Size Embedded into Oleogels on the Physico-Functional Properties of Hydrogel-in-Oleogel (Bigels). LWT 2022, 163, 113501.
- Martins, A.J.; Silva, P.; Maciel, F.; Pastrana, L.M.; Cunha, R.L.; Cerqueira, M.A.; Vicente, A.A. Hybrid Gels: Influence of Oleogel/Hydrogel Ratio on Rheological and Textural Properties. Food Res. Int. 2019, 116, 1298–1305.
- Alves Barroso, L.; Grossi Bovi Karatay, G.; Dupas Hubinger, M. Effect of Potato Starch Hydrogel:Glycerol Monostearate Oleogel Ratio on the Physico-Rheological Properties of Bigels. Gels 2022, 8, 694.
- Pérez-Salas, J.L.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; Rosas-Flores, W.; González-Laredo, R.F.; Medina-Torres, L.; Gallegos-Infante, J.A. Effect of Storage Time on the Mechanical and Microstructural Properties of Bigels Based on Xanthan Gum and Castor Oil. JAOCS J. Am. Oil Chem. Soc. 2023.
- Samui, T.; Goldenisky, D.; Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The Development and Characterization of Novel In-Situ Bigel Formulation. Food Hydrocoll. 2021, 113, 106416.
- Yang, J.; Zheng, H.; Mo, Y.; Gao, Y.; Mao, L. Structural Characterization of Hydrogel-Oleogel Biphasic Systems as Affected by Oleogelators. Food Res. Int. 2022, 158, 111536.
- Saffold, A.C.; Acevedo, N.C. Development of Novel Rice Bran Wax/Gelatin-Based Biphasic Edible Gels and Characterization of Their Microstructural, Thermal, and Mechanical Properties. Food Bioproc. Tech. 2021, 14, 2219–2230.
- Paul, S.R.; Qureshi, D.; Yogalakshmi, Y.; Nayak, S.K.; Singh, V.K.; Syed, I.; Sarkar, P.; Pal, K. Development of Bigels Based on Stearic Acid–Rice Bran Oil Oleogels and Tamarind Gum Hydrogels for Controlled Delivery Applications. J. Surfactants Deterg. 2018, 21, 17–29.
- Martinez, R.M.; Magalhães, W.V.; Sufi, B.d.S.; Padovani, G.; Nazato, L.I.S.; Velasco, M.V.R.; Lannes, S.C.d.S.; Baby, A.R. Vitamin E-Loaded Bigels and Emulsions: Physicochemical Characterization and Potential Biological Application. Colloids Surf. B Biointerfaces 2021, 201, 111651.
- Singh, V.K.; Anis, A.; Banerjee, I.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Preparation and Characterization of Novel Carbopol Based Bigels for Topical Delivery of Metronidazole for the Treatment of Bacterial Vaginosis. Mater. Sci. Eng. C 2014, 44, 151–158.
- Kodela, S.P.; Pandey, P.M.; Nayak, S.K.; Uvanesh, K.; Anis, A.; Pal, K. Novel Agar–Stearyl Alcohol Oleogel-Based Bigels as Structured Delivery Vehicles. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 669–678.
- Andonova, V.; Peneva, P.; Georgiev, G.S.; Toncheva, V.T.; Apostolova, E.; Peychev, Z.; Dimitrova, S.; Katsarova, M.; Petrova, N.; Kassarova, M. Ketoprofen-Loaded Polymer Carriers in Bigel Formulation: An Approach to Enhancing Drug Photostability in Topical Application Forms. Int. J. Nanomed. 2017, 12, 6221–6238.
- Cui, H.; Tang, C.; Wu, S.; Julian McClements, D.; Liu, S.; Li, B.; Li, Y. Fabrication of Chitosan-Cinnamaldehyde-Glycerol Monolaurate Bigels with Dual Gelling Effects and Application as Cream Analogs. Food Chem. 2022, 384, 132589.
- Mousavi, S.N.; Hosseini, E.; Seyed Dorraji, M.S.; Sheikh Mohammadi, S.; Pourmansouri, Z.; Rasoulifard, M.H.; Doosti, M.; Chiti, H. Synthesis of a Green Bigel Using Cottonseed Oil/Cannabis Oil/Alginate/Ferula Gum for Quercetin Release: Synergistic Effects for Treating Infertility in Rats. Int. J. Biol. Macromol. 2021, 177, 157–165.
- Bakonyi, M.; Gácsi, A.; Kovács, A.; Szűcs, M.B.; Berkó, S.; Csányi, E. Following-up Skin Penetration of Lidocaine from Different Vehicles by Raman Spectroscopic Mapping. J. Pharm. Biomed. Anal. 2018, 154, 1–6.
- Gravelle, A.J.; Nicholson, R.A.; Barbut, S.; Marangoni, A.G. The Impact of Model Rigid Fillers in Acid-Induced Sodium Caseinate/Xanthan Gum Cooperative Protein Gels. Food Hydrocoll. 2021, 113, 106439.
- Gravelle, A.J.; Nicholson, R.A.; Barbut, S.; Marangoni, A.G. Considerations for Readdressing Theoretical Descriptions of Particle-Reinforced Composite Food Gels. Food Res. Int. 2019, 122, 209–221.
- Lupi, F.R.; De Santo, M.P.; Ciuchi, F.; Baldino, N.; Gabriele, D. A Rheological Modelling and Microscopic Analysis of Bigels. Rheol. Acta 2017, 56, 753–763.
- Lupi, F.R.; Gentile, L.; Gabriele, D.; Mazzulla, S.; Baldino, N.; de Cindio, B. Olive Oil and Hyperthermal Water Bigels for Cosmetic Uses. J. Colloid Interface Sci. 2015, 459, 70–78.
- Rehman, K.; Amin, M.C.I.M.; Zulfakar, M.H. Development and Physical Characterization of Polymer-Fish Oil Bigel (Hydrogel/Oleogel) System as a Transdermal Drug Delivery Vehicle. J. Oleo Sci. 2014, 63, 961–970.
- Wakhet, S.; Singh, V.K.; Sahoo, S.; Sagiri, S.S.; Kulanthaivel, S.; Bhattacharya, M.K.; Kumar, N.; Banerjee, I.; Pal, K. Characterization of Gelatin–Agar Based Phase Separated Hydrogel, Emulgel and Bigel: A Comparative Study. J. Mater. Sci. Mater. Med. 2015, 26, 118.
- Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Veiga, M.D. Smart Freeze-Dried Bigels for the Prevention of the Sexual Transmission of HIV by Accelerating the Vaginal Release of Tenofovir during Intercourse. Pharmaceutics 2019, 11, 232.
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M.A. Mucoadhesive Hydrogel Nanoparticles as Smart Biomedical Drug Delivery System. Appl. Sci. 2019, 9, 825.
- Ilomuanya, M.O.; Hameedat, A.T.; Akang, E.N.; Ekama, S.O.; Silva, B.O.; Akanmu, A.S. Development and Evaluation of Mucoadhesive Bigel Containing Tenofovir and Maraviroc for HIV Prophylaxis. Futur. J. Pharm. Sci. 2020, 6, 81.
- Zulfakar, M.H.; Chan, L.M.; Rehman, K.; Wai, L.K.; Heard, C.M. Coenzyme Q10-Loaded Fish Oil-Based Bigel System: Probing the Delivery Across Porcine Skin and Possible Interaction with Fish Oil Fatty Acids. AAPS PharmSciTech 2018, 19, 1116–1123.
- Rehman, K.; Zulfakar, M.H. Novel Fish Oil-Based Bigel System for Controlled Drug Delivery and Its Influence on Immunomodulatory Activity of Imiquimod Against Skin Cancer. Pharm. Res. 2017, 34, 36–48.
- Lu, Y.; Zhong, Y.; Guo, X.; Zhang, J.; Gao, Y.; Mao, L. Structural Modification of O/W Bigels by Glycerol Monostearate for Improved Co-Delivery of Curcumin and Epigallocatechin Gallate. ACS Food Sci. Technol. 2022, 2, 975–983.




