You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hussein Sakr | -- | 2107 | 2023-11-06 01:58:47 | | | |
| 2 | Catherine Yang | Meta information modification | 2107 | 2023-11-06 02:04:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sakr, H.F.; Sirasanagandla, S.R.; Das, S.; Bima, A.I.; Elsamanoudy, A.Z. Diet-Induced Insulin Resistance. Encyclopedia. Available online: https://encyclopedia.pub/entry/51165 (accessed on 28 December 2025).
Sakr HF, Sirasanagandla SR, Das S, Bima AI, Elsamanoudy AZ. Diet-Induced Insulin Resistance. Encyclopedia. Available at: https://encyclopedia.pub/entry/51165. Accessed December 28, 2025.
Sakr, Hussein F., Srinivasa Rao Sirasanagandla, Srijit Das, Abdulhadi I. Bima, Ayman Z. Elsamanoudy. "Diet-Induced Insulin Resistance" Encyclopedia, https://encyclopedia.pub/entry/51165 (accessed December 28, 2025).
Sakr, H.F., Sirasanagandla, S.R., Das, S., Bima, A.I., & Elsamanoudy, A.Z. (2023, November 06). Diet-Induced Insulin Resistance. In Encyclopedia. https://encyclopedia.pub/entry/51165
Sakr, Hussein F., et al. "Diet-Induced Insulin Resistance." Encyclopedia. Web. 06 November, 2023.
Copy Citation
Insulin resistance (IR) is defined as the decreased ability of insulin to insert the glucose transporter (glut4) on the cell membrane facilitating glucose entry into body cells. IR in the hepatocytes is associated with increased de novo lipogenesis and gluconeogenesis, leading to hypertriglyceridemia and hyperglycemia.
hypertension
insulin
resistance
diet
mechanism
1. Fructose
Along with the increase in daily calorie consumption that has occurred over the past three decades, the prevalence of obesity, diabetes, and IR has grown considerably [1]. Unmatched by physical activity or energy demand, the constant supply of energy from dietary carbohydrates, lipids, and protein fuels may lead to a backlog of mitochondrial oxidation products, which is a mechanism linked to increasing mitochondrial malfunction and IR [2].
High-fructose corn syrup (HFCS), which contains 42% or 55% fructose and glucose respectively, and sucrose, which includes 50% fructose and 50% glucose, are the two most common forms of fructose consumption [3]. The secular increases in fructose intake, in contrast to those of trans fats or ethanol, have coincided with the growth of obesity and metabolic syndrome (MetS), particularly in children. Before World War II, Americans consumed 24 g of fructose per day; by the middle of the 1970s, the amount had increased to 37 g; and by the middle of the 1990s, it had reached 55 g (representing a gradual increase from 5% to 7% to 10% of total calories) [4]. In fact, according to current National Health and Nutrition Examination Survey (NHANES) statistics, almost 15% of US citizens obtain 25% of their daily calories from added sugars [5]. Interestingly, excessive fructose ingestion has been linked to weight gain, visceral adiposity, dyslipidemia, IR/glucose intolerance, and NAFLD [6]. This is especially true of drinks with added sugar. Independent of adenosine triphosphate hydrolysis and salt absorption, fructose is delivered into the enterocyte in the small intestine via the fructose transporter Glut5 [7]. Nevertheless, most of the fructose that is consumed is absorbed into the portal circulation and transported to the liver. There, fructokinase quickly converts fructose to fructose-1-phosphate (F1P), a mechanism that is insulin-independent and sidesteps the glycolytic pathway’s negative feedback control of phosphofructokinase [7]. Thus, in an uncontrolled manner, the mitochondria are directly supplied with lipogenic substrates (such as glyceraldehyde-3-phosphate and acetyl-CoA) produced by fructose metabolism. Due to the overproduction of mitochondrial precursors, hepatic DNL can overpower apoB and the lipid export system, resulting in intrahepatic lipid accumulation and steatosis [8]. Hepatic DNL also restricts further fatty acid oxidation in the liver through excess production of malonyl-CoA, which reduces the entry of fatty acids into the mitochondria. F1P also activates SREBP-1c via peroxisome proliferator-activated receptor-g coactivator-1 independently of insulin, which activates the genes involved in DNL [9]. Furthermore, JNK-1, a liver enzyme that links hepatic metabolism and inflammation, is stimulated when F1P activates dual specificity mitogen-activated protein kinase [10]. Additionally, PKC is activated by the lipogenic intermediate diacylglycerol, which is created during the liver’s processing of fructose. Both of these occurrences promote IRS-1’s serine phosphorylation, which results in hepatic IR [11].
When fructose enters hepatocytes, fructokinase quickly phosphorylates it into fructose-1-phosphate. As part of this process, ATP contributes phosphate, creating ADP, which is then converted into uric acid [12]. A fructose-mediated rise in AMP deaminase facilitates this process [13]. Elevated uric acid levels may cause oxidative stress in hepatocyte mitochondria, enhancing the generation of ROS and eventually leading to mitochondrial dysfunction [14]. Uric acid stimulates hepatic gluconeogenesis through the activation of AMPD2 and AMPK blockade [15]. Uric acid accumulation leads to insulin resistance [15][16], decreased FFA oxidation, endoplasmic reticulum stress, and decreased NO generation, leading to elevated blood pressure [17]. The involvement of fructose in the pathogenesis of IR is depicted in Figure 1.
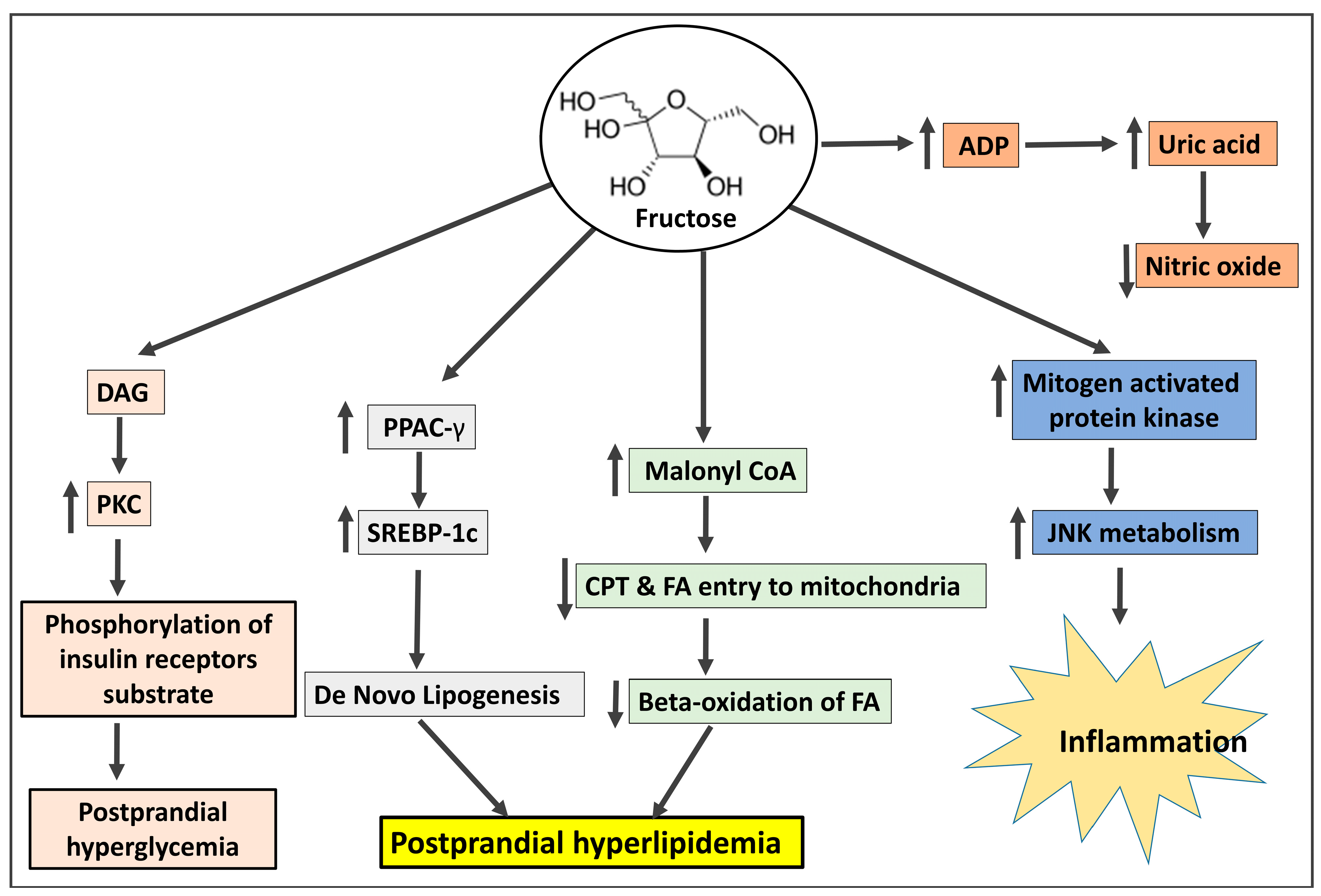
Figure 1. The mechanism by which fructose shares in the pathogenesis of insulin resistance. DAG: diacylglycerol; PKC: protein kinase C; PPAC-γ: peroxisome proliferator-activated receptor γ; SREBP-1c: sterol regulatory element-binding protein 1c; CPT: carnitine-palmitoyl-CoA transferase; FA: fatty acids; ADP: Adenosine di-phosphate; JNK: Jun N-terminal kinase.
2. Omega-6 Fatty Acids
Vegetable oils like canola, sunflower, corn, soy, and safflower oils are rich sources of omega-6 fatty acids, especially linoleic acid [18][19]. Since 1900, vegetable oils derived from seeds and flowers have been extensively used to replace saturated fats such as butter, tallow, and lard. The use of these oils gives processed food more stability. Linoleic acid, the primary omega-6 polyunsaturated fat found in vegetable oils like seed oils [19], increased by more than a factor of two as a result, and it currently accounts for 8% to 10% of the total energy consumed in the West [18]. Conjugated linoleic acid, which is present in pastured animal diets, should not be mistaken for the omega-6 fatty acid linoleic acid. In addition, because linoleic acid has a half-life of approximately two years in adipose tissue, the content of linoleic acid in adipose tissue is a trustworthy indicator of ingestion [20]. Oxidative stress, damage to the tissues, and mitochondrial dysfunction resulting from excess linoleic acid are responsible for various cardiovascular diseases, including Alzheimer’s disease, cancer, dementia, obesity, and diabetes [21]. Excessive consumption of linoleic acid in recent years has led to a reduction in the levels of omega-3 fatty acid intake, with an increasing ratio between omega 6 and omega 3 from 4:1 to 25:1 [22]. Significantly, as alpha-linolenic acid interacts with linoleic acid for conversion to longer-chain polyunsaturated fats, greater ingestion of omega-6 polyunsaturated fat linoleic acid can decrease omega-3 in the body [23]. Since it has long been known that individuals with CAD had lower levels of linoleic acid relative to total fatty acids in lipids, this finding has been used to support the theory that linoleic acid deficiency may be a risk factor for heart disease [24]. However, linoleic acid levels in both adipose tissue and platelets are favorably correlated with coronary artery disease (CAD), but long-chain omega-3 levels in platelets (eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) are negatively correlated with CAD [25]. Linoleic acid produces mitochondrial toxicity and catastrophic cardiolipin modifications [26], which are predisposed to exaggerate oxidative stress and overproduction of reactive oxygen species (ROS). ROS consume natural antioxidants and also produce lipid peroxidation of the cell membrane and DNA damage. Oxidative stress predisposes to IR, obesity, T2DM, heart failure, and cancer. A schematic diagram (Figure 2) shows how linoleic acid may cause inflammation and oxidative stress conditions.
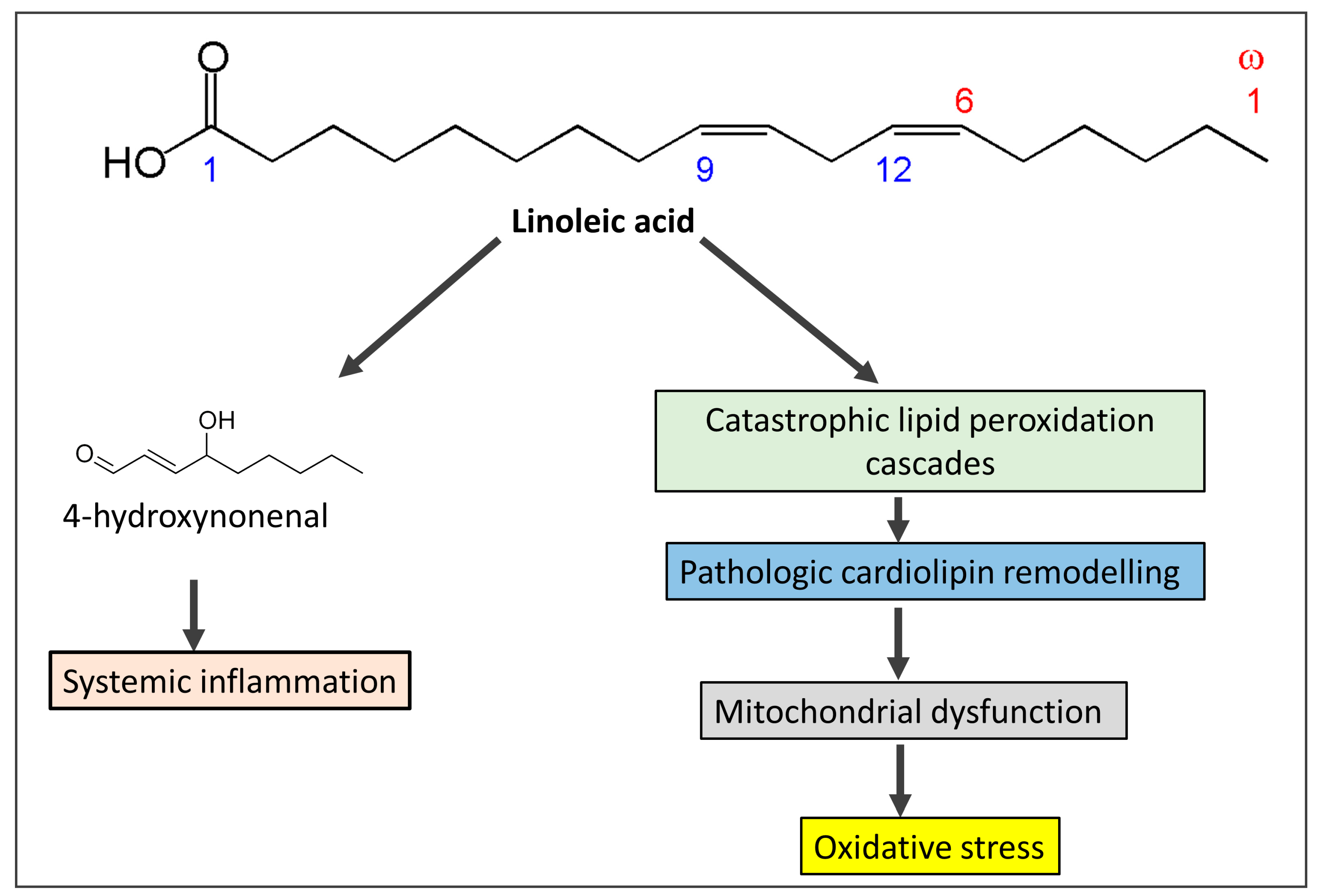
Figure 2. The mechanism by which omega-6 fatty acids derived from seed oils produces systemic inflammation and oxidative stress and share in the pathogenesis of insulin resistance.
3. Grains
Beginning as far back as ten thousand years ago, the use of wheat and other grains was linked to increased infections, bone illnesses such as osteoporosis, diabetes, metabolic syndrome, increased newborn mortality, and a shortening of life span [27]. Grains affect human health in different ways according to contents including amylopectin A and gluten protein.
While gluten is sometimes held responsible for all of the issues associated with wheat, in reality, gliadin, a smaller protein found inside gluten, is to blame for a number of the negative effects of contemporary wheat on human health. Gliadin proteins come in over 200 different varieties, many of which are weakly and imperfectly digested by humans and are therefore compatible with being found in grass seeds. The most potent cause of celiac disease, which is intestinal damage of the small intestine caused by wheat, rye, and barley, is a gene for a kind of gliadin called Glia-9 [28]. Only a few strains of wheat from the early 20th century included the Glia-9 gene, but it is now found in the majority of contemporary cultivars [29]. Many of the proteins found in grains are either not degraded at all or are broken down into minute fragments or peptides (short sequences of amino acids) rather than single amino acids. New gliadin variants are partially digested to produce small peptides that then enter the bloodstream and bind to opiate receptors in the neural network. These receptors are stimulated by heroin and morphine and have effects such as “mind fog”, paranoia, anxiety, the mania of bipolar disorder, depression, and appetite stimulation [30]. People who battle an insatiable hunger, such as those with bulimia and binge-eating disorders, are examples of the final impact of appetite stimulation, which is frequent and can be overpowering. These peptides are known as exorphins, which are exogenous morphine-like substances. However, gliadin-derived peptides do not provide a “high”, but rather an increase in appetite and caloric intake, with studies showing steady increases of 400 calories per day, largely from carbohydrates and grains [31]. Amylopectin is a complex carbohydrate composed of 25% amylose, a linear chain of glucose units, and 75% amylose, a chain of branching glucose units. Both amylopectin and amylose can be broken down by pancreatic and salivary amylase [32]. While part of the amylose is not completely digested, some of it goes to the colon undigested when the amylase enzymes hydrolyze amylopectin into glucose monomers [33]. Because it is most effectively digested, the complex carbohydrate amylopectin is immediately converted to glucose and transported into the circulation, resulting in an abrupt rise in blood glucose in response to the consumption of grains. According to a previous study, non-diabetic people with hyperglycemia are considerably more likely to experience brain shrinkage. IR is induced by hyperglycemia, which also causes hyperinsulinemia [34].
Gluten, a large protein made up of gliadin and glutenins, gives wheat dough its distinctive stretchiness. Since the long-branching glutenin proteins affect baking properties, gluten has undergone genetic manipulation [35]. Therefore, using unexpected breeding techniques, geneticists have crossed different wheat strains, mated wheat with non-wheat grasses to introduce novel genes, and employed chemicals and radiation to cause mutations in the glutenin component of gluten. Up to 14 distinct glutenin proteins that have never been consumed by humans are produced when two different wheat plants are hybridized [36]. As a result, novel glutenin protein genes not present in previous strains of wheat have been discovered in current strains of wheat; none of these genes, naturally, have been evaluated for acceptability for human consumption [37].
Wheat germ agglutinin (WGA), a protein found in wheat (as well as barley, rye, and rice) that defends the plant against molds and insects, has undergone structural alterations as a result of the genetic modifications imposed on wheat [38]. Due to agribusiness manipulation, the structure of modern wheat’s WGA varies from that of ancient wheat strains [39]. Ironically, geneticists have attempted to raise the amount of WGA in grains to boost pest resistance, which has increased the toxicity of such grains for human gastrointestinal systems. No matter how it is prepared (i.e., cooking, baking, sprouting the seeds, sourdough fermentation), people consume intact WGA, which belongs to the lectin protein family and is extraordinarily tough, entirely indigestible, and resistant to any breakdown by the body. WGA lectins that are not digested are hazardous because they cause harm without the necessity for hereditary predisposition [40]. Even in the absence of gliadin/gluten, WGA alone is sufficient to cause intestinal damage similar to celiac disease by altering microvilli, the absorptive “hairs” of intestinal cells [41].
Grain breeding efforts over the past 50 years have favored strains with higher phytate contents because phytic acid, or phytates, like WGA, protect the plant from pests. Additionally, since phytate content and fiber content are inversely correlated, recommendations to increase dietary fiber by consuming more “healthy whole grains” also result in a rise in phytate content. For instance, modern wheat, corn, and millet have the equivalent phytate levels of 800 mg/100 gm (312 ounces) of flour [42]. Iron, zinc, calcium, and magnesium absorption are all stopped by phytates at doses as low as 50 mg [43]. While passionate grain-consuming cultures can consume up to 5000 mg of phytates/per day, amounts that are related to nutritional deficits like zinc deficiency, osteoporosis, and iron deficiency anemia, children normally consume 600 to 1900 mg of phytates per day [41].
One of the most prevalent allergens is a group of proteins known as alpha-amylase inhibitors, which can lead to hives, asthma, cramps, diarrhea, and eczema [44]. Modern alpha-amylase inhibitors differ from conventional strains structurally by 10%, which can correspond to up to a dozen amino acid changes. Just a few amino acids can make the difference between the absence of an allergic reaction and a severe allergic reaction, even anaphylaxis. Baker’s asthma is a disorder that often strikes those who work in the baking business [44]. Additionally, there is a strange syndrome known as wheat-dependent exercise-induced anaphylaxis, which is a severe and sometimes fatal allergy brought on by physical activity after consuming wheat. Gliadin protein allergy is the cause of both diseases [45]. The schematic diagram in Figure 3 depicts the mechanism by which grains are involved with IR.
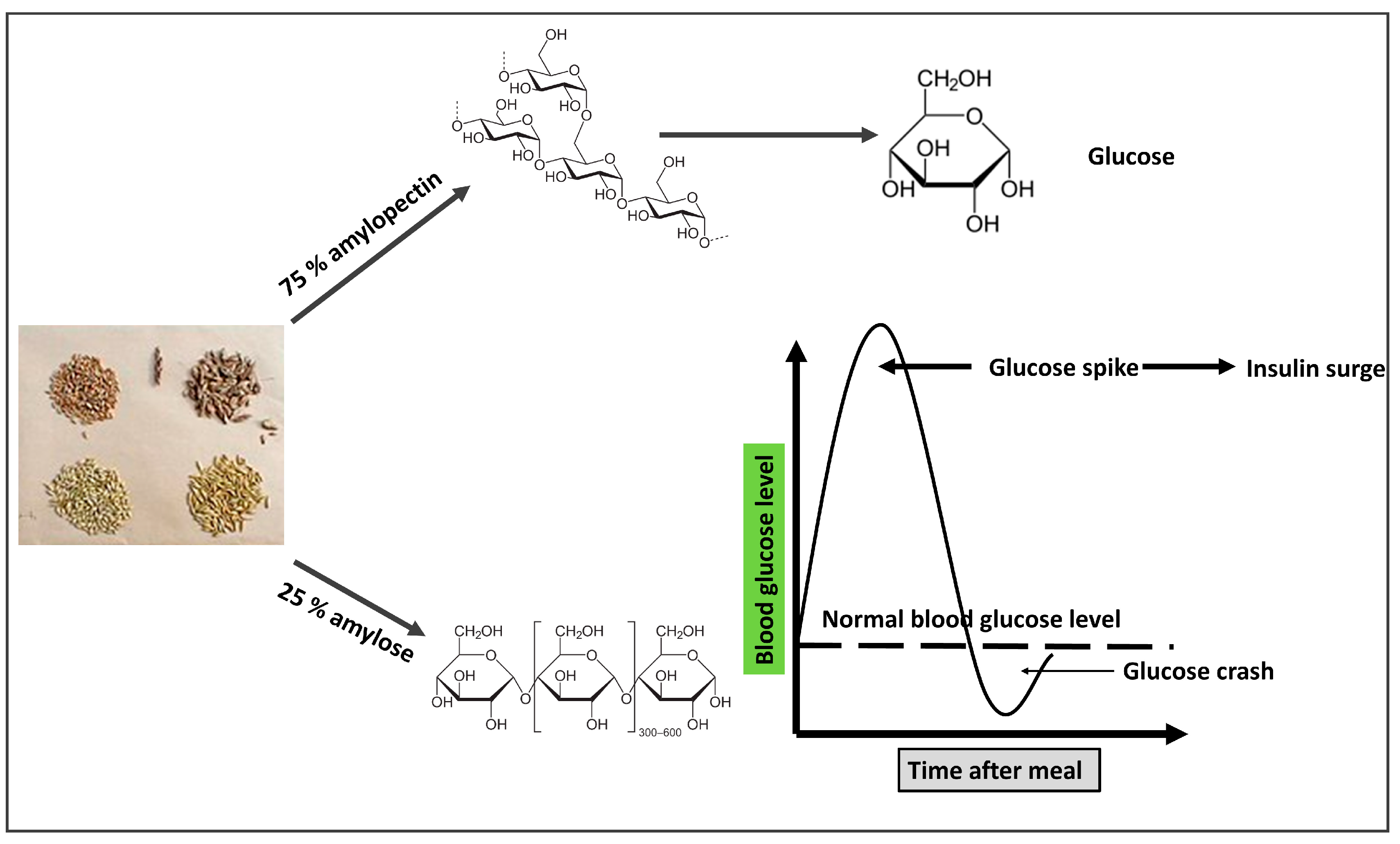
Figure 3. The mechanism by which grains seeds (clockwise from top-left: wheat, spelt, oat, barley) are involved in the pathogenesis of insulin resistance. The branched structure of amylopectin allows more glucose molecules to be hydrolyzed to increase its rate of digestion and increased blood glucose. A straight chain of amylose decreases its digestion rate, so most of it is directed to the large intestine.
References
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in dietary carbohydrate, protein, and fat intake and diet quality among us adults, 1999–2016. JAMA 2019, 322, 1178–1187.
- Bonnard, C.; Durand, A.; Peyrol, S.; Chanseaume, E.; Chauvin, M.A.; Morio, B.; Vidal, H.; Rieusset, J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Investig. 2008, 118, 789–800.
- Serna-Saldivar, S.O. Maize: Foods from Maize. Ref. Modul. Food Sci. 2016.
- Pereira, R.M.; Botezelli, J.D.; da Cruz Rodrigues, K.C.; Mekary, R.A.; Cintra, D.E.; Pauli, J.R.; da Silva, A.S.R.; Ropelle, E.R.; de Moura, L.P. Fructose consumption in the development of obesity and the effects of different protocols of physical exercise on the hepatic metabolism. Nutrients 2017, 9, 405.
- Marriott, B.P.; Olsho, L.; Hadden, L.; Connor, P. Intake of added sugars and selected nutrients in the united states, national health and nutrition examination survey (nhanes) 2003–2006. Crit. Rev. Food Sci. Nutr. 2010, 50, 228–258.
- Bremer, A.A.; Mietus-Snyder, M.; Lustig, R.H. Toward a unifying hypothesis of metabolic syndrome. Pediatrics 2012, 129, 557–570.
- Tappy, L.; Lê, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46.
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.M.; Lustig, R.H. The role of fructose in the pathogenesis of nafld and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264.
- Faeh, D.; Minehira, K.; Schwarz, J.M.; Periasamy, R.; Park, S.; Tappy, L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005, 54, 1907–1913.
- Wei, Y.; Wang, D.; Pagliassotti, M.J. Fructose selectively modulates c-jun n-terminal kinase activity and insulin signaling in rat primary hepatocytes. J. Nutr. 2005, 135, 1642–1646.
- Wei, Y.; Wang, D.; Topczewski, F.; Pagliassotti, M.J. Fructose-mediated stress signaling in the liver: Implications for hepatic insulin resistance. J. Nutr. Biochem. 2007, 18, 1–9.
- Hallfrisch, J. Metabolic effects of dietary fructose. Faseb J. 1990, 4, 2652–2660.
- Smith, C.M.; Rovamo, L.M.; Raivio, K.O. Fructose-induced adenine nucleotide catabolism in isolated rat hepatocytes. Can. J. Biochem. 1977, 55, 1237–1240.
- Yang, Y.; Zhou, Y.; Cheng, S.; Sun, J.L.; Yao, H.; Ma, L. Effect of uric acid on mitochondrial function and oxidative stress in hepatocytes. Genet. Mol. Res. 2016, 15, 15028644.
- Cicerchi, C.; Li, N.; Kratzer, J.; Garcia, G.; Roncal-Jimenez, C.A.; Tanabe, K.; Hunter, B.; Rivard, C.J.; Sautin, Y.Y.; Gaucher, E.A.; et al. Uric acid-dependent inhibition of amp kinase induces hepatic glucose production in diabetes and starvation: Evolutionary implications of the uricase loss in hominids. Faseb J. 2014, 28, 3339–3350.
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322.
- Jia, G.; Aroor, A.R.; Whaley-Connell, A.T.; Sowers, J.R. Fructose and uric acid: Is there a role in endothelial function? Curr. Hypertens. Rep. 2014, 16, 014–0434.
- DiNicolantonio, J.J.; O’Keefe, J.H. Omega-6 vegetable oils as a driver of coronary heart disease: The oxidized linoleic acid hypothesis. Open Heart 2018, 5, e000898.
- Fritsche, K.L. Linoleic acid, vegetable oils & inflammation. Mo. Med. 2014, 111, 41–43.
- Aglago, E.K.; Biessy, C.; Torres-Mejía, G.; Angeles-Llerenas, A.; Gunter, M.J.; Romieu, I.; Chajès, V. Association between serum phospholipid fatty acid levels and adiposity in Mexican women. J. Lipid Res. 2017, 58, 1462–1470.
- Mercola, J.; D’Adamo, C.R. Linoleic Acid: A Narrative Review of the Effects of Increased Intake in the Standard American Diet and Associations with Chronic Disease. Nutrients 2023, 15, 3129.
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128.
- Gibson, R.A.; Muhlhausler, B.; Makrides, M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (lcpufas), with a focus on pregnancy, lactation and the first 2 years of life. Matern. Child. Nutr. 2011, 2, 17–26.
- Schwertner, H.A.; Mosser, E.L. Comparison of lipid fatty acids on a concentration basis vs. weight percentage basis in patients with and without coronary artery disease or diabetes. Clin. Chem. 1993, 39, 659–663.
- Hodgson, J.M.; Wahlqvist, M.L.; Boxall, J.A.; Balazs, N.D. Can linoleic acid contribute to coronary artery disease? Am. J. Clin. Nutr. 1993, 58, 228–234.
- El-Hafidi, M.; Correa, F.; Zazueta, C. Mitochondrial dysfunction in metabolic and cardiovascular diseases associated with cardiolipin remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165744.
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529.
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023.
- Sehgel, N.L.; Sun, Z.; Hong, Z.; Hunter, W.C.; Hill, M.A.; Vatner, D.E.; Vatner, S.F.; Meininger, G.A. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension 2015, 65, 370–377.
- Wirth, A.; Wang, S.; Takefuji, M.; Tang, C.; Althoff, T.F.; Schweda, F.; Wettschureck, N.; Offermanns, S. Age-dependent blood pressure elevation is due to increased vascular smooth muscle tone mediated by g-protein signalling. Cardiovasc. Res. 2016, 109, 131–140.
- Drewnowski, A.; Krahn, D.D.; Demitrack, M.A.; Nairn, K.; Gosnell, B.A. Naloxone, an opiate blocker, reduces the consumption of sweet high-fat foods in obese and lean female binge eaters. Am. J. Clin. Nutr. 1995, 61, 1206–1212.
- Winkler, S.; Kaplan, D.L. Biosynthesized Materials: Properties and Processing. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 609–615.
- Farrell, J.J. Digestion and Absorption of Nutrients and Vitamins. In Sleisenger and Fordtran’s Gastrointestinal and Liver Disease; Elsevier: Amsterdam, The Netherlands, 2010.
- Roriz-Filho, J.S.; Sá-Roriz, T.M.; Rosset, I.; Camozzato, A.L.; Santos, A.C.; Chaves, M.L.; Moriguti, J.C.; Roriz-Cruz, M. (Pre)diabetes, brain aging, and cognition. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2009, 1792, 432–443.
- Urade, R.; Sato, N.; Sugiyama, M. Gliadins from wheat grain: An overview, from primary structure to nanostructures of aggregates. Biophys. Rev. 2018, 10, 435–443.
- Gao, X.; Liu, S.W.; Sun, Q.; Xia, G.M. High frequency of hmw-gs sequence variation through somatic hybridization between agropyron elongatum and common wheat. Planta 2010, 231, 245–250.
- Zhao, X.L.; Xia, X.C.; He, Z.H.; Gale, K.R.; Lei, Z.S.; Appels, R.; Ma, W. Characterization of three low-molecular-weight glu-d3 subunit genes in common wheat. Theor. Appl. Genet. 2006, 113, 1247–1259.
- Monsigny, M.; Roche, A.C.; Sene, C.; Maget-Dana, R.; Delmotte, F. Sugar-lectin interactions: How does wheat-germ agglutinin bind sialoglycoconjugates? Eur. J. Biochem. 1980, 104, 147–153.
- Peumans, W.J.; Stinissen, H.M.; Carlier, A.R. Isolation and partial characterization of wheat-germ-agglutinin-like lectins from rye (Secale cereale) and barley (Hordeum vulgare) embryos. Biochem. J. 1982, 203, 239–243.
- Hamid, R.; Masood, A. Dietary lectins as disease causing toxicants. Pak. J. Nutr. 2009, 8, 293–303.
- Lorenzsonn, V.; Olsen, W.A. In Vivo responses of rat intestinal epithelium to intraluminal dietary lectins. Gastroenterology 1982, 82, 838–848.
- Longin, C.F.H.; Afzal, M.; Pfannstiel, J.; Bertsche, U.; Melzer, T.; Ruf, A.; Heger, C.; Pfaff, T.; Schollenberger, M.; Rodehutscord, M. Mineral and phytic acid content as well as phytase activity in flours and breads made from different wheat species. Int. J. Mol. Sci. 2023, 24, 2770.
- Al Hasan, S.M.; Hassan, M.; Saha, S.; Islam, M.; Billah, M.; Islam, S. Dietary phytate intake inhibits the bioavailability of iron and calcium in the diets of pregnant women in rural bangladesh: A cross-sectional study. BMC Nutr. 2016, 2, 24.
- James, J.M.; Sixbey, J.P.; Helm, R.M.; Bannon, G.A.; Burks, A.W. Wheat alpha-amylase inhibitor: A second route of allergic sensitization. J. Allergy Clin. Immunol. 1997, 99, 239–244.
- Pastorello, E.A.; Farioli, L.; Conti, A.; Pravettoni, V.; Bonomi, S.; Iametti, S.; Fortunato, D.; Scibilia, J.; Bindslev-Jensen, C.; Ballmer-Weber, B.; et al. Wheat ige-mediated food allergy in european patients: Alpha-amylase inhibitors, lipid transfer proteins and low-molecular-weight glutenins. Allergenic molecules recognized by double-blind, placebo-controlled food challenge. Int. Arch. Allergy Immunol. 2007, 144, 10–22.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
729
Revisions:
2 times
(View History)
Update Date:
06 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




