
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alison Ingraldi | -- | 6112 | 2023-11-03 19:30:22 | | | |
| 2 | Catherine Yang | Meta information modification | 6112 | 2023-11-14 01:53:16 | | | | |
| 3 | Aaron J. Tabor | Meta information modification | 6112 | 2023-11-15 17:48:58 | | |
Video Upload Options
Biological tissues from various anatomical sources have been utilized for tissue transplantation and have developed into an important source of extracellular scaffolding material for regenerative medicine applications. Tissue scaffolds ideally integrate with host tissue and provide a homeostatic environment for cellular infiltration, growth, differentiation, and tissue resolution. The human amniotic membrane is considered an important source of scaffolding material due to its 3D structural architecture and function and as a source of growth factors and cytokines. This tissue source has been widely studied and used in various areas of tissue repair including intraoral reconstruction, corneal repair, tendon repair, microvascular reconstruction, nerve procedures, burns, and chronic wound treatment.
1. Introduction
2. Clinical Applications
2.1. Introduction
2.2. Periodontal and Oral Surgery
Differences between Membrane Types in Periodontal Application
2.3. Ophthalmology and Ocular Surgery
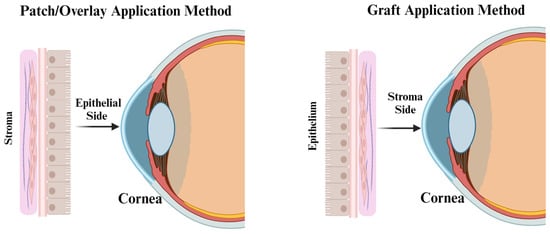
Differences between Membrane Types for Ophthalmic Applications
2.4. Chronic Wounds
Difference between Membrane Types for Chronic Wound Applications
2.5. Plastic Surgery
Differences between Membrane Types for Plastic Applications
References
- Hu, Z.; Luo, Y.; Ni, R.; Hu, Y.; Yang, F.; Du, T.; Zhu, Y. Biological importance of human amniotic membrane in tissue engineer-ing and regenerative medicine. Mater. Today Bio. 2023, 22, 100790.
- Jay, R.M.; Huish, J.P.; Wray, J.H. Amniotic membrane in clinical medicine: History, current status, and future use. In Extracellu-lar Matrix-Derived Implants in Clinical Medicine, 1st ed.; Mooradian, D.L., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 151–176.
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of human amni-otic membrane for tissue engineering. Membranes 2021, 11, 387.
- Tehrani, F.D.; Firouzeh, A.; Shabani, I.; Shabani, A. A review on modifications of amniotic membrane for biomedical applica-tions. Front. Bioeng. Biotechnol. 2021, 8, 606982.
- Elkhenany, H.; El-Derby, A.; Elkodous, M.A.; Salah, R.A.; Lotfy, A.; El-Badri, N. Applications of the amniotic membrane in tissue engineering and regeneration: The hundred-year challenge. Stem Cell Res. Ther. 2022, 13, 8.
- Munoz-Torres, J.R.; Martínez-González, S.B.; Lozano-Luján, A.D.; Martinez-Vázquez, M.C.; Velasco-Elizondo, P.; Garza-Veloz, I.; Martinez-Fierro, M.L. Biological properties and surgical applications of the human amniotic membrane. Front. Bioeng. Biotechnol. 2023, 10, 1067480.
- Davis, J.S. Skin grafting at the Johns Hopkins Hospital. Ann. Surg. 1909, 50, 542–549.
- Stern, M. The grafting of preserved amniotic membrane to burned and ulcerated surfaces, substituting skin grafts: A preliminary report. JAMA 1913, 60, 973–974.
- Sabella, N. Use of the fetal membranes in skin grafting. Med. Rec. 1913, 83, 478.
- Troensegaard-Hansen, E. Amniotic grafts in chronic skin ulceration. Lancet 1950, 1, 859–860.
- de Rötth, A. Plastic Repair of Conjunctival Defects with Fetal Membranes. Arch. Ophthalmol. 1940, 23, 522–525.
- Sorsby, A.; Symons, H.M. Amniotic membrane grafts in caustic burns of the eye: (Burns of the second degree). Br. J. Ophthalmol. 1946, 30, 337–345.
- John, T. Human amniotic membrane transplantation: Past, present, and future. Ophthalmol. Clin. N. Am. 2003, 16, 43–65.
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.J.; Botelho, M.F. Amniotic membrane: From structure and functions to clinical applications. Cell Tissue Res. 2012, 349, 447–458.
- Dua, H.S.; Gomes, J.A.P.; King, A.J.; Maharajan, V.S. The amniotic membrane of ophthalmology. Surv. Ophthalmol. 2004, 49, 51–77.
- Eichberg, D.G.; Ali, S.C.; Buttrick, S.S.; Komotar, R.J. The use of dehydrated amniotic membrane allograft for the augmenta-tion of dural repair in craniotomies. Cureus 2018, 10, e2586.
- Halim, A.S.; Ming, L.A.; Dorai, A.A.; Sulaiman, W.A.W. Use of amnion in plastic surgery. In Human Amniotic Membrane: Basic Science and Clinical Application, 1st ed.; Hilmy, N., Yusof, N., Nather, A., Eds.; World Scientific: Singapore, 2017; pp. 227–239.
- Mirzayan, R.; Russo, F.; Yang, S.-J.T.; Lowe, N.; Shean, C.J.; Harness, N.G. Human amniotic membrane wrapping of the ulnar nerve during cubital tunnel surgery reduces recurrence of symptoms. Arch. Bone Jt. Surg. 2022, 10, 969–975.
- Fairbairn, N.G.; Randolph, M.A.; Redmond, R.W. The clinical applications of human amnion in plastic surgery. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 662–675.
- Malhotra, C.; Jain, A.K. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J. Transpl. 2014, 4, 111–121.
- Tighe, S.; Mead, O.G.; Lee, A.; Tseng, S.C.G. Basic science review of birth tissue uses in ophthalmology. Taiwan. J. Ophthalmol. 2020, 10, 3–12.
- Burman, S.; Tejwani, S.; Vemuganti, G.K.; Gopinathan, U.; Sangwan, V.S. Ophthalmic applications of preserved human am-niotic membrane: A review of current indications. Cell Tissue Bank. 2004, 5, 161–175.
- Kesting, M.R.; Wolff, K.D.; Nobis, C.P.; Rohleder, N.H. Amniotic membrane in oral and maxillofacial surgery. Oral. Maxillofac. Surg. 2014, 18, 153–164.
- Gulameabasse, S.; Gindraux, F.; Catros, S.; Fricain, J.C.; Fénelon, M. Chorion and amnion/chorion membranes in oral and periodontal surgery: A systematic review. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 11216–11229.
- Regulski, M. Utilization of a viable human amnion membrane allograft in elderly patients with chronic lower extremity wounds of various etiologies. Wounds 2018, 30, E36–E40.
- Castellanos, G.; Bernabé-García, A.; Moraleda, J.M.; Nicolás, F.J. Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta 2017, 59, 146–153.
- Laurent, I.; Astère, M.; Wang, K.R.; Cheng, Q.F.; Li, Q.F. Efficacy and time sensitivity of amniotic membrane treatment in patients with diabetic foot ulcers: A systematic review and meta-analysis. Diabetes Ther. 2017, 8, 967–979.
- Mermet, I.; Pottier, N.; Sainthillier, J.M.; Malugani, C.; Cairey-Remonnay, S.; Maddens, S.; Riethmuller, D.; Tiberghien, P.; Humbert, P.; Aubin, F. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair. Regen. 2007, 15, 459–464.
- Sawhney, C.P. Amniotic membrane as a biological dressing in the management of burns. Burns 1989, 15, 339–342.
- Mohammadi, A.A.; Jafari, S.M.S.; Kiasat, M.; Tavakkolian, A.R.; Imani, M.T.; Ayaz, M.; Tolide-ie, H.R. Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns 2013, 39, 349–353.
- Lyons, A.B.; Chipps, L.K.; Moy, R.L.; Herrmann, J.L. Dehydrated human amnion/chorion membrane allograft as an aid for wound healing in patients with full-thickness scalp defects after Mohs micrographic surgery. JAAD Case Rep. 2018, 4, 688–691.
- Seaton, K.; Mullens, D.; Barr, J.; Hull, E.; Averitte, R. Use of amniotic tissue-derived allografts post-mohs micrographic sur-gery: A preliminary study assessing wound closure rate. Wounds 2021, 33, 185–191.
- Chao, Y.C.; Humphreys, S.; Penfield, W. A new method of preventing adhesions: The use of amnioplastin after craniotomy. Br. Med. J. 1940, 1, 517–538.1.
- Patel, V.R.; Samavedi, S.; Bates, A.S.; Kumar, A.; Coelho, R.; Rocco, B.; Palmer, K. Dehydrated human amnion/chorion membrane allograft nerve wrap around the prostatic neurovascular bundle accelerates early return to continence and poten-cy following robot-assisted radical prostatectomy: Propensity score-matched analysis. Eur. Urol. 2015, 67, 977–980.
- Liu, C.; Bai, J.; Yu, K.; Liu, G.; Tian, S.; Tian, D. Biological amnion prevents flexor tendon adhesion in zone II: A controlled, multicenter clinical trial. Biomed. Res. Int. 2019, 2019, 2354325.
- Roy, A.; Mantay, M.; Brannan, C.; Griffiths, S. Placental tissues as biomaterials in regenerative medicine. BioMed Res. Int. 2022, 2022, 6751456.
- Gruss, J.S.; Jirsch, D.W. Human amniotic membrane: A versatile wound dressing. Can. Med. Assoc. J. 1978, 118, 1237–1246.
- Mohammadi, A.A.; Riazi, H.; Hasheminasab, M.J.; Sabet, B.; Mohammadi, M.K.; Abbasi, S.; Amini, M. Amniotic membrane dressing vs. conventional topical antibiotic dressing in hospitalized burn patients. Iran. Red. Crescent Med. J. 2009, 11, 66–70.
- Gupta, A.; Kedige, S.D.; Jain, K. Amnion and chorion membranes: Potential stem cell reservoir with wide applications in per-iodontics. Int. J. Biomater. 2015, 2015, 274082.
- Mohan, R.; Bajaj, A.; Gundappa, M. Human amnion membrane: Potential applications in oral and periodontal field. J. Int. Soc. Prev. Community Dent. 2017, 7, 15–21.
- Odet, S.; Louvrier, A.; Meyer, C.; Nicolas, F.J.; Hofman, N.; Chatelain, B.; Mauprivez, C.; Laurence, S.; Kerdjoudj, H.; Zwetyenga, N.; et al. Surgical appli-cation of human amniotic membrane and amnion-chorion membrane in the oral cavity and efficacy evaluation: Corollary with ophthalmological and wound healing experiences. Front. Bioeng. Biotechnol. 2021, 9, 685128.
- Fénelon, M.; Catros, S.; Fricain, J.C. What is the benefit of using amniotic membrane in oral surgery? A comprehensive re-view of clinical studies. Clin. Oral. Investig. 2018, 22, 1881–1891.
- McQuilling, J.P.; Kammer, M.; Kimmerling, K.A.; Mowry, K.C. Characterisation of dehydrated amnion chorion membranes and evaluation of fibroblast and keratinocyte responses in vitro. Int. Wound J. 2019, 16, 827–840.
- Koob, T.J.; Lim, J.J.; Massee, M.; Zabek, N.; Denozière, G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1353–1362.
- Jain, A.; Jaiswal, G.R.; Kumathalli, K.; Kumar, R.; Singh, A.; Sarwan, A. Comparative Evaluation of Platelet Rich Fibrin and Dehydrated Amniotic Membrane for the Treatment of Gingival Recession- A Clinical Study. J. Clin. Diagn. Res. 2017, 11, ZC24–ZC28.
- Esteves, J.; Bhat, K.M.; Thomas, B.; Varghese, J.M.; Jadhav, T. Efficacy of human chorion membrane allograft for recession coverage: A case series. J. Periodontal 2015, 86, 941–944.
- Pundir, A.J.; Agrawal, V.; Pundir, S.; Diwan, V.; Bodhi, S. Comparative evaluation of the efficacy of human chorion and amnion with coronally advanced flap for recession coverage: A case series. Clin. Adv. Periodontics 2016, 6, 118–126.
- Maity, S.; Priyadharshini, V. Comparison of chorion allograft and subepithelial connective tissue autograft in the treatment of gingival recession—A randomized controlled clinical trial. J. Oral. Biol. Craniofac Res. 2023, 13, 104–110.
- Velez, I.; Parker, W.B.; Siegel, M.A.; Hernandez, M. Cryopreserved amniotic membrane for modulation of periodontal soft tissue healing: A pilot study. J. Periodontol. 2010, 81, 1797–1804.
- Kiany, F.; Moloudi, F. Amnion membrane as a novel barrier in the treatment of intrabony defects: A controlled clinical trial. Int. J. Oral. Maxillofac. Implant. 2015, 30, 639–647.
- Samandari, M.H.; Yaghmaei, M.; Ejlali, M.; Moshref, M.; Saffar, A.S. Use of amnion as a graft material in vestibuloplasty: A preliminary report. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontol. 2004, 97, 574–578.
- Ashraf, H.; Font, K.; Powell, C.; Schurr, M. Antimicrobial activity of an amnion-chorion membrane to oral microbes. Int. J. Dent. 2019, 2019, 1269534.
- Schwab, I.R.; Reyes, M.; Isseroff, R.R. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea 2000, 19, 421–426.
- Letko, E.; Stechschulte, S.U.; Kenyon, K.R.; Sadeq, N.; Romero, T.R.; Samson, C.M.; Nguyen, Q.D.; Harper, S.L.; Primack, J.D.; Azar, D.T.; et al. Amniotic membrane inlay and overlay grafting for corneal epithelial defects and stromal ulcers. Arch. Ophthalmol. 2001, 119, 659–663.
- Hanada, K.; Shimazaki, J.; Shimmura, S.; Tsubota, K. Multilayered amniotic membrane transplantation for severe ulceration of the cornea and sclera. Am. J. Ophthalmol. 2001, 131, 323–331.
- Jirsova, K.; Jones, G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting—A review. Cell Tissue Bank. 2017, 18, 193–204.
- Nubile, M.; Dua, H.S.; Lanzini, M.; Ciancaglini, M.; Calienno, R.; Said, D.G.; Pocobelli, A.; Mastropasqua, R.; Carpineto, P. In vivo analysis of stromal integration of multilayer amniotic membrane transplantation in corneal ulcers. Am. J. Ophthalmol. 2011, 151, 809–822.
- Shay, E.; Kheirkhah, A.; Liang, L.; Sheha, H.; Gregory, D.G.; Tseng, S.C.G. Amniotic membrane transplantation as a new therapy for the acute ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis. Surv. Ophthalmol. 2009, 54, 686–696.
- Kheirkhah, A.; Johnson, D.A.; Paranjpe, D.R.; Raju, V.K.; Casas, V.; Tseng, S.C.G. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch. Ophthalmol. 2008, 126, 1059–1066.
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155.
- Datar, S.G.; Godse, G.N. Modified supportive simple limbal epithelial transplantation (M-SLET): A surgical technique modi-fied for limbal stem cell deficiency. Indian. J. Ophthalmol. 2022, 70, 4434–4437.
- Thokala, P.; Singh, A.; Singh, V.K.; Rathi, V.M.; Basu, S.; Singh, V.; MacNeil, S.; Sangwan, V.S. Economic, clinical and social impact of simple limbal epithelial transplantation for limbal stem cell deficiency. Br. J. Ophthalmol. 2022, 106, 923–928.
- Kotomin, I.; Valtink, M.; Hofmann, K.; Frenzel, A.; Morawietz, H.; Werner, C.; Funk, R.H.W.; Engelmann, K. Sutureless fixa-tion of amniotic membrane for therapy of ocular surface disorders. PLoS ONE 2015, 10, e0125035.
- Colatrella, N. Insertion and removal of sutureless amniotic membranes. J. Dry. Eye Dis. 2019, 2, 17–19.
- Ramos, T.; Scott, D.; Ahmad, S. An update on ocular surface epithelial stem cells: Cornea and conjunctiva. Stem Cells Int. 2015, 2015, 601731.
- Niederkorn, J.Y.; Wang, S. Immune privilege of the eye and fetus: Parallel universes? Transplantation 2005, 80, 1139–1144.
- Azuara-Blanco, A.; Pillai, C.T.; Dua, H.S. Amniotic membrane transplantation for ocular surface reconstruction. Br. J. Ophthalmol. 1999, 83, 399–402.
- Koizumi, N.; Fullwood, N.J.; Bairaktaris, G.; Inatomi, T.; Kinoshita, S.; Quantock, A.J. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest. Ophthalmol. Sci. 2000, 41, 2506–2513.
- Eslani, M.; Baradaran-Rafii, A.; Cheung, A.Y.; Kurji, K.H.; Hasani, H.; Djalilian, A.R.; Holland, E.J. Amniotic membrane transplantation in acute severe ocular chemical injury: A randomized clinical trial. Am. J. Ophthalmol. 2019, 199, 209–215.
- Hao, Y.; Ma, D.H.-K.; Hwang, D.G.; Kim, W.S.; Zhang, F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2000, 19, 348–352.
- Gündüz, K.; Uçakhan, O.O.; Kanpolat, A.; Günlap, I. Nonpreserved human amniotic membrane transplantation for conjunc-tival reconstruction after excision of extensive ocular surface neoplasia. Eye 2006, 20, 351–357.
- Morkin, M.I.; Hamrah, P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul. Surf. 2018, 16, 132–138.
- Mejía, L.F.; Santamaría, J.P.; Acosta, C. Symptomatic management of postoperative bullous keratopathy with nonpreserved human amniotic membrane. Cornea 2002, 21, 342–345.
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Percoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair. Regen. 1994, 2, 165–170.
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair. Regen. 2009, 17, 763–771.
- Dinh, T.; Lewis, C. Amnion applications in the foot and ankle. Clin. Podiatr. Med. Surg. 2019, 36, 563–576.
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378.
- Pang, C.; Ibrahim, A.; Bulstrode, N.W.; Ferretti, P. An overview of the therapeutic potential of regenerative medicine in cu-taneous wound healing. Int. Wound J. 2017, 14, 450–459.
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health 2018, 21, 27–32.
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206.
- Avishai, E.; Yeghiazaryan, K.; Golubnitschaja, O. Impaired wound healing: Facts and hypotheses for multi-professional con-siderations in predictive, preventive, and personalised medicine. EPMA J. 2017, 8, 23–33.
- Garoufalis, M.; Nagesh, D.; Sanchez, P.J.; Lenz, R.; Park, S.J.; Ruff, J.G.; Tien, A.; Goldsmith, J.; Seat, A. Use of dehydrated human amnion/chorion membrane allografts in more than 100 patients with six major types of refractory nonhealing wounds. J. Am. Podiatr. Med. Assoc. 2018, 108, 84–89.
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116.
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228.
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound dressings: A comprehensive review. Curr. Dermatol. Rep. 2016, 5, 287–297.
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Prim. 2022, 8, 50.
- Sheikh, E.S.; Sheikh, E.S.; Fetterolf, D.E. Use of dehydrated human amniotic membrane allografts to promote healing in pa-tients with refractory non-healing wounds. Int. Wound. J. 2014, 11, 711–717.
- Zelen, C.M.; Serena, T.E.; Denoziere, G.; Fetterolf, D.E. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int. Wound. J. 2013, 10, 502–507.
- Serena, T.E.; Carter, M.J.; Le, L.T.; Sabo, M.J.; DiMarco, D.T. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer com-pression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014, 22, 688–693.
- Zelen, C.M. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J. Wound Care 2013, 22, 347–348, 350–351.
- Snyder, R.J.; Shimozaki, K.; Tallis, A.; Kerzner, M.; Reyzelman, A.; Lintzeris, D.; Bell, D.; Rutan, R.L.; Rosenblum, B. A pro-spective, randomized, multicenter, controlled evaluation of the use of dehydrated amniotic membrane allograft compared to standard of care for the closure of chronic diabetic foot ulcers. Wounds 2016, 28, 70–77.
- Tettelbach, W.H.; Cazzell, S.M.; Hubbs, B.; De Jong, J.L.; Forsyth, R.A.; Reyzelman, A.M. The influence of adequate debride-ment and placental-derived allografts on diabetic foot ulcers. J. Wound Care 2022, 31 (Suppl. S9), S16–S26.
- Niknejad, H.; Paeini-Vayghan, G.; Tehrani, F.A.; Khayat-Khoei, M.; Peirovi, H. Side dependent effects of the human amnion on angiogenesis. Placenta 2013, 34, 340–345.
- Duan-Arnold, Y.; Uveges, T.E.; Gyurdieva, A.; Johnson, A.; Danilkovich, A. Angiogenic potential of cryopreserved amniotic membrane is enhanced through retention of all tissue components in their native state. Adv. Wound Care 2015, 4, 513–522.
- Kjaergaard, N.; Hein, M.; Hyttel, L.; Helmig, R.B.; Schonheyder, H.C.; Uldbjerg, N.; Madsen, H. Antibacterial properties of human amnion and chorion in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 94, 224–229.
- Frazão, L.P.; Viera de Castro, J.; Nogueira-Silva, C.; Neves, N.M. Decellularized human chorion membrane as a novel bio-material for tissue regeneration. Biomolecules 2020, 10, 1208.
- McQuilling, J.P.; Vines, J.B.; Kimmerling, K.A.; Mowry, K.C. Proteomic comparison of amnion and chorion and evaluation of the effects of processing on placental membranes. Wounds 2017, 29, E36–E40.
- de Weerd, L.; Weum, S.; Sjåvik, K.; Acharya, G.; Hennig, R.O. A new approach in the repair of a myelomeningocele using amnion and a sensate perforator flap. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 860–863.
- Turchan, A.; Rochman, T.F.; Ibrahim, A.; Fauziah, D.; Wahyuhadi, J.; Parenrengi, M.A.; Fauzi, A.A.; Sufarnap, E.; Bajamal, A.H.; Ferdiansyah; et al. Duraplasty using amniotic mem-brane versus temporal muscle fascia: A clinical comparative study. J. Clin. Neurosci. 2018, 50, 272–276.
- Marton, E.; Giordan, E.; Gallinaro, P.; Curzi, C.; Trojan, D.; Paolin, A.; Guerriero, A.; Rossi, S.; Bendini, M.; Longatti, P.; et al. Homologous amniotic membrane as a dural substitute in decompressive craniectomies. J. Clin. Neurosci. 2021, 89, 412–421.
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cell Mater. 2008, 15, 88–99.
- Yang, L.; Shirakata, Y.; Shudou, M.; Dai, X.; Tokumaru, S.; Hirakawa, S.; Sayama, K.; Hamuro, J.; Hashimoto, K. New skin-equivalent model from de-epithelialized amnion membrane. Cell Tissue Res. 2006, 326, 69–77.
- Tanaka, Y.; Kubota, A.; Yokokura, S.; Uematsu, M.; Shi, D.; Yamato, M.; Okano, T.; Quantock, A.J.; Nishida, K. Optical me-chanical refinement of human amniotic membrane by dehydration and cross-linking. J. Tissue Eng. Regen. Med. 2012, 6, 731–737.
- Sarvari, R.; Keyhanvar, P.; Agbolaghi, S.; Roshangar, L.; Bahremani, E.; Keyhanvar, N.; Haghdoost, M.; Keshel, S.H.; Taghi-khani, A.; Firouzi, N.; et al. A comprehensive review on methods for promotion of mechani-cal features and biodegradation rate in amniotic membrane scaffolds. J. Mater. Sci. Mater. Med. 2022, 33, 32.
- Spoerl, E.; Wollensak, G.; Reber, F.; Pillunat, L. Cross-linking of human amniotic membrane by glutaraldehyde. Ophthalmic Res. 2004, 36, 71–77.
- Lai, J.Y.; Ma, D.-H.K. Glutaraldehyde cross-linking of amniotic membranes affects their nanofibrous structures and limbal epithelial cell culture characteristics. Int. J. Nanomed. 2013, 8, 4157–4168.
- Wilmas, K.M.; Patel, J.; Silapunt, S.; Doan, H.Q.; Migden, M.R. The use of a dehydrated complete human placental mem-brane allograft for Mohs surgical defects of the nose. Dermatol. Surg. 2023, 49, 343–347.
- Ingraldi, A.L.; Lee, D.; Tabor, A.J. Post-Mohs Surgical Defect Repair with Dehydrated Human Amnion-Amnion Membrane: A Retrospective Clinical Case Study. J. Med. Case Rep. Case Ser. 2023, 4.
- Toman, J.; Michael, G.M.; Wisco, O.J.; Adams, J.R.; Hubbs, B.S. Mohs defect repair with dehydrated human amnion/chorion membrane. Facial Plast. Surg. Aesthetic Med. 2022, 24, 48–53.
- Klama-Baryła, A.; Rojczyk, E.; Kitala, D.; Łabuś, W.; Smętek, W.; Wilemska-Kucharzewska, K.; Kucharzewski, M. Preparation of placental tissue transplants and their application in skin wound healing and chosen skin bullous diseases—Stevens-Johnson syndrome and toxic epidermal necrolysis treatment. Int. Wound J. 2020, 17, 491–507.
- Charlton, O.A.; Harris, V.; Phan, K.; Mewton, E.; Jackson, C.; Cooper, A. Toxic epidermal necrolysis and Steven-Johnson syndrome: A comprehensive review. Adv. Wound Care 2020, 9, 426–439.
- Aliabadi, S.B.; Dogahe, Z.H.; Feizkhah, A.; Mobayen, M.; Mirbolouk, B. Management of toxic epidermal necrolysis using early combination therapy of intravenous immunoglobulin and amniotic membrane grafting: A case report. J. Burn. Care Res. 2023, 44, 467–470.
- Branski, L.K.; Herndon, D.N.; Celis, M.M.; Norbury, W.B.; Masters, O.E.; Jeschke, M.G. Amnion in the treatment of pediatric partial-thickness facial burns. Burns 2008, 34, 393–399.
- Mostaque, A.K.; Rahman, K.B. Comparisons of the effects of biological membrane (amnion) and silver sulfadiazine in the management of burn wounds in children. J. Burn. Care Res. 2011, 32, 200–209.
- Tenenhaus, M. The use of dehydrated human amnion/chorion membranes in the treatment of burns and complex wounds. Ann. Plast. Surg. 2017, 78 (Suppl. S1), S11–S13.
- Yang, C.; Xiong, A.B.; He, X.C.; Ding, X.B.; Tian, X.L.; Li, Y.; Yan, H. Efficacy and feasibility of amniotic membrane for the treatment of burn wounds: A meta-analysis. J. Trauma. Acute Care Surg. 2021, 90, 744–755.
- Ravishankar, R.; Bath, A.S.; Roy, R. Amnion bank—The use of long-term glycerol preserved amniotic membranes in the man-agement of superficial and superficial partial thickness burns. Burns 2003, 29, 369–374.
- Kesting, M.R.; Wolff, K.D.; Hohlweg-Majert, B.; Steinstraesser, L. The role of allogenic amniotic membrane in burn treatment. J. Burn. Care Res. 2008, 29, 907–916.




