Bones are composite materials consisting of organic polymers (mainly type I collagen), carbonated apatite, and water, with volume fractions of 35–45%, 35–45%, and 15–25%, respectively. Carbonated apatite in bone contributes to rigidity, while organic polymers and water contribute to toughness. The inorganic crystal, carbonated apatite, is a plate-shaped crystal with dimensions of 50 nm × 25 nm × 1–4 nm, generating a significant organic–inorganic interface, due to its nanoscale size. This interface is believed to absorb externally applied forces to dissipate mechanical energy to thermal energy. Crystallization of calcium phosphate in a solution or in a dispersion of a polymer attracts interest as a biomimetic synthesis of the bone-like composites.
1. Introduction
Plastics are extensively used as mechanical materials in numerous products, due to their excellent mechanical properties, lightweight nature, affordability, and good processability
[1]. Before the invention of plastics, materials like wood and bamboo were commonly used, but plastics have largely replaced them in many applications. However, there are several issues associated with the continued use of plastics. Firstly, plastics rely on fossil resources, such as petroleum, leading to concerns about resource depletion and carbon dioxide emissions during incineration. Additionally, many plastics lack biodegradability, causing marine pollution with microplastics, for example
[2]. Another concern about the use of plastics as a structural material is the generation of poisonous gas when burning. Potential alternatives to plastics include cellulose and biodegradable plastics. However, the mass production of the parts of industrial products using bone and wood is challenging because their anisotropic mechanical properties would make a molding process difficult.
On the other hand, aside from cellulose, materials such as bones, teeth, shells, horns, and crustacean shells have been biosynthesized by living organisms, and they are integral parts of the biological structural materials. These materials are composites of organic polymers and inorganic crystals
[3]. The inorganic crystals are calcium phosphates or calcium carbonates, while the organic polymers are proteins or polysaccharides, such as collagen and chitin. These composites form through the growth of inorganic crystals on the surface of organic polymers
[4], resulting in composites with relatively lightweight and mechanically robust properties. If these materials can be industrially produced, they could serve as environmentally friendly alternatives to plastics
[5][6].
2. General Features of Organic–Inorganic Composites
Organic–inorganic composite materials can be broadly classified into two categories: ceramics-reinforced plastics and polymer-reinforced ceramics (
Table 1). For the former, the low rigidity of the organic phase is enhanced through the addition of the inorganic phase. Glass fiber-reinforced plastics, clay–polyamide composites
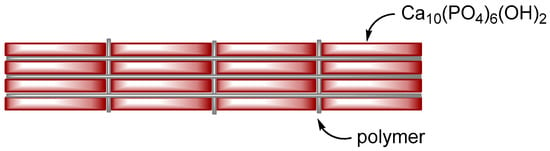
[7], and rubber, an important component of automobile tires, for example, are commonly used in daily life. If plastic is too soft to resist external stress, we can add inorganic fillers, such as glass fibers, to increase its stiffness. The major component of the composites of this category is an organic phase, and the minor component is an inorganic phase. For “polymer-reinforced ceramics”, the brittleness of ceramics is improved by incorporating organic polymers, as seen in laminated glass. For laminated glass, due to the sandwiched structure of glass plate–polymer film–glass plate, it would not be broken into pieces if a shock is given, owing to the organic polymer film. The major component of this category is an inorganic phase, and the minor component is an organic phase. Bone is polymer-reinforced ceramics and can be roughly described as nanometer-sized laminated glass with a brick-and-mortar structure (
Figure 1). According to the brick-and-mortar model, ceramic in bone is a dispersed phase, and polymer is a continuous phase. When attempting to prepare a bone-like composite, as the weight fraction of ceramics increases and is close to one, the ceramic phase becomes a continuous phase, resulting in fragile materials. Therefore, the fabrication of the brick-and-mortar structure becomes difficult, as the weight fraction of the ceramic phase is close to one.
Table 1. Classification of organic–inorganic composites.
| |
Ceramic-Reinforced Polymer |
Polymer-Reinforced Ceramics |
| Major component |
Polymer (matrix, continuous phase) |
Ceramics (filler, dispersed phase) |
| Minor component |
Ceramics (filler, dispersed phase) |
Polymers (matrix, continuous phase) |
| Examples |
Glass–fiber reinforced plastics |
Laminated glass |
| Polyamide–clay composites |
Bones |
| Tire |
Teeth |
Figure 1. Brick-and-mortar structure of bone and polymer–HAP composites prevent fragile fracture.
3. Preparation of Organic–Inorganic Composites
For the preparation of organic–inorganic composites, the following three protocols have been reported:
-
Direct mixing of polymer and inorganic crystals.
-
Polymerization of the monomer in the presence of the inorganic crystal powder.
-
Crystallization of the inorganic phase in the presence of the organic polymer.
As an example of the first protocol, Bonfield and coworkers reported that a composite of polyethylene and apatite was prepared by mixing both components
[8]. The elastic modulus of the composite was linearly related to the volume fraction of apatite. When the volume fraction was varied from 0 to 0.6, the elastic modulus ranged from 1 to 12 GPa, demonstrating that the filler enhanced the stiffness of the composites. The second protocol was employed to prepare the composite of poly(L-lactic acid-co-glycolic acid) and hydroxyapatite
[9]. Polyamide–clay composites were prepared by the ring-open polymerization of ε-caprolactam in the presence of clay minerals and montmorillonite, which was ion-exchanged by ω-carboxyalkylammonium. Thus, the polyamide–clay composite was also prepared using the second protocol
[7].
The third protocol is a biomimetic, bottom-up approach and is suitable for the preparation of nanometer-sized inorganic crystals that are bound to the polymer phase. Stupp et al. reported that precipitation occurred when an aqueous solution of polyglutamic acid was mixed with calcium hydroxide and phosphoric acid, and the resulting precipitate was the composite of the polymer and the apatite
[10]. Polyglutamic acid sodium salt, polylysine hydrochloride, and polyacrylic acid aqueous solution (1 mM) were mixed with calcium hydroxide and phosphoric acid, and the pH of the mixture was adjusted to 7.4 at 37 °C to obtain polymer–HAP composites as precipitates. The composites were called organoapatite, and X-ray diffraction showed that the composites contained poorly crystalline hydroxyapatite. The ratio of Ca/P was close to 1.6, the theoretical value of hydroxyapatite in the absence of polymers, while the ratio was 1.4 to 1.6 in the presence of the polymers, showing that calcium-deficient hydroxyapatite formed.
Anionic functional groups of proteins play a crucial role in the biomineralization of bones and teeth
[11][12][13][14][15][16][17][18][19][20][21][22][23][24]. Akkus and coworkers crystallized HAP in the presence of charged peptides and performed a systematic investigation on the effects of charged peptides on the HAP morphology
[25]. They found that the amounts of anionic peptides, such as poly-L-Asp and poly-L-Glu bound to HAP, were larger than those of cationic peptides, such as poly-L-Lys and poly-L-Arg. Negatively charged peptides led to smaller crystals in HAP than positively charged ones. Research involving the complexation of hydroxyapatite with various polymers and polymer gels was carried out and reviewed
[26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49].
Carbonated apatite in bone is a hexagonal crystal, and citrate anions are adsorbed onto the
a and
b faces
[50]. Anions are adsorbed onto the
a and
b faces of hydroxyapatite, while cations are adsorbed onto the
c face
[18][51]. Thus, by adding calcium ions and phosphate ions to solutions of polymers with anionic functional groups, such as carboxylate, or phosphate groups, under an alkaline condition, the polymers are adsorbed onto the
a and
b faces of apatite through their anions, leading to crystal growth along the
c-axis. As a result, it might be possible to control the crystal size in the nanometer range and achieve the composite formation of organic polymers and needle-like or plate-like inorganic crystals bonded with each other with aligned orientations. Formation of the brick-and-mortar structure is crucial for the toughness of the composites
[3][52][53][54]. The polymers used here can include petroleum-derived synthetic polymers, proteins, and polysaccharides, but the use of biomass-derived polysaccharides synthesized from carbon dioxide through photosynthesis could address carbon dioxide emission and environmental issues.
4. Composite of Hydroxyapatite and Polysaccharide
The use of polysaccharides as an organic component of the composite is an attractive strategy because they are carbon neutral and degrade biologically. There have been several studies on the preparation of the composites of hydroxyapatite and polysaccharides
[23][47][55][56][57]. Yao and coworkers crystallized HAP in the presence of polysaccharides and found that the crystal size of HAP decreased in the order of amylose (−OH) > chitosan (−NH
2, −NHCOCH
3, and −OH) > carrageenan (−OSO
3− and −OH) > pectin (−COO
− and −OH)
[23]. The polar functional groups of these polysaccharides are shown in the parentheses. It seems that the –COO
− group is the most effective in suppressing the crystal growth of HAP. We can avoid environmental issues, such as the microplastic pollution of sea water, by employing polysaccharide-based structural materials. The annual production of cellulose is 1.5 × 10
12 tons
[58], while that of starch is 8 × 10
8 tons (corn starch)
[59]. Considering the large annual production of cellulose, the use of cellulose as an organic phase of the composite seems attractive. Yu and coworkers reported a composite of cellulose nanofibers and TiO
2-coated mica. It showed a bending strength of 281 MPa and an elastic modulus of 20 GPa
[5].
5. Introduction of Hydrophobic Groups for Water-Resistance Enhancement
The surfaces of polysaccharides and hydroxyapatite are hydrophilic and readily hydrated. Thus, when molded composites are immersed in water, they often undergo substantial swelling, resulting in significant changes in mechanical properties. In bone’s apatite, the carboxylate groups of citric acid are adsorbed to the calcium ions on the
a and
b faces through ionic bonding. Accordingly, the methylene groups of citric acid face outward, contributing to the hydrophobicity of apatite crystals
[50]. Bone contains around 10% water, and when it is dried, it becomes more elastic but also more brittle, compromising its toughness
[60]. This implies that the water content plays a significant role in bone’s mechanical properties, especially toughness. The synthesized composites of cellulose or starch and hydroxyapatite demonstrated significant water absorption, leading to a near-complete loss of mechanical strength. Therefore, the acylation of the hydroxyl groups of polysaccharides was considered as a means to improve the water resistance of the composites.