Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dina Hamdan | -- | 1153 | 2023-11-06 23:25:10 | | | |
| 2 | Catherine Yang | Meta information modification | 1153 | 2023-11-07 01:32:03 | | | | |
| 3 | Dina Hamdan | + 2 word(s) | 1155 | 2023-12-01 23:30:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khasawneh, H.; Ferreira Dalla Pria, H.R.; Miranda, J.; Nevin, R.; Chhabra, S.; Hamdan, D.; Chakraborty, J.; Biachi De Castria, T.; Horvat, N. Treatment of Pancreatic Adenocarcinoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/51218 (accessed on 07 February 2026).
Khasawneh H, Ferreira Dalla Pria HR, Miranda J, Nevin R, Chhabra S, Hamdan D, et al. Treatment of Pancreatic Adenocarcinoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/51218. Accessed February 07, 2026.
Khasawneh, Hala, Hanna Rafaela Ferreira Dalla Pria, Joao Miranda, Rachel Nevin, Shalini Chhabra, Dina Hamdan, Jayasree Chakraborty, Tiago Biachi De Castria, Natally Horvat. "Treatment of Pancreatic Adenocarcinoma" Encyclopedia, https://encyclopedia.pub/entry/51218 (accessed February 07, 2026).
Khasawneh, H., Ferreira Dalla Pria, H.R., Miranda, J., Nevin, R., Chhabra, S., Hamdan, D., Chakraborty, J., Biachi De Castria, T., & Horvat, N. (2023, November 06). Treatment of Pancreatic Adenocarcinoma. In Encyclopedia. https://encyclopedia.pub/entry/51218
Khasawneh, Hala, et al. "Treatment of Pancreatic Adenocarcinoma." Encyclopedia. Web. 06 November, 2023.
Copy Citation
Pancreatic ductal adenocarcinoma (PDAC) accounts for the majority of pancreatic cancers and is associated with poor prognosis, a high mortality rate, and a substantial number of healthy life years lost. Surgical resection is the primary treatment option for patients with resectable disease; however, only 10–20% of all patients with PDAC are eligible for resection at the time of diagnosis.
pancreatic cancer
neoadjuvant therapy
computed tomography
1. Introduction
Treatment plans for PDAC should be made following a comprehensive evaluation, including primary staging of the disease and assessment of the patient’s overall health status. At primary staging, PDAC is classified as a resectable disease, borderline resectable disease without metastases, locally advanced disease, or metastatic disease. Accuracy in the primary staging setting is crucial, as disease staging significantly affects treatment decisions. Guidelines for the management of pancreatic cancer have been published by the National Comprehensive Cancer Network (NCCN) in the United States [1]. Of note, considering the complexity of PDAC, the NCCN also strongly recommends that the management of patients with PDAC should involve high-quality imaging and multidisciplinary discussion at a high-volume center [1]. The NCCN guidelines pertaining to the resectability of pancreatic cancer, which are based on vascular involvement, are widely accepted and used at multidisciplinary team discussions in the United States [1][2] (Table 1). Below, the different PDAC resectability groups and the most common treatment options for each group are detailed (see also Figure 1 for a summary).
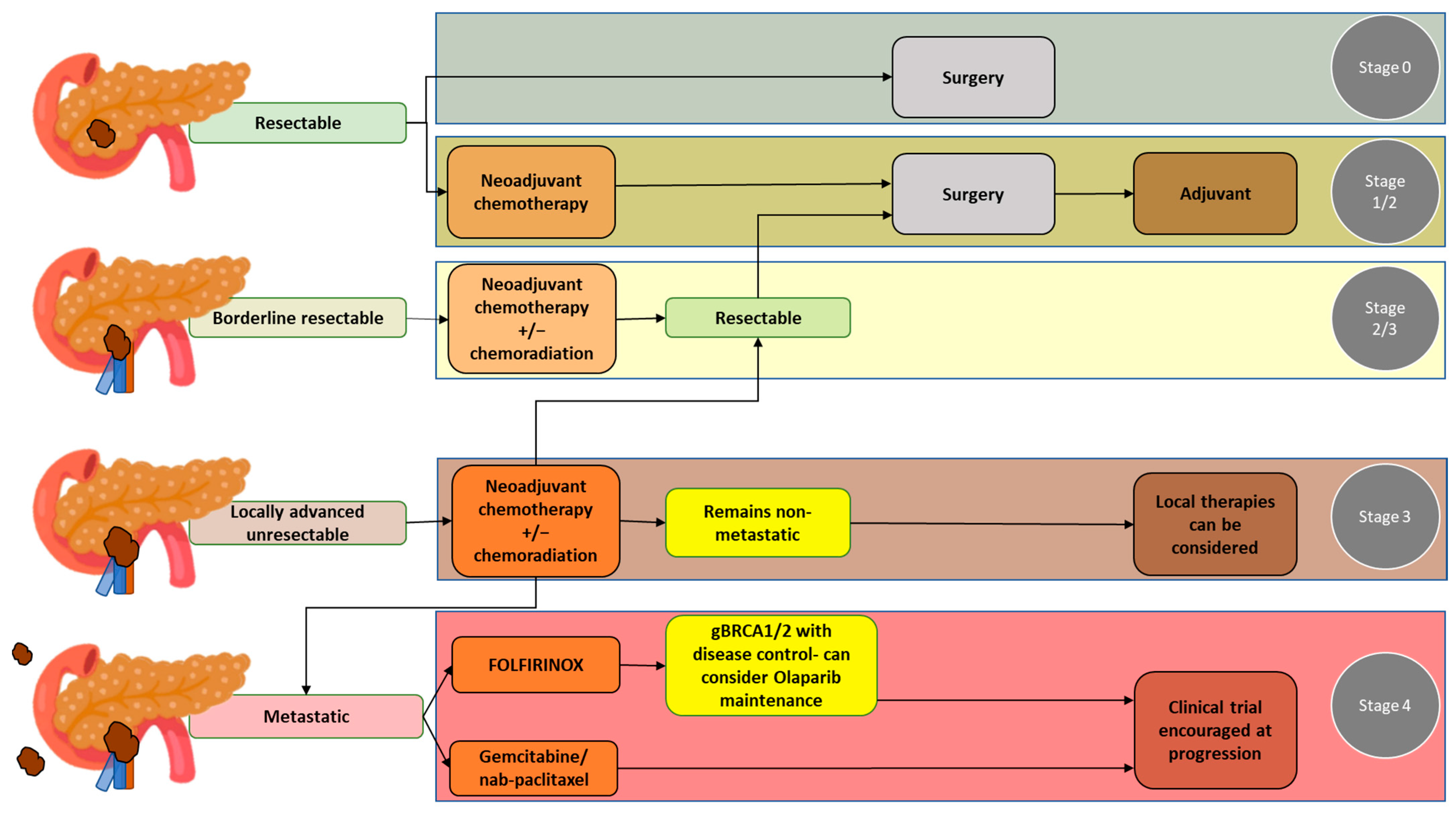
Figure 1. Pancreatic ductal adenocarcinoma groups according to resectability status and the most common treatment options for each group.
Table 1. NCCN criteria regarding the resectability status of pancreatic adenocarcinoma at diagnosis (primary staging).
| Resectability Status | Arterial | Venous |
|---|---|---|
| Resectable | No tumor contact |
|
| Borderline Resectable | CA & branches:
|
SMV or PV:
|
| Locally Advanced |
|
|
Abbreviations: CA: celiac axis; CHA: common hepatic artery; IVC: inferior vena cava; PV: portal vein; SMA: superior mesenteric artery; SMV: superior mesenteric vein.
2. Resectable PDAC
Surgery is the primary treatment option for patients with resectable PDAC. The goal of surgery is to obtain R0 resection, which entails the complete resection of the primary tumor and regional lymph nodes, since positive margins are associated with poor long-term survival [3][4] (Figure 2). After surgery, adjuvant chemotherapy is indicated. Of note, while surgical resection and adjuvant chemotherapy can be beneficial for improving survival outcomes, the median survival ranges from 20 to 53.5 months even under optimal conditions [1][5][6]. Some studies have shown that neoadjuvant therapy followed by surgery and adjuvant therapy further improves overall survival [7]; thus, neoadjuvant chemotherapy may also be considered in these patients.
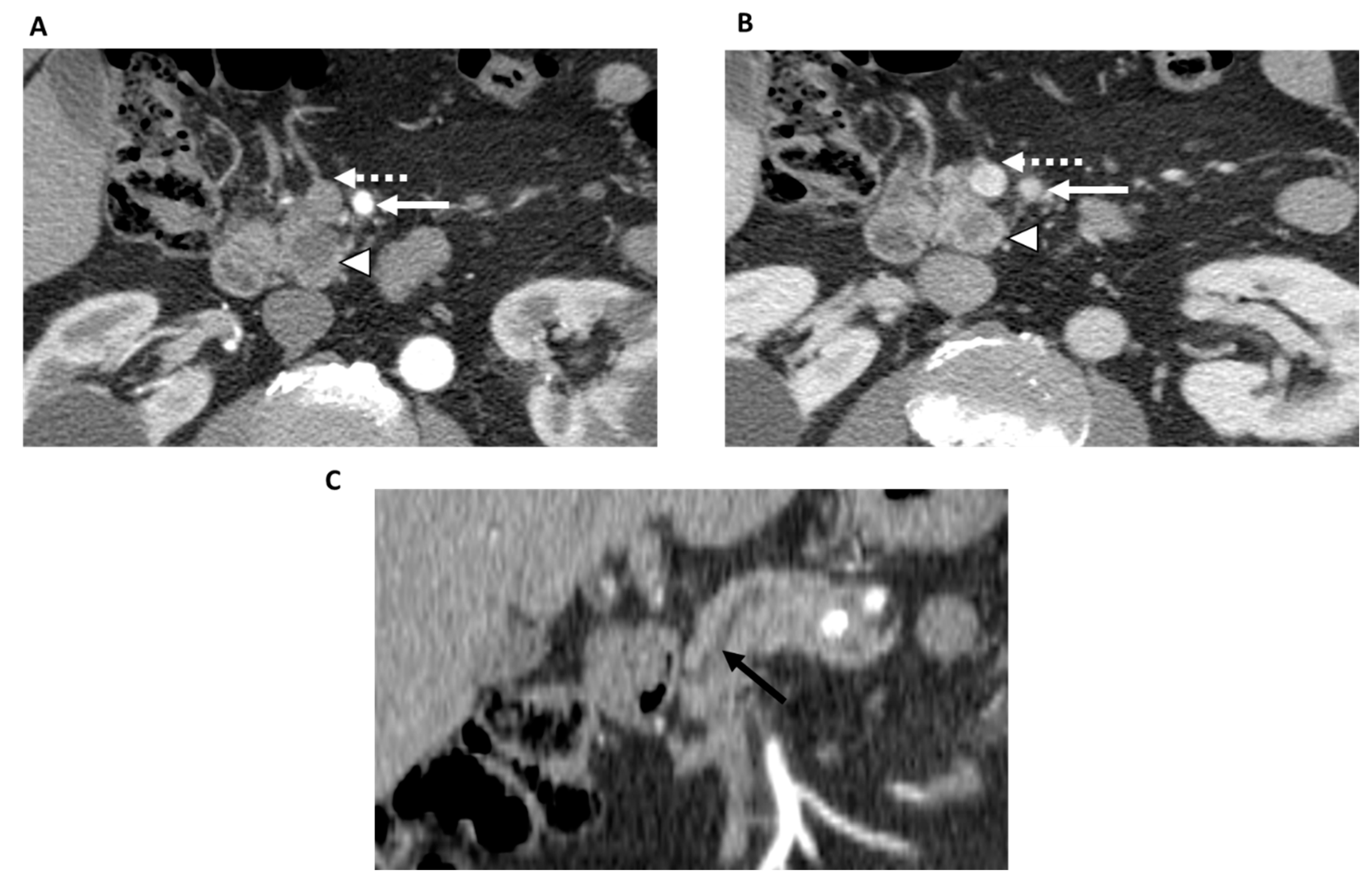
Figure 2. 78-year-old man with resectable pancreatic adenocarcinoma within the uncinate process. Contrast-enhanced CT in the axial plane shows a tumor within the uncinate process (arrowhead) in both the arterial (A) and portal venous phases (B). The superior mesenteric artery (white solid arrow) and superior mesenteric vein (dashed arrow) are not involved by the tumor. Contrast-enhanced CT in the coronal plane (C) shows upstream main pancreatic duct dilatation (black arrow). There was no evidence of metastatic disease. Based on the NCCN criteria, the patient was classified as resectable and underwent upfront surgery with R0 resection.
3. Borderline Resectable PDAC
Considering the complexity of borderline resectable PDAC, multidisciplinary discussion between medical oncologists, radiologists, surgeons, and radiation oncologists is especially necessary to determine the best treatment strategy. Treatment options include upfront surgery as well as neoadjuvant chemotherapy with or without radiation therapy. It is also advisable to refer borderline PDAC patients to participate in a clinical trial, as clinical trials offer the opportunity to undergo innovative treatment approaches, new drug combinations, and emerging therapies that can potentially improve patient outcomes.
The goal of neoadjuvant therapy is the downstaging of the tumor, which improves the likelihood of an R0 surgical resection, thereby prolonging survival rates; in this way, neoadjuvant therapy can also identify patients with rapid progression and poor response to treatment [8][9]. While the comparison between neoadjuvant and adjuvant therapies is challenging due to the different patient populations that receive these therapies, recent systematic reviews and meta-analyses underscore the benefits of neoadjuvant therapy over adjuvant therapy, including improved overall survival, higher R0 resection rates, and fewer pathological lymph nodes [10][11][12]. Further, with the literature suggesting an increasing shift towards adopting a neoadjuvant approach, numerous ongoing trials are examining the effectiveness of different neoadjuvant chemotherapy approaches, such as neoadjuvant therapy involving FOLFIRINOX, neoadjuvant therapy involving gemcitabine/nab-paclitaxel, and neoadjuvant therapy combining chemotherapy and radiation therapy [13]. Promising outcomes from neoadjuvant therapy involving chemotherapy indicate that chemotherapy should be an integral component of the neoadjuvant strategy. The use of stereotactic radiotherapy and emerging techniques like proton therapy are also being explored as part of the neoadjuvant strategy, with encouraging results [14].
Given the various neoadjuvant strategies, molecular profiling at diagnosis and the evaluation of the immune environment are emerging as potential tools to personalize neoadjuvant strategies. Encouragingly, combining neoadjuvant therapy with immunotherapy has shown promise to improve patient outcomes, particularly in the context of localized disease [13].
4. Locally Advanced PDAC
For patients with locally advanced PDAC and poor performance status, treatment options include best supportive care or palliative care (e.g., palliative radiotherapy, chemotherapy using a single agent, or polychemotherapy) (Figure 3). For patients with locally advanced PDAC and good or intermediate performance status, treatment options include systemic chemotherapy, chemoradiation therapy, or therapy as part of a clinical trial. Among the patients with locally advanced PDAC and good or intermediate performance status, if there is no disease progression, surgical resection may also be feasible [1].
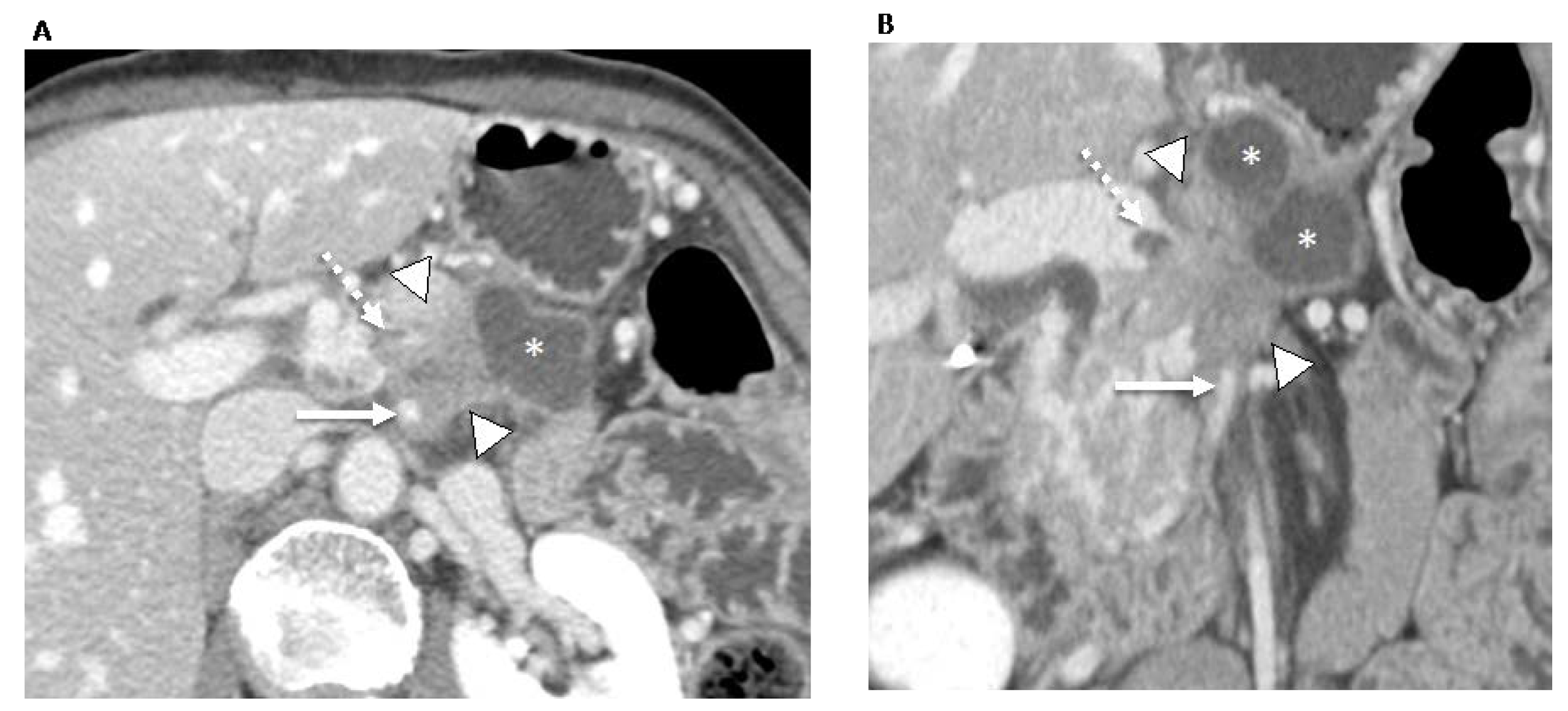
Figure 3. 70-year-old man with locally advanced pancreatic ductal adenocarcinoma. Contrast-enhanced CT in the axial (A) and coronal (B) planes in the portal venous phase shows an ill-defined pancreatic neck and body tumor (arrowheads) with an adjacent pseudocyst (asterisks). The tumor involves the locoregional vessels including encasement (>180°) of the superior mesenteric artery (arrow) and superior mesenteric vein/portomesenteric junction, which was severely narrowed with small non-occlusive thrombus (dashed arrow). The patient underwent palliative radiotherapy.
5. Metastatic PDAC
For patients with metastatic PDAC, treatment options include systemic chemotherapy (e.g., involving FOLFIRINOX or gemcitabine with nab-paclitaxel), targeted therapy, or palliative care. Treatment for these patients is aimed towards managing the disease and improving their quality of life. The choice of treatment will depend on the patient’s performance status, the extent of the disease, and the patient’s treatment response
References
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Pancreatic Adenocarcinoma. Version 2. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 15 July 2023).
- Al-Hawary, M.M.; Francis, I.R.; Chari, S.T.; Fishman, E.K.; Hough, D.M.; Lu, D.S.; Macari, M.; Megibow, A.J.; Miller, F.H.; Mortele, K.J.; et al. Pancreatic ductal adenocarcinoma radiology reporting template: Consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014, 270, 248–260.
- Windsor, J.A.; Barreto, S.G. The concept of ‘borderline resectable’ pancreatic cancer: Limited foundations and limited future? J. Gastrointest. Oncol. 2017, 8, 189–193.
- Balthazar, E.J. Pancreatitis associated with pancreatic carcinoma. Preoperative diagnosis: Role of CT imaging in detection and evaluation. Pancreatology 2005, 5, 330–344.
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C.; et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA 2010, 304, 1073–1081.
- Conroy, T.; Castan, F.; Lopez, A.; Turpin, A.; Ben Abdelghani, M.; Wei, A.C.; Mitry, E.; Biagi, J.J.; Evesque, L.; Artru, P.; et al. Five-Year Outcomes of FOLFIRINOX vs Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1571–1578.
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J. Clin. Oncol. 2022, 40, 1220–1230.
- Dhir, M.; Malhotra, G.K.; Sohal, D.P.S.; Hein, N.A.; Smith, L.M.; O’Reilly, E.M.; Bahary, N.; Are, C. Neoadjuvant treatment of pancreatic adenocarcinoma: A systematic review and meta-analysis of 5520 patients. World J. Surg. Oncol. 2017, 15, 183.
- Brown, Z.J.; Heh, V.; Labiner, H.E.; Brock, G.N.; Ejaz, A.; Dillhoff, M.; Tsung, A.; Pawlik, T.M.; Cloyd, J.M. Surgical resection rates after neoadjuvant therapy for localized pancreatic ductal adenocarcinoma: Meta-analysis. Br. J. Surg. 2022, 110, 34–42.
- Yang, B.; Chen, K.; Liu, W.; Long, D.; Wang, Y.; Liu, X.; Ma, Y.; Tian, X.; Yang, Y. The benefits of neoadjuvant therapy for patients with resectable pancreatic cancer: An updated systematic review and meta-analysis. Clin. Exp. Med. 2023.
- Ratnayake, B.; Savastyuk, A.Y.; Nayar, M.; Wilson, C.H.; Windsor, J.A.; Roberts, K.; French, J.J.; Pandanaboyana, S. Recurrence Patterns for Pancreatic Ductal Adenocarcinoma after Upfront Resection Versus Resection Following Neoadjuvant Therapy: A Comprehensive Meta-Analysis. J. Clin. Med. 2020, 9, 2132.
- Lindemann, J.; du Toit, L.; Kotze, U.; Bernon, M.; Krige, J.; Jonas, E. Survival equivalence in patients treated for borderline resectable and unresectable locally advanced pancreatic ductal adenocarcinoma: A systematic review and network meta-analysis. HPB 2021, 23, 173–186.
- Versteijne, E.; de Hingh, I.; Homs, M.Y.V.; Intven, M.P.W.; Klaase, J.M.; van Santvoort, H.C.; de Vos-Geelen, J.; Wilmink, J.W.; van Tienhoven, G. Neoadjuvant Treatment for Resectable and Borderline Resectable Pancreatic Cancer: Chemotherapy or Chemoradiotherapy? Front. Oncol. 2021, 11, 744161.
- Motoi, F.; Unno, M. Adjuvant and neoadjuvant treatment for pancreatic adenocarcinoma. Jpn. J. Clin. Oncol. 2020, 50, 483–489.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
867
Revisions:
3 times
(View History)
Update Date:
01 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




