
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilgiz Gareev | -- | 3166 | 2023-11-09 10:44:30 | | | |
| 2 | Peter Tang | Meta information modification | 3166 | 2023-11-10 03:04:07 | | |
Video Upload Options
Venous thromboembolic complications (VTCs), which include deep vein thrombosis (DVT) and pulmonary embolism (PE), have remained a pressing problem in modern clinical medicine for a long time. Despite the already wide arsenal of modern methods for diagnosing and treating this disease, VTCs rank third in the structure of causes of death among all cardiovascular diseases, behind myocardial infarction (MI) and ischemic stroke (IS). Numerous studies have confirmed the importance of understanding the molecular processes of VTCs for effective therapy and diagnosis. Significant progress has been made in VTC research, where the relative contribution of microRNAs (miRNAs) in the mechanism of thrombus formation and their consideration as therapeutic targets have been well studied.
1. Introduction
|
Thrombosis/Conditions Associated with Thrombosis |
miRNA |
Regulation |
Study |
Targets |
Effect |
References |
|---|---|---|---|---|---|---|
|
AAF |
miR-200b-3p |
Up |
In vitro |
VEGF and Ang-II |
Increases intimal hyperplasia to induce autogenous arteriovenous fistula thrombosis |
[7] |
|
Thrombosis |
miR-1915-3p |
Up |
In vitro |
RHOB |
Enhances megakaryocyte (megakaryopoiesis) differentiation and platelet generation |
[8] |
|
COVID-19 |
miR-145 and miR-885 |
Down |
Ex vivo (human serum) and in vitro |
D-dimer |
Increase endothelial cell apoptosis and display significantly impaired angiogenetic properties |
[9] |
|
Inflammation and arterial thrombosis |
miR-146b-3p |
Up |
In vitro |
P38MAPK/COX-2 pathway |
TNF-α activates miR-146b-3p and then induces thrombosis |
[10] |
|
COVID-19 |
miR-16-5p |
Down |
In silico and bioinformatic analysis |
EGFR, HSP90AA1, APP, TP53, PTEN, UBC, FN1, ELAVL1, and CALM1 |
Thrombosis-related predictive biomarker |
[11] |
|
Multiple myeloma |
miR-532 |
Down |
Ex vivo (human serum) and in vitro |
TF, EPCR, ERK 1/2, MAPK, and NF-κB |
Increases multiple myeloma-induced endothelial cell thrombosis |
[12] |
|
Photochemical-induced carotid thrombosis |
miR-223 |
Down |
In vivo |
IGF-1R |
Attenuates thrombosis |
[13] |
|
Primary antiphospholipid syndrome |
miR-483-3p miR-326 |
Up Down |
In vitro |
PI3K/AKT and NOTCH |
Contribute to primary antiphospholipid syndrome pathogenesis by producing endothelial cell proliferation, monocyte activation, and adhesion/procoagulant factors |
[14] |
|
Atherosclerotic plaque rupture with thrombosis |
miR-466h-5p |
Up |
In vivo |
Bcl2 |
Promotes atherosclerotic plaque rupture and thrombosis |
[15] |
|
Catheter-related thrombosis |
miR-92a-3p |
Down |
In vivo |
MAPK/NF-κB |
Inhibits oxidative stress injury and prevents catheter-related thrombosis formation |
[16] |
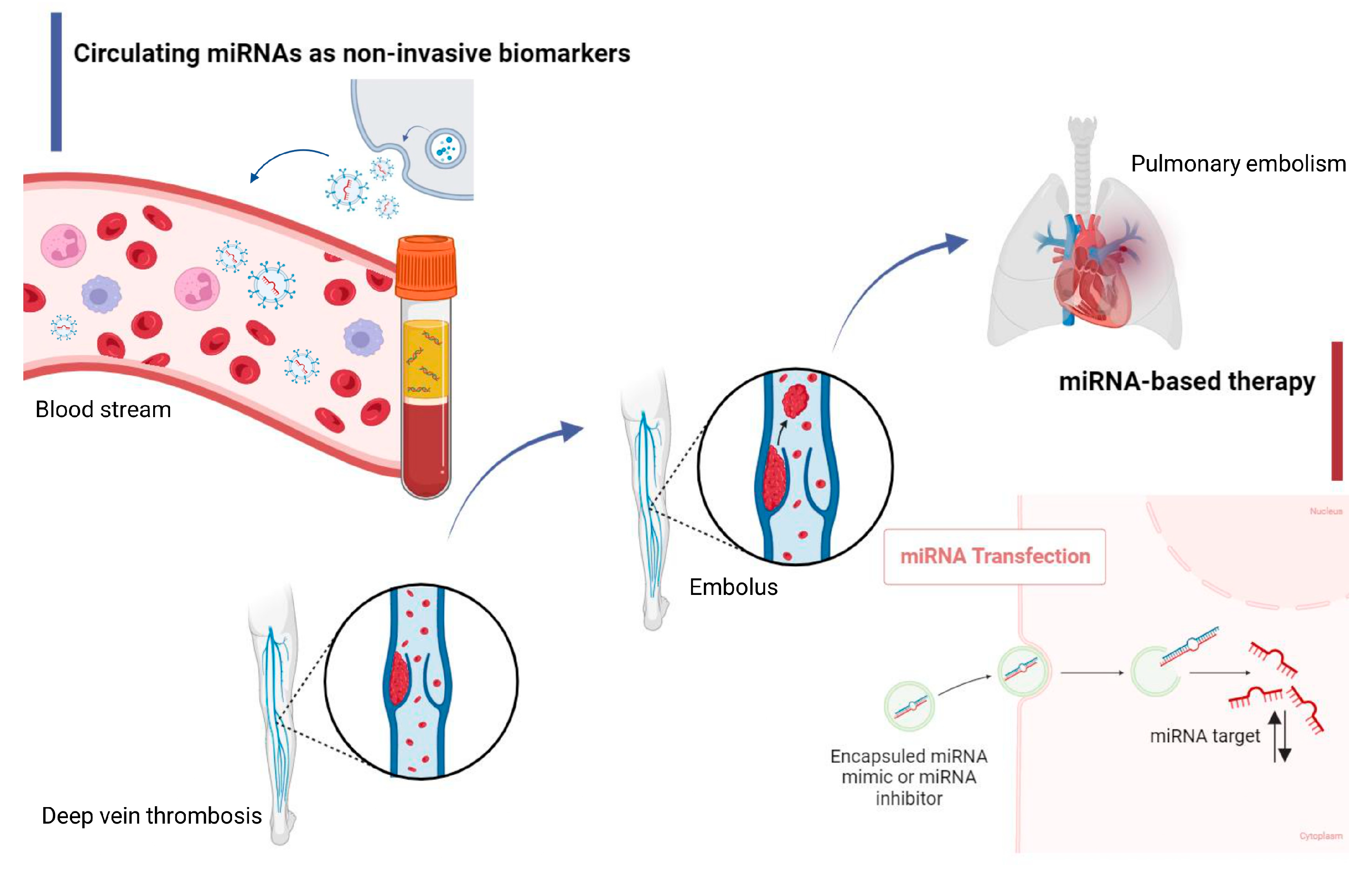
2. MiRNAs and Hemostasis



3. MiRNA and DVT
4. MiRNA and PE
5. Circulating miRNAs vs. Traditional Biomarkers
References
- Lutsey, P.L.; Zakai, N.A. Epidemiology and prevention of venous thromboembolism. Nat. Rev. Cardiol. 2023, 20, 248–262.
- Westafer, L.M.; Long, B.; Gottlieb, M. Managing Pulmonary Embolism. Ann. Emerg. Med. 2023, 82, 394–402.
- Navarrete, S.; Solar, C.; Tapia, R.; Pereira, J.; Fuentes, E.; Palomo, I. Pathophysiology of deep vein thrombosis. Clin. Exp. Med. 2023, 23, 645–654.
- Larsson, A.E.; Andréasson, B.; Holmberg, H.; Liljeholm, M.; Själander, A. Erythrocytosis, thrombocytosis, and rate of recurrent thromboembolic event-A population based cohort study. Eur. J. Haematol. 2023, 110, 608–617.
- Xiao, Q.; Wang, D.; Sheng, Y.; Huang, J.; Ha, X. MicroRNA-126 Regulates Thrombosis Through Endothelial Progenitor Cells. DNA Cell Biol. 2023, 42, 315–321.
- Gareev, I.; Beylerli, O.; Yang, G.; Sun, J.; Pavlov, V.; Izmailov, A.; Shi, H.; Zhao, S. The current state of MiRNAs as biomarkers and therapeutic tools. Clin. Exp. Med. 2020, 20, 349–359.
- Luo, M.; Mo, C.; Tang, D.; Liu, S.Z.; Yang, T. Exosomal miRNA-200b-3p regulated autogenous arteriovenous fistula thrombosis in maintenance hemodialysis patients. J. Vasc. Access 2022, 21, 11297298221092951.
- Qu, M.; Zou, X.; Fang, F.; Wang, S.; Xu, L.; Zeng, Q.; Fan, Z.; Chen, L.; Yue, W.; Xie, X.; et al. Platelet-derived microparticles enhance megakaryocyte differentiation and platelet generation via miR-1915-3p. Nat. Commun. 2020, 11, 4964.
- Gambardella, J.; Kansakar, U.; Sardu, C.; Messina, V.; Jankauskas, S.S.; Marfella, R.; Maggi, P.; Wang, X.; Mone, P.; Paolisso, G.; et al. Exosomal miR-145 and miR-885 Regulate Thrombosis in COVID-19. J. Pharmacol. Exp. Ther. 2023, 384, 109–115.
- Su, Z.; Wu, F. Inflammatory Factors Induce Thrombosis through the miR-146b-3p/p38MAPK/COX-2 Pathway. Biomed. Res. Int. 2020, 2020, 8718321.
- Eyileten, C.; Wicik, Z.; Simões, S.N.; Martins-Jr, D.C.; Klos, K.; Wlodarczyk, W.; Assinger, A.; Soldacki, D.; Chcialowski, A.; Siller-Matula, J.M.; et al. Thrombosis-related circulating miR-16-5p is associated with disease severity in patients hospitalised for COVID-19. RNA Biol. 2022, 19, 963–979.
- Gao, L.; Li, L.; Hu, J.; Li, G.; Zhang, Y.; Dai, X.; De, Z.; Xu, F. Metformin Inhibits Multiple Myeloma Serum-induced Endothelial Cell Thrombosis by Down-Regulating miR-532. Ann. Vasc. Surg. 2022, 85, 347–357.e2.
- Wang, H.; Wang, Q.; Kleiman, K.; Guo, C.; Eitzman, D.T. Hematopoietic Deficiency of miR-223 Attenuates Thrombosis in Response to Photochemical Injury in Mice. Sci. Rep. 2017, 7, 1606.
- Solé, C.; Royo, M.; Sandoval, S.; Moliné, T.; Cortés-Hernández, J. Small-Extracellular-Vesicle-Derived miRNA Profile Identifies miR-483-3p and miR-326 as Regulators in the Pathogenesis of Antiphospholipid Syndrome (APS). Int. J. Mol. Sci. 2023, 24, 11607.
- Nie, P.; Yang, F.; Wan, F.; Jin, S.; Pu, J. Analysis of MicroRNAs Associated with Carotid Atherosclerotic Plaque Rupture with Thrombosis. Front. Genet. 2021, 12, 599350.
- Wen, C.; Ying, Y.; Zhao, H.; Jiang, Q.; Gan, X.; Wei, Y.; Wei, J.; Huang, X. Resistance exercise affects catheter-related thrombosis in rats through miR-92a-3p, oxidative stress and the MAPK/NF-κB pathway. BMC Cardiovasc. Disord. 2021, 21, 440.
- Martinez Licha, C.R.; McCurdy, C.M.; Maldonado, S.M.; Lee, L.S. Current Management of Acute Pulmonary Embolism. Ann. Thorac. Cardiovasc. Surg. 2020, 26, 65–71.
- Trott, T.; Bowman, J. Diagnosis and Management of Pulmonary Embolism. Emerg. Med. Clin. N. Am. 2022, 40, 565–581.
- Kruger, P.C.; Eikelboom, J.W.; Douketis, J.D.; Hankey, G.J. Deep vein thrombosis: Update on diagnosis and management. Med. J. Aust. 2019, 210, 516–524.
- Wu, J.; Al-Zahrani, A.; Beylerli, O.; Sufianov, R.; Talybov, R.; Meshcheryakova, S.; Sufianova, G.; Gareev, I.; Sufianov, A. Circulating miRNAs as Diagnostic and Prognostic Biomarkers in High-Grade Gliomas. Front. Oncol. 2022, 12, 898537.
- Danese, E.; Montagnana, M.; Gelati, M.; Lippi, G. The Role of Epigenetics in the Regulation of Hemostatic Balance. Semin. Thromb. Hemost. 2021, 47, 53–62.
- Nourse, J.; Braun, J.; Lackner, K.; Hüttelmaier, S.; Danckwardt, S. Large-scale identification of functional microRNA targeting reveals cooperative regulation of the hemostatic system. J. Thromb. Haemost. 2018, 16, 2233–2245.
- Schulte, C.; Mayr, M. MicroRNAs: A New Understanding of Platelet Physiology and Pathology. Thromb. Haemost. 2019, 119, 191.
- Garcia, A.; Dunoyer-Geindre, S.; Fish, R.J.; Neerman-Arbez, M.; Reny, J.L.; Fontana, P. Methods to Investigate miRNA Function: Focus on Platelet Reactivity. Thromb. Haemost. 2021, 121, 409–421.
- Khodadi, E. Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 2020, 20, 1–10.
- De Los Reyes-García, A.M.; Arroyo, A.B.; Teruel-Montoya, R.; Vicente, V.; Lozano, M.L.; González-Conejero, R.; Martínez, C. MicroRNAs as potential regulators of platelet function and bleeding diatheses. Platelets 2019, 30, 803–808.
- Ender, F.; Freund, A.; Quecke, T.; Steidel, C.; Zamzow, P.; von Bubnoff, N.; Gieseler, F. Tissue factor activity on microvesicles from cancer patients. J. Cancer Res. Clin. Oncol. 2020, 146, 467–475.
- Zhang, X.; Yu, H.; Lou, J.R.; Zheng, J.; Zhu, H.; Popescu, N.I.; Lupu, F.; Lind, S.E.; Ding, W.Q. MicroRNA-19 (miR-19) regulates tissue factor expression in breast cancer cells. J. Biol. Chem. 2011, 286, 1429–1435.
- Yu, G.; Li, H.; Wang, X.; Wu, T.; Zhu, J.; Huang, S.; Wan, Y.; Tang, J. MicroRNA-19a targets tissue factor to inhibit colon cancer cells migration and invasion. Mol. Cell Biochem. 2013, 380, 239–247.
- Li, S.; Chen, H.; Ren, J.; Geng, Q.; Song, J.; Lee, C.; Cao, C.; Zhang, J.; Xu, N. MicroRNA-223 inhibits tissue factor expression in vascular endothelial cells. Atherosclerosis 2014, 237, 514–520.
- Chuang, T.D.; Luo, X.; Panda, H.; Chegini, N. miR-93/106b and their host gene, MCM7, are differentially expressed in leiomyomas and functionally target F3 and IL-8. Mol. Endocrinol. 2012, 26, 1028–1042.
- Teruel, R.; Pérez-Sánchez, C.; Corral, J.; Herranz, M.T.; Pérez-Andreu, V.; Saiz, E.; García-Barberá, N.; Martínez-Martínez, I.; Roldán, V.; Vicente, V.; et al. Identification of miRNAs as potential modulators of tissue factor expression in patients with systemic lupus erythematosus and antiphospholipid syndrome. J. Thromb. Haemost. 2011, 9, 1985–1992.
- Luo, M.; Li, R.; Ren, M.; Chen, N.; Deng, X.; Tan, X.; Li, Y.; Zeng, M.; Yang, Y.; Wan, Q.; et al. Hyperglycaemia-induced reciprocal changes in miR-30c and PAI-1 expression in platelets. Sci. Rep. 2016, 6, 36687.
- Fort, A.; Borel, C.; Migliavacca, E.; Antonarakis, S.E.; Fish, R.J.; Neerman-Arbez, M. Regulation of fibrinogen production by microRNAs. Blood 2010, 116, 2608–2615.
- Zhang, Y.; Zhang, Z.; Wei, R.; Miao, X.; Sun, S.; Liang, G.; Chu, C.; Zhao, L.; Zhu, X.; Guo, Q.; et al. IL (Interleukin)-6 Contributes to Deep Vein Thrombosis and Is Negatively Regulated by miR-338-5p. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 323–334.
- Tang, K.C.; Yang, Z.P.; Zeng, Q.; Wang, J.; Guo, F.; Zhao, Y. Effect of miR-495 on lower extremity deep vein thrombosis through the TLR4 signaling pathway by regulation of IL1R1. Biosci. Rep. 2018, 38, BSR20180598.
- Ding, Y.; Li, X. Resistin Promotes Thrombosis in Rats with Deep Vein Thrombosis via Up-Regulating MMP-2, MMP-9, and PAI-1. Clin. Lab. 2019, 65, 1789–1796.
- Zhang, T.; Li, Q.; Wang, L.; Li, G. Expression variations and clinical significance of MMP-1, MMP-2 and inflammatory factors in serum of patients with deep venous thrombosis of lower extremity. Exp. Ther. Med. 2019, 17, 181–186.
- Franciscis, S.; Gallelli, L.; Amato, B.; Butrico, L.; Rossi, A.; Buffone, G.; Caliò, F.G.; De Caridi, G.; Grande, R.; Serra, R. Plasma MMP and TIMP evaluation in patients with deep venous thrombosis: Could they have a predictive role in the development of post-thrombotic syndrome? Int. Wound J. 2016, 13, 1237–1245.
- Ai, P.; Shen, B.; Pan, H.; Chen, K.; Zheng, J.; Liu, F. MiR-411 suppressed vein wall fibrosis by downregulating MMP-2 via targeting HIF-1α. J. Thromb. Thrombolysis 2018, 45, 264–273.
- Fei, J.; Qin, X.; Ma, H.; Zhang, X.; Wang, H.; Han, J.; Yu, C.; Jiang, J. Resveratrol Ameliorates Deep Vein Thrombosis-Induced Inflammatory Response Through Inhibiting HIF-1α/NLRP3 Pathway. Inflammation 2022, 45, 2268–2279.
- Qi, J.; Pan, T.; You, T.; Tang, Y.; Chu, T.; Chen, J.; Fan, Y.; Hu, S.; Yang, F.; Ruan, C.; et al. Upregulation of HIF-1α contributes to complement activation in transplantation-associated thrombotic microangiopathy. Br. J. Haematol. 2022, 199, 603–615.
- Gu, W.; Qi, J.; Zhang, S.; Ding, Y.; Qiao, J.; Han, Y. Inhibition of Hypoxia-Inducible Factor Prolyl-Hydroxylase Modulates Platelet Function. Thromb. Haemost. 2022, 122, 1693–1705.
- Akhter, M.S.; Biswas, A.; Iqbal, J.; Hamali, H.A.; Mobarki, A.A.; Abdullah, S.M.; Dobie, G.; Saxena, R. Endothelial Nitric Oxide Synthase Gene Polymorphisms Increase Risk of Deep Vein Thrombosis by Altering Homocysteine Levels. Clin. Lab. 2022, 68, 575–582.
- Qin, J.Z.; Wang, S.J.; Xia, C. microRNAs regulate nitric oxide release from endothelial cells by targeting NOS3. J. Thromb. Thrombolysis 2018, 46, 275–282.
- Mandras, S.A.; Mehta, H.S.; Vaidya, A. Pulmonary Hypertension: A Brief Guide for Clinicians. Mayo Clin. Proc. 2020, 95, 1978–1988.
- Zhu, R.; Qi, W.Y.; Liu, T.W.; Liu, F. MicroRNA 449a can Attenuate Protective Effect of Urokinase Against Pulmonary Embolism. Front. Pharmacol. 2022, 13, 713848.
- Li, Y.; Shao, J.; Song, J.; Yu, S.; Wang, J.; Sun, K. MiR-34a-3p suppresses pulmonary vascular proliferation in acute pulmonary embolism rat by targeting DUSP1. Biosci. Rep. 2022, 42, BSR20210116.
- Teerapuncharoen, K.; Bag, R. Chronic Thromboembolic Pulmonary Hypertension. Lung 2022, 200, 283–299.
- Wang, L.; Guo, L.J.; Liu, J.; Wang, W.; Yuan, J.X.; Zhao, L.; Wang, J.; Wang, C. MicroRNA expression profile of pulmonary artery smooth muscle cells and the effect of let-7d in chronic thromboembolic pulmonary hypertension. Pulm. Circ. 2013, 3, 654–664.
- Chen, Z.; Nakajima, T.; Tanabe, N.; Hinohara, K.; Sakao, S.; Kasahara, Y.; Tatsumi, K.; Inoue, Y.; Kimura, A. Susceptibility to chronic thromboembolic pulmonary hypertension may be conferred by miR-759 via its targeted interaction with polymorphic fibrinogen alpha gene. Hum. Genet 2010, 128, 443–452.
- Johnson, E.D.; Schell, J.C.; Rodgers, G.M. The D-dimer assay. Am. J. Hematol. 2019, 94, 833–839.
- Favresse, J.; Lippi, G.; Roy, P.M.; Chatelain, B.; Jacqmin, H.; Ten Cate, H.; Mullier, F. D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Crit. Rev. Clin. Lab. Sci. 2018, 55, 548–577.
- Zhou, Q.; Zhang, D.; Chen, X.; Yang, Z.; Liu, Z.; Wei, B.; Jin, M.; Feng, K.; Guo, C.; Sun, J.; et al. Plasma D-dimer predicts poor outcome and mortality after spontaneous intracerebral hemorrhage. Brain Behav. 2021, 11, 462–468.
- Price, C.P.; Fay, M.; Hopstaken, R.M. Point-of-Care Testing for D-Dimer in the Diagnosis of Venous Thromboembolism in Primary Care: A Narrative Review. Cardiol. Ther. 2021, 10, 27–40.
- Gareev, I.; Yang, G.; Sun, J.; Beylerli, O.; Chen, X.; Zhang, D.; Zhao, B.; Zhang, R.; Sun, Z.; Yang, Q.; et al. Circulating MicroRNAs as Potential Noninvasive Biomarkers of Spontaneous Intracerebral Hemorrhage. World Neurosurg. 2020, 133, e369–e375.
- Wu, J.; Gareev, I.; Beylerli, O.; Mukhamedzyanov, A.; Pavlov, V.; Khasanov, D.; Khasanova, G. Circulating miR-126 as a Potential Non-invasive Biomarker for Intracranial Aneurysmal Rupture: A Pilot Study. Curr. Neurovasc. Res. 2021, 18, 525–534.





