Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marek Sebela | -- | 3771 | 2023-11-06 16:30:32 | | | |
| 2 | Lindsay Dong | Meta information modification | 3771 | 2023-11-08 01:57:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Šebela, M.; Rašková, M. Cytotoxicity of Polyamine-Derived Aminoaldehydes and Acrolein. Encyclopedia. Available online: https://encyclopedia.pub/entry/51200 (accessed on 07 February 2026).
Šebela M, Rašková M. Cytotoxicity of Polyamine-Derived Aminoaldehydes and Acrolein. Encyclopedia. Available at: https://encyclopedia.pub/entry/51200. Accessed February 07, 2026.
Šebela, Marek, Michaela Rašková. "Cytotoxicity of Polyamine-Derived Aminoaldehydes and Acrolein" Encyclopedia, https://encyclopedia.pub/entry/51200 (accessed February 07, 2026).
Šebela, M., & Rašková, M. (2023, November 06). Cytotoxicity of Polyamine-Derived Aminoaldehydes and Acrolein. In Encyclopedia. https://encyclopedia.pub/entry/51200
Šebela, Marek and Michaela Rašková. "Cytotoxicity of Polyamine-Derived Aminoaldehydes and Acrolein." Encyclopedia. Web. 06 November, 2023.
Copy Citation
Polyamines participate in the processes of cell growth and development. The degradation branch of their metabolism involves amine oxidases. The oxidation of spermine, spermidine and putrescine releases hydrogen peroxide and the corresponding aminoaldehyde. Polyamine-derived aminoaldehydes have been found to be cytotoxic.
acrolein
aldehyde dehydrogenase
amine oxidase
aminoaldehyde
3-aminopropanal
1. Introduction
Aldehydes represent electrophilic carbonyl compounds with a hydrogen atom substituent on the carbonyl carbon [1]. The exposure of living cells to aldehydes may occur easily as the compounds are produced in metabolism or occur as natural dietary constituents, contaminants, drugs and pollutants. The major sources of endogenous aldehydes are lipid oxidation, oxidative degradation of amino acids (e.g., via polyamines) and sugar metabolism [2]. These metabolic products belong to a complex group of compounds that can covalently modify or cross-link proteins, yielding advanced lipoxidation end products (ALEs) and advanced glycoxidation/glycation end products (AGEs). ALEs and AGEs are involved in the development and progression of different oxidation-based diseases such as atherosclerosis, chronic renal failure, neurological disorders and diabetes [2]. For example, methylglyoxal (pyruvaldehyde) is formed as a byproduct in glycolysis but can also arise from the metabolism of threonine, fatty acids or keto compounds such as acetoacetate and acetone [3]. Its increased formation occurs under hyperglycemia, which is associated with, e.g., diabetes, liver and kidney diseases. Exogenous sources include food processing (cooking, fermentation) and storage as well as combustion processes. In proteins, methylglyoxal primarily reacts with Arg, Lys, Cys and Trp residues [2].
Reactive short-chain aldehydes, which arise as secondary products after the peroxidation of polyunsaturated fatty acids in lipids under oxidative stress conditions, can be classified into several groups [4]: alkanals, alkenals, substituted alkenals and alkanedials (dialdehydes, e.g., malondialdehyde). 2-alkenals (e.g., acrolein, crotonaldehyde) contain an α-β double bond in addition to the carbonyl function; substituted alkenals contain an extra hydroxyl or oxo group (e.g., 4-hydroxy-2-nonenal, 4-oxo-2-nonenal). Acrolein is also an environmental pollutant released by smoking cigarettes, the combustion of fuels, the production of plastics and the prolonged heating of frying oils [2][5]. It has been shown to inhibit cell proliferation, enhance apoptosis and disrupt gene expression. There is an association with several inflammation-related diseases such as atherosclerosis and Alzheimer’s disease [6]. The α,β-unsaturated carbonyl species react covalently with Cys, His, Lys and Arg residues in proteins [5]. Simple Michael adducts can react with another acrolein molecule to form cyclic products [7][8]. These aldehydes can promote protein carbonylation. Thus, determining the presence of carbonyl groups in proteins has been widely used to measure the level of oxidative stress [9].
Polyamines have been recognized as important regulators involved in various growth and developmental processes (e.g., cell proliferation and differentiation, embryogenesis) and in responses to environmental stress [10][11][12][13]. Intracellular polyamine concentrations are maintained by a highly regulated metabolic network (at multiple levels, starting from gene transcription) consisting of biosynthetic and catabolic enzymes as well as transport systems for both import and export. Putrescine (1,4-butanediamine; PUT), which is produced by ornithine decarboxylase (EC 4.1.1.17), is converted to spermidine (4-azaoctane-1,8-diamine; SPD) and spermine (4,9-diaza-1,12-dodecanediamine; SPM) by the corresponding synthases (EC 2.5.1.16 and 2.5.1.22, respectively). Decarboxylated S-adenosylmethionine provides the necessary aminopropyl groups [11]. There are three enzymes that facilitate polyamine catabolism in mammals, namely the inducible cytosolic spermidine/spermine N1-acetyltransferase 1 (SSAT, EC 2.3.1.57) and spermine oxidase (SMO, EC 1.5.3.16) and the constitutively expressed peroxisomal N1-acetylpolyamine oxidase (AcPAO, EC 1.5.3.13). The induction is based on various stimuli, including free polyamines, polyamine analogs, hormones, natural products and drugs. The above oxidases produce 3-aminopropanal (APAL) and 3-acetamidopropanal (AcAPAL), respectively, as well as hydrogen peroxide and the corresponding shortened polyamine. APAL can easily generate acrolein through the elimination of ammonia [14]. Elevated polyamine levels are needed for continued proliferation and tumor progression, especially in oncogene-driven cancers. Accordingly, antitumor polyamine analogs increase SSAT and SMO activities, which leads to a drop in cellular polyamines [15]. Copper-containing amine oxidases such as the intracellular kidney diamine oxidase (DAO; EC 1.4.3.22, formerly EC 1.4.3.6) and extracellular serum amine oxidase (SAO; EC 1.4.3.21, formerly EC 1.4.3.6 as well) can oxidize polyamines at their primary amino groups with a different efficiency. PUT belongs to the best substrates of DAO, which is in accordance with the name of the enzyme, but is only very weakly oxidized by SAO [16][17].
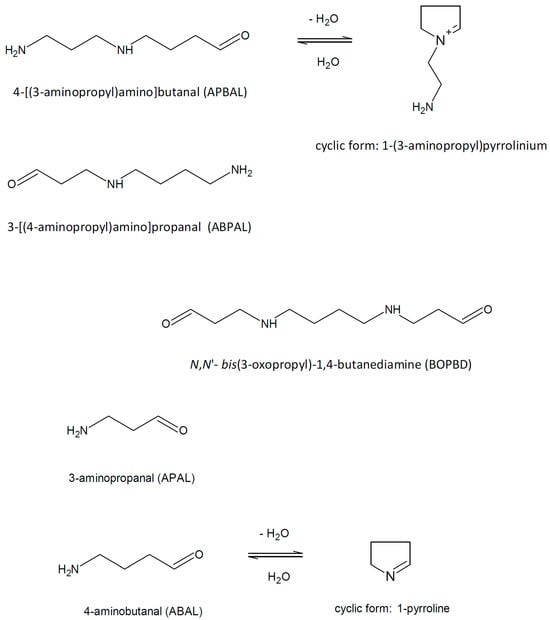
2. 3-Aminopropanal and Other Aminoaldehydes Produced in Polyamine Metabolism
Polyamine catabolism in living organisms is accompanied by the formation of several aminoaldehydes (Figure 1) such as N,N′-bis(3-oxopropyl)-1,4-butanediamine (BOPBD), N-(3-oxopropyl)-1,4-butanediamine, i.e., 3-[(4-aminobutyl)amino]propanal (ABPAL), 4-[(3-aminopropyl)amino]butanal (APBAL), 3-aminopropanal (APAL) and 4-aminobutanal (ABAL), as described further in the text.

Figure 1. Polyamine-derived aminoaldehydes and their chemical structures.
BOPBD and ABPAL are produced by ruminant copper-containing SAOs when SPM and SPD are oxidized, respectively. The chemical nature of the aminoaldehydes was discovered by experiments with the bovine enzyme (BSAO) and clearly demonstrated that the oxidation occurred at the primary amino groups and resulted in the oxopropyl moieties of the products [18]. BOPBD and ABPAL were analyzed using paper chromatography after their reduction in the reaction mixture to more stable hydroxypropyl counterparts, and their chromatographic properties agreed with those of synthetic standards. Other chemicals properties of the reduced products, such as the melting points or infrared spectrum (measured for BOPBD), were also in accordance with the standards [18]. All of the above aminoaldehyde compounds were purified from BSAO reaction mixtures by means of an ion-exchange chromatography on SE-Sephadex. The purified aminoaldehydes (ca. 90% purity) were analyzed using thin layer chromatography (TLC) after their reduction to the corresponding hydroxypropyl derivatives by sodium borohydride and were assigned to the synthetic standards. Stability tests confirmed their spontaneous decomposition yielding acrolein and demonstrated the formation of larger condensation products [19].
High-performance liquid chromatography (HPLC) of rat tissue extracts with a post-column derivatization of amines by dansyl chloride and fluorescence detection demonstrated that SPD is oxidized by DAO at its terminal amino groups, yielding ABPAL or APBAL, which are converted via an aldehyde dehydrogenase (ALDH) reaction into the amino acids putreanine and isoputreanine, respectively [20]. The latter compound is formed by the hydrolysis of N-(3-aminopropyl)pyrrolidin-2-one, which was detected as an intermediate. APAL is produced by the oxidative deamination of 1,3-propanediamine (DAP), as has been shown using the ion-exchange chromatography of the reaction mixture containing this diamine and DAO from porcine kidney [21]. Similarly, APAL was purified from the reaction mixture of this enzyme through chromatographic steps and was obtained as a crystalline 2,4-dinitrophenylhydrazone. This colored product was analyzed using TLC and found to be identical to a derivative produced from enzymatically oxidized (by an alcohol dehydrogenase) 3-aminopropanol and also to a derivative isolated from blood serum after treatment with As2O3 and 2,4-dinitrophenylhydrazine (DNPH) [22]. DAP is released in the reaction of DAP-forming PAOs, which occur in plants and bacteria (see below). Additionally, it is supposed to be produced in vertebrates by the oxidative cleavage of isoputreanine, which is formed by APBAL oxidation, at the secondary amino group [23].
A report based on 1H-NMR plus liquid chromatography data and describing the formation of APAL by BSAO in a successive conversion of SPM into SPD and SPD into PUT [21] was later challenged by pointing to sources of possible experimental artifacts. Lee and Sayre performed a reaffirmation of the chemical structure of the aminoaldehyde products of a BSAO reaction with SPM and SPD [24]. Uncertain action of BSAO on the secondary amino groups of the substrates was ruled out as homospermidine conversion by the enzyme yielded cyclic 1-(4-aminobutyl)pyrrolinium (no β-elimination of acrolein is possible in this case because of the non-existing oxopropyl moieties in the aminoaldehyde), indicating that the oxidative deamination occurred at the primary amino group. They also demonstrated by 1H-NMR that acrolein, which is released by the spontaneous decomposition of aminoaldehyde products generated from SPM and SPD (i.e., BOPBD and ABPAL, respectively), can react with ammonia and produce APAL, which results in the biasing of the interpretation of the chromatographic and NMR data acquired with reaction mixtures. Another NMR-based study of substrate oxidation by BSAO confirmed that the enzyme oxidized SPD and homospermidine at the primary amino groups [25]. Clear 1H-NMR signals of hydrated ABPAL were obtained. The authors further demonstrated that the presence of the N-aminopropyl group in BSAO substrates is reflected in their cytotoxic effect on human endothelial cells [25].
ABAL emerges from the reaction of PUT oxidation by DAOs [26]. However, the free aldehyde form of ABAL is difficult to detect and quantify as a metabolite due to the inherent reactivity [27]. SPD is a good substrate of plant DAOs [16]. The expected product aminoaldehyde is APBAL in this case, which is in contrast to the above-mentioned reaction of BSAO producing the isomeric and oxopropyl group-containing ABPAL. ABAL and APBAL are known to cyclize spontaneously to 1-pyrroline and its N-aminopropyl derivative, i.e., 1-(3-aminopropyl)pyrrolinium, respectively, in the reaction mixtures of DAO or PAO [24][28]. As a proof, MALDI-TOF (matrix-assisted laser desorption ionization time-of-flight) mass spectrometry demonstrates that SPD (m/z 146) is oxidized by pea seedling DAO to a product with m/z 127, suggesting a water loss and cyclization as the observed mass is consistent with the structure of the cationic 1-(3-aminopropyl)pyrrolinium. Aqueous solutions of ABAL were characterized by NMR spectroscopy in the pH range of 1 and 13 to demonstrate the existence of two neutral species (1-pyrroline, pyrroline trimer) and four protonated species (1-pyrrolinium, free aldehyde, hemiaminal and hydrate). The pyrroline (predominant) and its trimer were present at basic pH values, whereas the open-chain forms, pyrrolinium and hemiaminal, appeared at the pH range of 1–6. Interestingly, 4-butylaminobutyraldehyde exclusively existed as N-butylpyrrolinium ion at acidic pH [27]. Lentil seedling DAO generates BOPBD and APBAL by the oxidative deamination of SPM and SPD, respectively. In the presence of high SPM amounts, BOPBD was shown to react with the primary amino groups of other SPM molecules, yielding a yellow-orange compound. Its analysis using MS and two-dimensional NMR experiments allowed for the identification of a pyrimidine-based cyclic structure with two side chains [29].
The maize enzyme is a representative of the class of DAP-forming PAOs (EC 1.5.3.14) from the monocotyledonous plants. These enzymes oxidize SPM to APBAL, hydrogen peroxide and DAP. SPD is oxidized to ABAL, H2O2 and DAP [30]. Because these products cannot be converted directly to other polyamines, the DAP-forming PAOs are considered to be involved in the terminal catabolism of polyamines. DAP formation was demonstrated by TLC of the reaction mixture components with a fluorescence detection upon dansylation [30]. In mammals, the cytosolic SMO (EC 1.5.3.16) is a highly inducible flavoprotein enzyme that participates in polyamine homeostasis. It exclusively oxidizes SPM and not SPD or N1-acetyl-SPM (the specificity is charge-based) and produces SPD, hydrogen peroxide and APAL as reactions products [31]. Non-specific PAOs (EC 1.5.3.17) oxidize SPM and SPD but may also accept acetylated polyamines such as N1-acetyl-SPM or N1-acetyl-SPD as substrates. Additionally, their reaction resembles that of mammalian APAL-producing PAOs. Examples have been found to occur in, e.g., Arabidopsis [32][33]. N1-acetylated SPM and SPD are preferentially oxidized by mammalian peroxisomal AcPAOs (EC 1.5.3.13). N1-acetyl-SPM and N1-acetyl-SPD are converted to SPD and PUT, respectively, 3-acetamidopropanal (AcAPAL) and H2O2 [34][35]. This process is known as polyamine back-conversion because of the formation of deacetylated polyamines. Maize PAO (EC 1.5.3.14) oxidizes N1-acetyl-SPM to 4-[(3-acetamidopropyl)amino]butanal and N8-acetyl-SPD to AcAPAL. The other products are DAP and H2O2 [30].
3. Cytotoxicity of Polyamine-Derived Aminoaldehydes
Polyamine-derived aminoaldehydes were purified on a cation exchanger and used in experiments, demonstrating their toxicity to the malarial parasite Plasmodium falciparum that was cultured in erythrocytes and treated at the young and mature developmental stages [36]. A strong lethal effect of the aminoaldehydes was observed at >100 µM concentrations after incubation for 105 min by inhibiting the incorporation of [3H]hypoxanthine, and it was more pronounced in the mature parasite [36]. Bovine serum is frequently used as a medium supplement for in vitro cell cultures. When SPD (30 μM) was exogenously added to cultured mouse cells in the presence of bovine fetal or calf serum, the contained BSAO activity caused considerable cytotoxic effects because of the generation of the products aminoaldehyde and hydrogen peroxide. This SPD-related toxicity was found to be dose-dependent and was inhibited by 1 mM of aminoguanidine as an inhibitor of BSAO [37]. The cytotoxic effects of the SPM oxidation products were analyzed in a study with mouse FM3A cells in the presence of bovine calf serum [38]. Exogenously added SPM (15 and 30 µM) as well as acrolein (7.5 and 15 µM), which is expected to be formed by the spontaneous decomposition of SPM-derived aminodialdehyde, largely inhibited FM3A cells growth.
A mouse lymphocytic leukemia cell line was used to study the cytotoxicity of the aminodialdehyde BOPBD, which was produced by BSAO reacting with SPM [39]. SPM exerted cytotoxicity at a concentration of 25 µM, and complete cell death was observed within 12 h. The cytotoxicity was prevented by aminoguanidine. Catalase delayed but did not prevent cell death by hydrogen peroxide scavenging; thus, its primary determinant was likely the aminoaldehyde generation. Reduced glutathione (GSH) was found to be the major target of both SPM oxidation products. The attributes of the observed cell death process (e.g., a lack of caspase activation involvement) as well as cell morphology indicated necrosis rather than apoptosis but with damage to mitochondria and phosphatidyl serine redistribution at the plasma membrane [39]. Exogenously added SPD has been shown to promote longevity by inducing autophagy in a series of in vitro studies with yeast, Drosophila and murine models [40].
The levels of PUT, SPD, SPM and acrolein were analyzed in the plasma of patients with renal failure. PUT was approximately increased twice compared with normal subjects, whereas SPD and particularly SPM were decreased, as determined by an HPLC analysis. This correlated with the observed increase in PAO activity [41]. Both free and protein-bound acrolein were analyzed by HPLC and by using specific antibodies, respectively. Interestingly, their levels were well correlated with the renal failure severity. The increased level of the free aldehyde was slightly above 1 µM compared with 0.5 µM in normal subjects; the conjugated acrolein reached about 170 µM, which is a five-fold increase [41]. Polyamine oxidation, represented by the activity levels of AcPAO and SMO as well as acrolein concentration, reflected in the formation of Nε-(3-formyl-3,4-dehydropiperidino)lysine (i.e., FDP-Lys adducts of proteins), was found to be elevated in the plasma of stroke patients and was correlated with the size of stroke. Hence, these levels were suggested as early diagnostic markers of the disease [42]. Both enzymes were assayed by HPLC, and the protein-conjugated acrolein was determined using specific antibodies in ELISA (enzyme-linked immunosorbent assay) tests. An increase in AcPAO occurred first, followed by increased SMO and then FDP-Lys [42]. Cigarette smoking has been long known to suppress immune responses in the lungs. Smokers are also more susceptible to respiratory infections [43]. The vapor phases from cigarette smoke extracts were found to inhibit the production of several proinflammatory cytokines in macrophages and T-cells. Both acrolein and crotonaldehyde, identified in the vapor phase by gas chromatography coupled with mass spectrometry (GC-MS) and quantified in the whole extract by reversed-phase HPLC after derivatization with 2,4-dinitrophenylhydrazine, exerted 50% inhibitory concentrations (IC50) at micromolar levels. The inhibition by these α,β-unsaturated aldehydes was completely abrogated by N-acetylcysteine. The inhibition of cytokine production was associated with affecting the expression of related genes, as demonstrated by an mRNA-level analysis [43].
Serum albumin, which is an abundant protein in blood serum (and also in lung lining fluid), has multiple physiological functions including the binding and transportation of molecules and ions, regulation of colloid osmotic pressure and maintenance of vascular pressure [44]. The carbonylation of bovine serum albumin (BSA) upon its reaction with acrolein was studied using a colorimetric assay with DNPH, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), Western blotting with anti-dinitrophenylhydrazone-adduct antibodies and nanoflow liquid chromatography coupled with electrospray ionization tandem mass spectrometry (nanoLC-ESI-MS/MS). Furthermore, this study was interested in the influence of acrolein on gene expression and its cytotoxicity towards cultured lung epithelial cells in the presence or absence of BSA as an aldehyde scavenger [45]. SDS-PAGE followed by Western blotting demonstrated the formation of carbonylated and cross-linked BSA forms with increased molecular masses after the reaction with acrolein. It was shown that the carbonylation of BSA by acrolein decreased the increased the transcription of two selected genes (a transcription factor, heme oxygenase 1), which was induced by acrolein itself. An increased in vivo BSA carbonylation was demonstrated in the bronchoalveolar lavage of rats exposed to acrolein. The in vivo adduction site in BSA was then found at Cys34 using nanoLC-ESI-MS/MS [45]. Similarly, multiple modifications of bovine ubiquitin by acrolein are detectable using MALDI-TOF MS.
4. Cytotoxicity of 3-Aminopropanal
Cerebral ischemia is caused by insufficient blood flow in the brain tissue, which leads to hypoxia and subsequently cell death. The area surrounding an ischemic event is called a penumbra [46]. The polyamines SPD and SPM are abundant brain metabolites, and they are implicated in the pathogenesis of the disease as their increased oxidation by upregulated PAO is accompanied by the production of APAL. The pKa value of APAL, which is approximately 9.3, suggests its lysosomotropic properties. A high intralysosomal concentration of the aminoaldehyde upon its accumulation is supposed to lead to reactions with thiol groups in proteins and subsequent damage to lysosomal integrity [47][48]. Ivanova et al. studied APAL as a cytotoxic mediator in ischemic rats [49]. They developed an HPLC method to quantify the compound in tissue samples as a 2,4-dinitrophenylhydrazone adduct. The APAL levels were found to be significantly elevated 2 h after the onset of ischemia, and the increase continued with cell death development and spreading around the ischemic core. Unlike glial cells, where apoptosis was activated via caspase 1, APAL did not induce apoptosis in neuronal cells but primarily caused necrosis. Adding PAO inhibitors effectively prevented ischemic cell damage [49].
A keyhole limpet hemocyanin was reacted with APAL in the presence of sodium borohydride and used to raise antibodies using rabbit immunization. These antibodies were then applied to detect and semi-quantify (by dot blotting using APAL-reacted BSA as a standard) the level of APAL-modified proteins in biological samples, such as in the cerebrospinal fluid of ischemic patients [50]. Patients with a good neurological prognosis had significantly lower levels of such protein modifications compared with those with the most severe forms of disease. The authors additionally screened a library of sulfhydryl compounds and found that N-(2-mercaptopropionyl)glycine (N-2-MPG) efficiently neutralized APAL in glial and neuronal cell cultures by forming a thioacetal product. N-2-MPG was also found using immunohistochemistry to significantly reduce the cytotoxicity of APAL in vivo in a rat ischemic model with middle cerebral artery occlusion [50]. Phenelzine is another example of a possible aldehyde sequestration reagent, which is efficient against both APAL and acrolein cytotoxicity. In this case, a hydrazone is produced by the reaction. Phenelzine avidly neutralizes APAL in retinal ganglion cells, even when administered with a delay of several hours [51]. N-2-MPG is an approved drug (tiopronin) for the treatment of cystinuria. It was applied as a study drug to treat aneurysmal subarachnoid hemorrhage [52]. No adverse effects were registered in phase I clinical trials. All enrolled patients were administered the compound within 96 h of the hemorrhage onset for a short period of 14 days. The patients were otherwise subjected to surgical or endovascular repair of their aneurysm and a standard therapy for cerebral vasospasm [52]. The efficacy of tiopronin to neutralize APAL in cerebrospinal fluid was evaluated in a subsequent phase II study utilizing LC-ESI-MS/MS to quantify the compound [53].
The molecular mechanism of APAL cytotoxicity, which is related to the apoptosis of neurons and glial cells as a consequence of degenerative or traumatic injuries to the central nervous system, was studied using histochemistry and immunohistochemistry to demonstrate that the compound primarily targets lysosomes [47]. Human astrocytoma cells were used as a model and were subjected to microscopy after the treatment with 75 µM of APAL, resulting in apoptosis. Specific antibodies allowed for the localization of high levels of APAL-modified proteins in lysosomes. The occurrence of lysosomal ruptures was demonstrated by a fluorescence staining with acridine orange, increased immunoreactivity of the lysosomal cathepsin B inside the whole cell, caspase activation and typical morphological signs of apoptosis.
Microscopic experiments with marker dyes and lysosome-rich murine macrophage cells demonstrated the lysosomotropic properties of APAL [54]. Treating cells with 100 µM of APAL resulted in a time-dependent increase in apoptosis. The induced lysosomal rupture led to a mitochondria-associated and enhanced production of reactive oxygen species, which was likely due to processes involving the release of cysteine-containing cathepsins, as their specific inhibition largely reduced the observed APAL-induced effects. Early lysosomal ruptures were detectable already after a short exposure to 100 µM of APAL for only 20 min, and apoptotic cells and caspase activation were observed after 3 h of treatment, indicating that apoptosis was not induced immediately. Necrosis was the predominant cell death form when a higher concentration of 200 µM of APAL was applied [54]. Several pieces of experimental evidence were obtained that indicated that the late endosomal/lysosomal membrane-associated protein Niemann–Pick C1 (NPC1) was involved in regulating the accumulation of amines in lysosomes [55]. Microscopic data for human fibroblasts demonstrated that NPC1 mediated the vacuolization of lysosomes induced by lysosomotropic amines. Moreover, these amines facilitated the formation of late endosomal/lysosomal hybrid organelles in the presence of NPC1 (monitored by fluorescence resonance energy transfer after lysosomal endocytosis of a fluorescently labeled and biotinylated dextran and the streptavidin-conjugated labeled latex beads localized in endosomes), and the subsequent lysosomal cargo secretion was stimulated. NPC1-depleted fibroblasts were found to be more susceptible to the toxic effects of APAL manifested by damage to the lysosomal membrane [55]. A rat retinal ganglion cell line was used as a model to compare the cytotoxicity of APAL and AcAPAL [56]. The cytotoxic effect was assayed by measuring the activity of lactate dehydrogenase (EC 1.1.1.27). The enzyme is cytosolic and is released into the cell culture medium upon damage to the plasma membrane. While APAL showed a strong effect at a concentration of 200 µM (although it was much less pronounced than those of acrolein or hydrogen peroxide at the same concentration), AcAPAL displayed no cytotoxicity up to a concentration of 1 mM.
5. Conclusions
The aminoaldehyde products of the enzymatic polyamine oxidation have been shown to be cytotoxic, although the concurrently generated hydrogen peroxide seems to be the major cytotoxic compound in the reaction mixtures (roughly 80% for human adenocarcinoma cells) as deduced from the moderating effect of catalase [57]. Regardless, the use of polyamine oxidation products to destroy cancer cells selectively and safely belongs to the ongoing experimental research directions on the road to innovative therapeutic strategies. Polyamine concentrations are high in growing tissues such as tumors (e.g., colorectal, breast and prostate cancer), which has been elucidated by their increased synthesis and uptake. When the production of polyamines in cancer cells is decreased by mutations or treatment, senescence and apoptosis occur. Polyamine metabolism is thus targeted with great interest in studies with anticancer compounds [13][58].
References
- LoPachin, R.M.; Gavin, T. Molecular mechanisms of aldehyde toxicity: A chemical perspective. Chem. Res. Toxicol. 2014, 27, 1081–1091.
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Rad. Res. 2013, 47 (Suppl. 1), 3–27.
- Lai, S.W.T.; Lopez Gonzalez, E.J.; Zoukari, T.; Ki, P.; Shuck, S.C. Methylglyoxal and its adducts: Induction, repair, and association with disease. Chem. Res. Toxicol. 2022, 35, 1720–1746.
- Domingues, R.M.; Domingues, P.; Melo, T.; Pérez-Sala, D.; Reis, A.; Spickett, C.M. Lipoxidation adducts with peptides and proteins: Deleterious modifications or signaling mechanisms? J. Proteom. 2013, 92, 110–131.
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, metabolism and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food. Res. 2008, 52, 7–25.
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124.
- Uchida, K.; Kanematsu, M.; Sakai, K.; Matsuda, T.; Hattori, N.; Mizuno, Y.; Suzuki, D.; Miyata, T.; Noguchi, N.; Niki, E.; et al. Protein-bound acrolein: Potential markers for oxidative stress. Proc. Natl. Acad. Sci. USA 1998, 95, 4882–4887.
- Furuhata, A.; Ishii, T.; Kumazawa, S.; Yamada, T.; Nakayama, T.; Uchida, K. Nε-(3-methylpyridinium)lysine, a major antigenic adduct generated in acrolein-modified protein. J. Biol. Chem. 2003, 278, 48658–48665.
- Suzuki, Y.J.; Carini, M.; Butterfield, D.A. Protein carbonylation. Antioxid. Redox Signal. 2010, 12, 323–325.
- Baron, K.; Stasolla, C. The role of polyamines during in vivo and in vitro development. In Vitro Cell. Dev. Biol.-Plant 2008, 44, 384–395.
- Casero, R.A., Jr.; Pegg, A.E. Polyamine catabolism and disease. Biochem. J. 2009, 421, 323–338.
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945.
- Sari, I.N.; Setiawan, T.; Kim, K.S.; Wijaya, Y.T.; Cho, K.W.; Kwon, H.Y. Metabolism and function of polyamines in cancer progression. Cancer Lett. 2021, 519, 91–104.
- O’Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–662.
- Casero, R.A., Jr.; Stewart, T.M.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695.
- Pietrangeli, P.; Federico, R.; Mondovì, B.; Morpurgo, L. Substrate specificity of copper-containing plant amine oxidases. J. Inorg. Biochem. 2007, 101, 997–1004.
- Pietrangeli, P.; Morpurgo, L.; Mondovì, B.; Di Paolo, M.L.; Rigo, A. Soluble copper amine oxidases from mammals. In Copper Amine Oxidases: Structures, Catalytic Mechanisms and Role in Pathophysiology; Floris, G., Mondovì, B., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 51–68.
- Tabor, C.W.; Tabor, H.; Bachrach, U. Identification of the aminoaldehydes produced by the oxidation of spermine and spermidine with purified plasma amine oxidase. J. Biol. Chem. 1964, 239, 2194–2203.
- Kimes, B.W.; Morris, D.R. Preparation and stability of oxidized polyamines. Biochim. Biophys. Acta 1971, 228, 223–234.
- Seiler, N.; Knödgen, B.; Haegele, K. N-(3-Aminopropyl)pyrrolidin-2-one, a product of spermidine catabolism in vivo. Biochem. J. 1982, 208, 189–197.
- Houen, G.; Bock, K.; Jensen, A.L. HPLC and NMR investigation of the serum amine oxidase catalyzed oxidation of polyamines. Acta Chem. Scand. 1994, 48, 52–60.
- Quash, G.; Taylor, D.R. Serum β-aminopropionaldehyde: Identification and origin. Clin. Chim. Acta 1970, 30, 17–23.
- Seiler, N. Oxidation of polyamines and brain injury. Neurochem. Res. 2000, 25, 471–490.
- Lee, Y.; Sayre, L.M. Reaffirmation that metabolism of polyamines by bovine plasma amine oxidase occurs strictly at the primary amino termini. J. Biol. Chem. 1998, 273, 19490–19494.
- Houen, G.; Struve, C.; Søndergaard, R.; Friis, T.; Anthoni, U.; Nielsen, P.H.; Christophersen, C.; Petersen, B.O.; Duus, J.Ø. Substrate specificity of the bovine serum amine oxidase and in situ characterization of aminoaldehydes by NMR spectroscopy. Biorg. Med. Chem. 2005, 13, 3783–3796.
- Šebela, M.; Brauner, F.; Radová, A.; Jacobsen, S.; Havliš, J.; Galuszka, P.; Peč, P. Characterisation of a homogeneous plant aminoaldehyde dehydrogenase. Biochim. Biophys. Acta-Protein Struct. Molec. Enzym. 2000, 1480, 329–341.
- Struve, C.; Christophersen, C. Structural equilibrium and ring-chain tautomerism of aqueous solutions of 4-aminobutyraldehyde. Heterocycles 2003, 60, 1907–1914.
- Šebela, M.; Radová, A.; Angelini, R.; Tavladoraki, P.; Frébort, I.; Peč, P. FAD-containing polyamine oxidases: A timely challenge for researchers in biochemistry and physiology of plants. Plant Sci. 2001, 160, 197–207.
- Padiglia, A.; Medda, R.; Paci, M.; Sette, M.; Lorrai, A.; Floris, G. Characterization of a cyclic compound fomed after spermine oxidation by lentil amine oxidase. Biochem. Mol. Biol. Int. 1997, 41, 407–413.
- Federico, R.; Ercolini, L.; Laurenzi, M.; Angelini, R. Oxidation of acetylpolyamines by maize polyamine oxidase. Phytochemistry 1996, 43, 339–341.
- Cervelli, M.; Amendola, R.; Polticelli, F.; Mariottini, P. Spermine oxidase: Ten years after. Amino Acids 2012, 42, 441–450.
- Moschou, P.N.; Sanmartin, M.; Andriopoulou, A.H.; Rojo, E.; Sanchez-Serrano, J.J.; Roubelakis-Angelakis, K.A. Bridging the gap between plant and mammalian polyamine catabolism: A novel peroxisomal polyamine oxidase responsible for a full back-conversion pathway in Arabidopsis. Plant Physiol. 2008, 147, 1845–1857.
- Fincato, P.; Moschou, P.N.; Spedaletti, V.; Tavazza, R.; Angelini, R.; Federico, R.; Roubelakis-Angelakis, K.A.; Tavladoraki, P. Functional diversity inside the Arabidopsis polyamine oxidase gene family. J. Exp. Bot. 2011, 62, 1155–1168.
- Häkkinen, M.R.; Hyvönen, M.T.; Auriola, S.; Casero, R.A., Jr.; Vepsäläinen, J.; Khomutov, A.R.; Alhonen, L.; Keinänen, T.A. Metabolism of N-alkylated spermine analogues by polyamine and spermine oxidases. Amino Acids 2010, 38, 369–381.
- Moriya, S.; Iwasaki, K.; Samejima, K.; Takao, K.; Kohda, K.; Hiramatsu, K.; Kawakita, M. A mass spectrometric method to determine activities of enzymes involved in polyamine catabolism. Anal. Chim. Acta 2012, 748, 45–52.
- Morgan, D.M.L.; Bachrach, U.; Assaraf, Y.G.; Harari, E.; Golenser, J. The effect of purified aminoaldehydes produced by polyamine oxidation on the development in vitro of Plasmodium falciparum in normal and glucose-6-phosphate-dehydrogenase-deficient erythrocytes. Biochem. J. 1986, 236, 97–101.
- Hegre, O.D.; Marshall, S.; Hickey, G.E. Spermidine cytotoxicity in vitro: Effect of serum and oxygen tension. In Vitro 1984, 20, 198–204.
- Sharmin, S.; Sakata, K.; Kashiwagi, K.; Ueda, S.; Iwasaki, S.; Shirahata, A.; Igarashi, K. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem. Biophys. Res. Commun. 2001, 282, 228–235.
- Bonneau, M.J.; Poulin, R. Spermine oxidation leads to necrosis with plasma membrane phosphatidylserine redistribution in mouse leukemia cells. Exp. Cell Res. 2000, 259, 23–34.
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314.
- Sakata, K.; Kashiwagi, K.; Sharmin, S.; Ueda, S.; Igarashi, K. Acrolein produced from polyamines as one of the uraemic toxins. Biochem. Soc. Trans. 2003, 31, 371–374.
- Tomitori, H.; Usui, T.; Saeki, N.; Ueda, S.; Kase, H.; Nishimura, K.; Kashiwagi, K.; Igarashi, K. Polyamine oxidase and acrolein as novel biochemical markers of cerebral stroke. Stroke 2005, 36, 2609–2613.
- Lambert, C.; McCue, J.; Portas, M.; Ouyang, Y.; Li, J.; Rosano, T.G.; Lazis, A.; Freed, B.M. Acrolein in cigarette smoke inhibits T-cell responses. J. Allergy Clin. Immunol. 2005, 116, 916–922.
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Aspects Med. 2012, 33, 209–290.
- Bein, K.; Birru, R.L.; Wells, H.; Larkin, T.P.; Cantrell, P.S.; Fagerburg, M.V.; Zeng, X.; Leikauf, G.D. Albumin protects lung cells against acrolein cytotoxicity and acrolein-adducted albumin increases heme oxygenase 1 transcripts. Chem. Res. Toxicol. 2020, 33, 1969–1979.
- Fisher, M. The ischemic penumbra: Identification, evolution and treatment concepts. Cerebrovasc. Dis. 2004, 17 (Suppl. 1), 1–6.
- Li, W.; Yuan, X.M.; Ivanova, S.; Tracey, K.J.; Eaton, J.W.; Brunk, U.T. 3-Aminopropanal, formed during cerebral ischaemia, is a potent lysosomotropic neurotoxin. Biochem. J. 2003, 371, 429–436.
- Yu, Z.; Li, W.; Hillman, J.; Brunk, U.T. Human neuroblastoma (SH-SY5Y) cells are highly sensitive to the lysosomotrophic aldehyde 3-aminopropanal. Brain Res. 2004, 1016, 163–169.
- Ivanova, S.; Botchkina, G.I.; Al-Abed, Y.; Meistrell, M., 3rd; Batliwalla, F.; Dubinsky, J.M.; Iadecola, C.; Wang, H.; Gregersen, P.K.; Eaton, J.W.; et al. Cerebral ischemia enhances polyamine oxidation: Identification of enzymatically formed 3-aminopropanal as an endogenous mediator of neuronal and glial cell death. J. Exp. Med. 1998, 188, 327–340.
- Ivanova, S.; Batliwalla, F.; Mocco, J.; Kiss, S.; Huang, J.; Mack, W.; Coon, A.; Eaton, J.W.; Al-Abed, Y.; Gregersen, P.K.; et al. Neuroprotection in cerebral ischemia by neutralization of 3-aminopropanal. Proc. Natl. Acad. Sci. USA 2002, 99, 5579–5584.
- Wood, P.L.; Khan, M.A.; Moskal, J.R.; Todd, K.C.; Tanay, V.A.M.I.; Baker, G. Aldehyde load in ischemia-reperfusion brain injury: Neuroprotection by neutralization of reactive aldehydes with phenelzine. Brain Res. 2006, 1122, 184–190.
- Kim, G.H.; Kellner, C.P.; Hickman, Z.L.; Zacharia, B.E.; Starke, R.M.; Hwang, B.Y.; Ducruet, A.F.; Fernandez, L.; Mayer, S.A.; Tracey, K.J.; et al. A phase I clinical trial of tiopronin, a putative neuroprotective agent, in aneurysmal subarachnoid hemorrhage. Neurosurgery 2010, 67, 182–185.
- Ironside, N.; Christophe, B.; Bruce, S.; Carpenter, A.M.; Robison, T.; Yoh, N.; Cremers, S.; Landry, D.; Frey, H.P.; Chen, C.J.; et al. A phase II randomized controlled trial of tiopronin for aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2020, 133, 351–359.
- Yu, Z.; Li, W.; Brunk, U.T. 3-Aminopropanal is a lysosomotropic aldehyde that causes oxidative stress and apoptosis by rupturing lysosomes. APMIS 2003, 111, 643–652.
- Kaufmann, A.M.; Krise, J.P. Niemann-Pick C1 functions in regulating lysosomal amine content. J. Biol. Chem. 2008, 283, 24584–24593.
- Wood, P.L.; Khan, M.A.; Moskal, J.R. The concept of “aldehyde load” in neurodegenerative mechanisms: Cytotoxicity of the polyamine degradation products hydrogen peroxide, acrolein, 3-aminopropanal, 3-acetamidopropanal and 4-aminobutanal in a retinal ganglion cell line. Brain Res. 2007, 1145, 150–156.
- Calcabrini, A.; Arancia, G.; Marra, M.; Crateri, P.; Befani, O.; Martone, A.; Agostinelli, E. Enzymatic oxidation products of spermine induce greater cytotoxic effects on human multidrug- resistant colon carcinoma cells (LoVo) than on their wild type counterparts. Int. J. Cancer 2002, 99, 43–52.
- Agostinelli, E.; Tempera, G.; Viceconte, N.; Saccoccio, S.; Battaglia, V.; Grancara, S.; Toninello, A.; Stevanato, R. Potential anticancer application of polyamine oxidation products formed by amine oxidase: A new therapeutic approach. Amino Acids 2010, 38, 353–368.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
466
Revisions:
2 times
(View History)
Update Date:
08 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




