Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | RADU ALEXANDRU VULPOI | -- | 2349 | 2023-11-07 09:22:51 | | | |
| 2 | Jessie Wu | + 16 word(s) | 2365 | 2023-11-07 09:43:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vulpoi, R.A.; Luca, M.; Ciobanu, A.; Olteanu, A.; Bărboi, O.; Iov, D.; Nichita, L.; Ciortescu, I.; Cijevschi Prelipcean, C.; Ștefănescu, G.; et al. Irritable Bowel Syndrome and Artificial Intelligence. Encyclopedia. Available online: https://encyclopedia.pub/entry/51231 (accessed on 08 February 2026).
Vulpoi RA, Luca M, Ciobanu A, Olteanu A, Bărboi O, Iov D, et al. Irritable Bowel Syndrome and Artificial Intelligence. Encyclopedia. Available at: https://encyclopedia.pub/entry/51231. Accessed February 08, 2026.
Vulpoi, Radu Alexandru, Mihaela Luca, Adrian Ciobanu, Andrei Olteanu, Oana Bărboi, Diana-Elena Iov, Loredana Nichita, Irina Ciortescu, Cristina Cijevschi Prelipcean, Gabriela Ștefănescu, et al. "Irritable Bowel Syndrome and Artificial Intelligence" Encyclopedia, https://encyclopedia.pub/entry/51231 (accessed February 08, 2026).
Vulpoi, R.A., Luca, M., Ciobanu, A., Olteanu, A., Bărboi, O., Iov, D., Nichita, L., Ciortescu, I., Cijevschi Prelipcean, C., Ștefănescu, G., Mihai, C., & Drug, V.L. (2023, November 07). Irritable Bowel Syndrome and Artificial Intelligence. In Encyclopedia. https://encyclopedia.pub/entry/51231
Vulpoi, Radu Alexandru, et al. "Irritable Bowel Syndrome and Artificial Intelligence." Encyclopedia. Web. 07 November, 2023.
Copy Citation
Irritable bowel syndrome (IBS) has a global prevalence of around 4.1% and is associated with a low quality of life and increased healthcare costs. Current guidelines recommend that IBS is diagnosed using the symptom-based Rome IV criteria. Despite this, when patients seek medical attention, they are usually over-investigated. This issue might be resolved by novel technologies in medicine, such as the use of Artificial Intelligence (AI).
irritable bowel syndrome
artificial intelligence
diagnostic
deep learning
1. Artificial Intelligence-Assisted Colonoscopy in Irritable Bowel Syndrome
Colonoscopy is considered the gold standard for diagnosing organic lesions of the colon. Direct visualization of the lesion and the ability to take biopsies represent the two greatest advantages of this investigation. Colorectal cancer (CCR) remains a major public health problem, responsible for over 900,000 deaths worldwide in 2020. According to GLOBOCAN 2020, it represents the second most common cause of cancer-related death [1]. Considering these data, clinicians may have a low threshold of suspicion in patients with chronic abdominal pain and altered bowel movements. In clinical practice, colonoscopy is frequently used in order to rule out organic lesions in patients with symptoms suggestive of IBS. This is in stark contrast with the recommendations of the American College of Gastroenterology (ACG) and British Society of Gastroenterology (BSG) on irritable bowel syndrome (IBS) management [2][3]. According to these guidelines, colonoscopy should be reserved solely for patients presenting with alarm signs such as iron deficiency anemia, rectal bleeding or melena, nocturnal diarrhea, unintentional weight loss, symptom onset at an older age (e.g., age ≥ 45 or 50 years), family history of CRC and inflammatory bowel diseases [4].
For a positive diagnosis of IBS, conventional colonoscopy has not proved to be of significant benefit. The results of a recent meta-analysis demonstrated that there is no difference in the yield of CRC or inflammatory bowel disease between individuals with or without IBS [4].
Recently, Tabata K et al. published a paper in which they used a free AI algorithm created with “Google Cloud Platform AutoML Vision” [5]. Their aim was to establish if the AI system is capable of detecting minute changes in the colon that cannot be detected by human investigators. This is usually possible by adding additional information in the training program such as the presence or absence of symptoms. In the study 4 different groups were created: Group N with healthy volunteers, Group I with patients with IBS, Group C patients with constipation-IBS and Group D for patients with diarrhea-IBS. A total of 2479, 382, 538 and 484 images from colonoscopy were randomly selected for groups N, I, C and D, respectively group for training, validation and testing of the AI System. The algorithm managed to discriminate between Group N and Group I with a total area under the curve (AUC) of 0.95 (Group I AUC 0.48, Group N AUC 0.97) and the sensitivity, specificity, positive prediction value and negative prediction value of Group I detection were 30.8%, 97.6%, 66.7% and 90.23%, respectively. Similar but slightly less accurate results were obtained when comparing Group C and D, and Group N vs. Group I + Group C + Group D. Although the experiment shows promising results, the authors themselves recognized that the way the AI classifier distinguishes between endoscopic features from model patients with IBS vs. healthy control is unclear. The problem with these results is also illustrated by the low sensitivity obtained (38%). When predicting whether a patient has IBS or not, it is important that the sensitivity be incredibly high so that as many positive cases as possible can be captured. Until now, no other studies have employed the use of AI technology developed for colonoscopy in order to investigate patients with IBS [5].
To enhance the colonoscopy examination, several other AI systems were developed and approved for use. Nonetheless, most of them were aimed at detecting and diagnosing organic lesions [6][7]. To achieve this, two concepts were introduced in the development of AI-enhanced colonoscopy: computer-assisted detection (CADe) and computer-assisted diagnosis (CADx). Using CADe, AI is able to assist endoscopists in colon lesion detection, thereby increasing the adenoma detection rate (ADR). Resaercehrs present an example from researchers' work of developing an AI system including a CADe module, in Figure 1 [8][9]. For this system, researchers used MobileNet1, a deep learning network with 4.2 million parameters already trained on the ImageNet dataset, retrained for detecting several types of polyps, lesions, water jet and endoscopic instruments [10]. On the other hand, an AI system using CADx assists the investigator in distinguishing lesions and assessing their potential for malignancy [11]. For this system, the authors used an architecture based on EfficientNet, a neural network with 19 million parameters [12]. This network was also pretrained using the open ImageNet dataset. However, IBS is not associated with any organic lesions. Therefore, it is likely that the benefits of using AI technology in IBS patients will be rather indirect. If no lesions are found during the colonoscopic examination, it may be possible to improve the reliability of an IBS diagnosis by using a tool that objectively analyses the images.
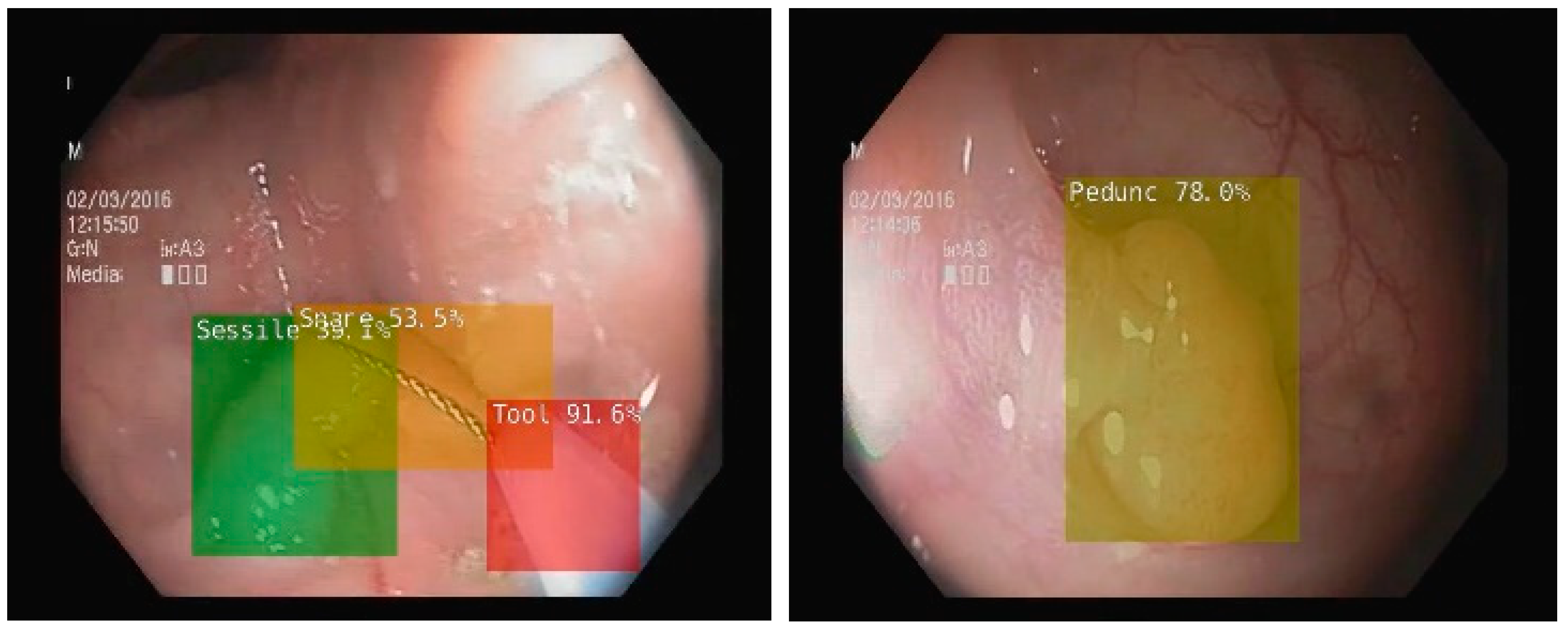
Figure 1. CADe in colonoscopy on NVIDIA Jetson Xavier NX microsystem using MobileNet1 deep learning network retrained to detect more types of polyps, bleedings, water jet, and endoscopic instruments (snare, tool) [9].
Additionally, AI technologies were developed in order to increase quality assurance in colonoscopy. These AI systems were specifically trained to evaluate quality indicators in colonoscopy such as the rate of cecum intubation and total colonoscopy, withdrawal time, as well as the degree of bowel cleansing according to established bowel preparation scales (e.g., Boston Bowel Preparation Score—BBPS). In the near future, researchers might be able to ensure that the colonoscopic investigation is of adequate quality by utilizing a tool that can objectively evaluate bowel preparation. In addition to that, AI is associated with a very low risk of missing an adenoma. As a result, both clinicians and patients can rest assured knowing that the IBS diagnosis is indeed accurate, and no further investigation is warranted.
Furthermore, artificial intelligence technology has been developed to indirectly assist colonoscopy in a laboratory setting. Baumgartner et al. [13] published a study in 2021 that evaluated the significance of intestinal mucosal biofilms present during colonoscopy. Biofilm formation is a unique form of microbial growth environment in which adherent prokaryotic communities embed themselves within an extracellular matrix. The researchers used an AI system to evaluate the characteristics of biofilms and to study their significance. When the bowel is adequately prepared, biofilms can be observed in white light high-resolution endoscopy as adherent layers on the intestinal surface. These are also resistant to jet washing and detach in a film-like manner [13].
The study, which included 1112 patients from two European medical centers, examined the association between IBS and endoscopically visible biofilms. Out of them, 117 patients (56 with IBS, 25 with ulcerative colitis, and 36 controls undergoing colorectal cancer screening with normal findings at colonoscopy) were selected for molecular and microscopic analyses. Biopsy samples were retrieved from both biofilm-positive and biofilm-negative patients. When no biofilm was present, the biopsies were taken from similar areas of the intestine (cecum or ascending colon) [13]. The AI system used in confocal microscopy employed a trained U-Net, which represents a convolutional network architecture, using skip connections between the encoding and the decoding paths, for fast and precise segmentation of biomedical images. This algorithm was effective in quantifying the number and density of bacteria in biofilm-positive biopsies, as well as confirming the direct contact between the bacteria and the epithelium, which was absent in biofilm-negative biopsies. According to the findings of this study, in 57% of the IBS patients, biofilm was present. Other clinical situations in which biofilm was identified included subjects with ulcerative colitis (34%), after organ transplantation (23%) and healthy individuals undergoing screening colonoscopies (6%) [13].
Briefly, several studies suggest that AI-assisted biofilm analysis could be a potential novel diagnostic tool for IBS.
2. The Analysis of Acoustic Bowel Movements Using Artificial Intelligence
Over the past few years, there has been a growing interest in research into the sounds of the bowel as a possible non-invasive, reliable, replicable and cost-effective diagnostic test for IBS. The paper published by Craine et al. at the beginning of the twenty-first century was a pivotal study on the topic. In their study, the researchers investigated bowel sounds from both healthy and IBS individuals using computer analysis. According to their findings, fasting sound-to-sound intervals were significantly different between groups. The sensitivity and specificity of this method for detecting IBS were 89% and 100%, respectively [14]. The study of bowel sounds has been undertaken using a variety of analytical approaches such as wavelet transformations, multilayer perceptrons, independent component analysis and autoregressive moving average models. Due to a lack of standardization regarding automated bowel sound analysis, healthcare providers still have a hard time accessing bowel sound devices despite technological advancements in this field [15].
Based on a systematic review of bowel sound analysis methods published in 2018, Inderjeeth et al. concluded that the methods available up to that point were not suitable for evaluating bowel sounds in the clinical setting [16]. A study by the same team, led by Marshall, demonstrated that piezoelectric transducers can be used as contact microphones to identify migrating motor complexes and tested their prototype using machine learning algorithms [17][18]. Their AI system used a logistic regression model based on a calculated IBS Acoustic Index derived from 26 bowel sound features. Using an independent cohort of participants (comprised of recordings from 31 IBS and 37 healthy participants), their proof-of-concept study on bowel sound analysis for diagnosing IBS using AI achieved 87% sensitivity and specificity [17].
Further studies are still needed before automated bowel sound identification procedures can be routinely applied in clinical practice.
3. Artificial Intelligence-Generated Personalized Diet in Irritable Bowel Syndrome
IBS is therapeutically challenging, which is reflected in the patients’ numerous medical appointments. Given the fact that multiple factors are suspected to contribute to disease progression and severity, alongside widely varying symptoms, it is often difficult to design a targeted treatment plan. A specific dietary approach is frequently one of the key components of IBS management, which has been shown to be effective and safe. Certain diets may lead to symptomatic improvement, such as the low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) diet [19]. Unfortunately, this diet is associated with rather low compliance rates as it is difficult to implement and a fair proportion of IBS patients have no positive response.
Constructing a targeted, specific diet for IBS patients seems a promising strategy. The human microbiome is influenced by ingested foods and may play a significant role in the pathogenicity of the disease. Therefore, it should constitute an essential factor to consider when choosing the right diet for the treatment of IBS. In their work published in Gut Microbes in November 2022, Karakan and his team focused their research on this topic [20]. However, choosing the appropriate diet according to the patient’s microbiome can be rather difficult. In a systematic review of the intestinal microbiota published in 2018, there were many differences and inconsistencies between individuals with and without IBS [21].
Karakan et al. developed an AI system (ENBIOSIS) that may optimize the personalized nutritional strategy based on a patient’s individual microbiota. Their system is a machine learning classifier that uses XGBoost, a stochastic gradient-boosting classification model. For training purposes, two databases were needed. Firstly, they compiled a microbiota database using available open-source data from the American Gut Project [22], the Human Microbiome Project [23] and the Flemish Gut Flora Project [24]. Afterward, they compiled a nutrient database containing various categories, such as carbohydrates, proteins, lipids, vitamins/minerals, phytochemicals, food additives, and specific and fermented foods. For validation purposes, the researchers included two groups of subjects. One was comprised of IBS patients (diagnosed according to the Rome IV criteria), while the other one consisted of healthy subjects. Stool samples were collected from each group at two time points (pre- and post-intervention). High-throughput 16S rRNA sequencing was performed on the fecal samples in order to evaluate the gut microbiota community. Different microbiota patterns were observed in IBS patients compared to healthy controls. In the interventional part of the study, the 34 IBS participants were further divided into two groups. The first group (n = 14) was given a personalized type of microbiota-specific diet, while the second group (n = 11) received a standard IBS diet (low-FODMAP diet). Both groups were followed up for six weeks [20].
The IBS severity scoring system (IBS-SSS) was used to evaluate the clinical status of the participants both pre- and post-intervention. Additionally, changes in the microbiota were followed pre- and post-intervention with the help of a microbiome-derived IBS index score created using a machine learning technique. Even though the microbiota-derived IBS index score and the IBS-SSS improved in both groups (AI-personalized diet vs. low-FODMAP diet), the results were more substantial in subjects receiving an AI-personalized diet [20].
4. Smartphone Application Using Artificial Intelligence to Monitor Irritable Bowel Syndrome Symptoms
In today’s world technology is practically ubiquitous, also reflected in the presence of gadgets at every step. Leaving home without a smartphone, a smartwatch, or even smart headphones seems almost inescapable. Nowadays, these gadgets are being used to provide medical assistance and monitor health conditions. Wearable devices and apps can help track heart rate, blood pressure, and other important health metrics. They can also be used to alert medical personnel in case of an emergency. For instance, the Apple Watch Series 6 includes a feature that can detect if the user has suffered a hard fall and will alert emergency services if they are immobile for more than a minute.
Integrating AI technology into these gadgets may also give rise to new opportunities in regard to monitoring and tailoring IBS treatment. In 2022, Pimentel et al. published a study on this topic in the American Journal of Gastroenterology [25]. They developed a smartphone application using AI (named Dieta mobile app) for self-reporting stool form assessment. Out of the 45 IBS subjects included in the study, 39 agreed to use the application. Using digital images of users’ stools as input, the AI was trained to identify the characteristics of the stool. The visual characteristics used to train the AI were the Bristol Stool Scale, consistency, fragmentation, edge fuzziness and volume. The results of the study indicate that the AI system was significantly more accurate than the subjects’ own reports when categorizing daily average Bristol Stool Scale scores as constipation, normal, or diarrhea (0.95 vs. 0.89) [25].
In conclusion, AI may be able to provide a more objective assessment of stool characteristics, allowing for a more efficient diagnosis and treatment follow-up of IBS patients.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2020, 116, 17–44.
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106.
- Wu, J.; Wang, C.; Lv, L. Diagnostic yield of colonoscopy for organic disease in irritable bowel syndrome and its risk factors: A meta-analysis. Neurogastroenterol. Motil. 2022, 35, e14481.
- Tabata, K.; Mihara, H.; Nanjo, S.; Motoo, I.; Ando, T.; Teramoto, A.; Fujinami, H.; Yasuda, I. Artificial intelligence model for analyzing colonic endoscopy images to detect changes associated with irritable bowel syndrome. PLoS Digit. Health 2023, 2, e0000058.
- Vulpoi, R.-A.; Luca, M.; Ciobanu, A.; Olteanu, A.; Barboi, O.-B.; Drug, V.L. Artificial Intelligence in Digestive Endoscopy—Where Are We and Where Are We Going? Diagnostics 2022, 12, 927.
- Taghiakbari, M.; Mori, Y.; von Renteln, D. Artificial intelligence-assisted colonoscopy: A review of current state of practice and research. World J. Gastroenterol. 2021, 27, 8103–8122.
- Repici, A.; Badalamenti, M.; Maselli, R.; Correale, L.; Radaelli, F.; Rondonotti, E.; Ferrara, E.; Spadaccini, M.; Alkandari, A.; Fugazza, A.; et al. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 2020, 159, 512–520.e7.
- Ciobanu, A.; Luca, M.; Barbu, T.; Drug, V.; Olteanu, A.; Vulpoi, R. Experimental Deep Learning Object Detection in Real-time Colonoscopies. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021; Institute of Electrical and Electronics Engineers (IEEE): Iasi, Romania, 2021.
- Kulkarni, U.; Meena, S.M.; Gurlahosur, S.V.; Bhogar, G. Quantization Friendly MobileNet (QF-MobileNet) Architecture for Vision Based Applications on Embedded Platforms. Neural Netw. 2020, 136, 28–39.
- van der Zander, Q.E.W.; Schreuder, R.M.; Fonollà, R.; Scheeve, T.; van der Sommen, F.; Winkens, B.; Aepli, P.; Hayee, B.; Pischel, A.B.; Stefanovic, M.; et al. Optical diagnosis of colorectal polyp images using a newly developed computer-aided diagnosis system (CADx) compared with intuitive optical diagnosis. Endoscopy 2020, 53, 1219–1226.
- Tan, M.; Le, Q.V. EfficientNet: Rethinking Model Scaling for Convolutional Neural Networks. PMLR, 24 May 2019; pp. 6105–6114. Available online: https://proceedings.mlr.press/v97/tan19a.html (accessed on 11 March 2023).
- Baumgartner, M.; Lang, M.; Holley, H.; Crepaz, D.; Hausmann, B.; Pjevac, P.; Moser, D.; Haller, F.; Hof, F.; Beer, A.; et al. Mucosal Biofilms Are an Endoscopic Feature of Irritable Bowel Syndrome and Ulcerative Colitis. Gastroenterology 2021, 161, 1245–1256.e20.
- Craine, B.L.; Silpa, M.; O’Toole, C.J. Computerized auscultation applied to irritable bowel syndrome. Dig. Dis. Sci. 1999, 44, 1887–1892.
- Nowak, J.K.; Nowak, R.; Radzikowski, K.; Grulkowski, I.; Walkowiak, J. Automated Bowel Sound Analysis: An Overview. Sensors 2021, 21, 5294.
- Inderjeeth, A.J.; Webberley, K.M.; Muir, J.; Marshall, B.J. The potential of computerised analysis of bowel sounds for diagnosis of gastrointestinal conditions: A systematic review. Syst. Rev. 2018, 7, 1–18.
- Du, X.; Allwood, G.; Webberley, K.M.; Inderjeeth, A.J.; Osseiran, A.; Marshall, B.J. Noninvasive Diagnosis of Irritable Bowel Syndrome via Bowel Sound Features: Proof of Concept. Clin. Transl. Gastroenterol. 2019, 10, e00017.
- Du, X.; Allwood, G.; Webberley, K.M.; Osseiran, A.; Marshall, B.J. Bowel Sounds Identification and Migrating Motor Complex Detection with Low-Cost Piezoelectric Acoustic Sensing Device. Sensors 2018, 18, 4240.
- Staudacher, H.M.; Scholz, M.; Lomer, M.C.; Ralph, F.S.; Irving, P.M.; Lindsay, J.O.; Fava, F.; Tuohy, K.; Whelan, K. Gut microbiota associations with diet in irritable bowel syndrome and the effect of low FODMAP diet and probiotics. Clin. Nutr. 2020, 40, 1861–1870.
- Karakan, T.; Gundogdu, A.; Alagözlü, H.; Ekmen, N.; Ozgul, S.; Tunali, V.; Hora, M.; Beyazgul, D.; Nalbantoglu, O.U. Artificial intelligence-based personalized diet: A pilot clinical study for irritable bowel syndrome. Gut Microbes 2022, 14, 2138672.
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531.
- McDonald, D.; Hyde, E.; Debelius, J.W.; Morton, J.T.; Gonzalez, A.; Ackermann, G.; Aksenov, A.A.; Behsaz, B.; Brennan, C.; Chen, Y.; et al. American Gut: An Open Platform for Citizen Science Microbiome Research. mSystems 2018, 3, e00031-18.
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323.
- Vandeputte, D.; Vanleeuwen, R.; Falony, G.; Joossens, M.; Raes, J. Perspectives and pitfalls of microbiome research through home based fecal sampling: The Flemish Gut Flora Project experience. Arch. Public Health 2015, 73 (Suppl. S1), P33.
- Pimentel, M.; Mathur, R.; Wang, J.; Chang, C.; Hosseini, A.; Fiorentino, A.; Rashid, M.; Pichetshote, N.; Basseri, B.; Treyzon, L.; et al. A Smartphone Application Using Artificial Intelligence Is Superior To Subject Self-Reporting When Assessing Stool Form. Am. J. Gastroenterol. 2022, 117, 1118–1124.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
954
Revisions:
2 times
(View History)
Update Date:
07 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




